Abstract
Success in resolving hepatitis C virus (HCV) infection has been correlated to vigorous, multispecific, and sustained CD8+ T-cell response in humans and chimpanzees. The efficacy of inducing T-cell-mediated immunity by recombinant serotype 5 adenovirus vector has been proven in many animal models of infectious diseases, but its immunogenicity can be negatively influenced by preexisting immunity against the vector itself. To evaluate the less prevalent adenovirus serotype 6 (Ad6) as an alternative vector for and HCV vaccine development, we have generated serotype 5 and 6 adenoviral vectors directing expression of the nonstructural region of HCV (MRKAd5-NSmut and MRKAd6-NSmut). Immunogenicity studies in mice showed that the two vectors induced comparable T-cell responses but that only MRKAd6-NSmut was not suppressed in the presence of anti-Ad5 immunity. In contrast, preexisting anti-Ad5 immunity dramatically blunted the immunogenicity of the serotype 5-based HCV vector. Furthermore, MRKAd6-NSmut showed equivalent potency, breadth, and longevity of HCV-specific T-cell responses in rhesus macaques as the corresponding Ad5-based vector over a wide range of doses and was capable of boosting DNA-primed animals even if administered at low doses. These data support the use of the MRKAd6-NSmut for anti-HCV immunotherapy and, more generally, for the Ad6 serotype as a better genetic vaccine vehicle than Ad5.
Liver disease caused by hepatitis C virus (HCV) infection is a major medical problem, affecting an estimated 170 million people worldwide (20, 30). No effective vaccine is available, and the consensus therapeutic treatment, consisting of PEGylated alpha interferon (IFN-α) in combination with ribavirin, is poorly effective against some viral genotypes (16, 30).
The current literature suggests that once chronic infection is established, the HCV-specific immune response exerts some control over viral load, but in most cases it is unable to terminate persistent infection and to resolve chronic hepatitis (16). As in the case of other pathogens, like human immunodeficiency virus type 1 (HIV-1), that are able to establish persistent infection, the outcome of HCV disease is the result of a balance between the kinetics and the magnitude of the immune response, the pathogen replication rate, and the accessibility of infected cells to the immune response. Anti-HCV preexisting immunity induced by vaccination may be more successful in preventing the establishment of HCV chronic infection. Toward this end, development of a B-cell-based vaccine is a very difficult task due to the high genetic variability of the virus. In fact, anti-HCV antibodies capable of neutralizing virus infectivity ex vivo have been described, but these antibodies are generally virus isolate specific (12).
Several studies indicated that virus-specific T-cell proliferative and cytotoxic responses are significantly stronger and target more HCV antigens in individuals who resolved acute infection compared to those who developed chronic infection (11, 14, 15, 21, 27, 42). Furthermore, duration of functional CD4+ and CD8+ T-cell responses following primary infection appears fundamental to achieving viral clearance (13a, 19, 22, 37, 41). In addition, there is now strong evidence that cellular immunity induced by primary infection in acute or resolving humans or chimpanzees provides protection from rechallenge with either homologous or heterologous viral strains in a large number of cases (5, 18, 23, 26, 28). Thus, HCV immunogens able to elicit strong and broad cellular-mediated immunity (CMI) represent a valid approach for an HCV vaccine. In particular the nonstructural (NS) region of HCV appears to be a good candidate immunogen in light of its sequence conservation among different isolates. The NS region encompasses about two thirds of the HCV genome and encodes five different proteins (NS3, NS4A, NS4B, NS5A, and NS5B) that result from the proteolytic cleavage of the HCV polyprotein by the encoded NS3 protease. Furthermore, despite the fact that cytotoxic T lymphocyte (CTL) epitopes have been identified in all viral proteins, recent data collected in chronically and acutely infected patients indicated that responses against the NS region are more prevalent in the latter group (37).
Viral delivery of genetic vaccines is a powerful mean of inducing antiviral T-cell immune responses. Extensive immunization experiments conducted in rodents and nonhuman primates utilizing vectors encoding the HIV gag antigen indicated that recombinant viral vectors were the most effective in eliciting specific CTL responses, particularly those based on replication-defective adenovirus (36). From more than 50 human adenovirus subtypes known, the most used one is serotype 5 (Ad5). Nonhuman primate immunization and challenge studies have shown that CTL responses elicited by an Ad5 vaccine vector can provide significant control of a simian AIDS virus (35). Similarly, Ad5-based vaccination with vectors encoding the Ebola glycoprotein and nucleoprotein prevented infection of cynomolgus macaques after challenge with either low or high doses of virus (38). However, preexisting anti-Ad immunity can significantly dampen vaccine responses (8). Epidemiological studies (9) suggest that most North Americans have anti-Ad5 neutralizing antibody (NAb) titers, and about one-third of them are relatively high. Other parts of the world typically exhibit even higher frequencies and levels of anti-Ad5 antibodies.
Considering the impact of anti-Ad5 neutralizing antibodies present in the human population, development of adenovirus vaccine vectors based on alternative serotypes is an important research priority. Data available in the literature suggest that high titers of neutralizing antibodies against Ad6 are less prevalent than those against other subgroup C human adenoviruses such as Ad5 and Ad2 (10). However, the extent of immunologic cross-reactivity between Ad5 and Ad6 and the immunogenicity of Ad6-based vaccine vectors have not been previously determined. In this study, we investigated the seroprevalence and the cross-neutralization of Ad5 and Ad6 in two cohorts of healthy individuals from Europe and from Egypt (where high frequency of chronic HCV infection has been reported [34]). We then tested the impact of anti-Ad5 immunity on the immunogenicity of serotype 5- and serotype 6-based HCV vaccine vectors in mice and compared the Ad5 and Ad6 HCV vectors for their ability to induce a strong and long lasting T-cell response in mice and rhesus macaques.
MATERIALS AND METHODS
Generation of MRKAd5 and MRKAd6 genome plasmids with HCV NSmut sequence.
The V1JNS3-5Akozak plasmid (S. Capone, I. Zampaglione, A. Vitelli, M. Pezzanera, L. Kierstead, J. Burns, L. Ruggeri, M. Arcuri, E. Cappelletti, A. Meola, B. Bruni Ercole, R. Tafi, C. Santini, A. Luzzago, T. M. Fu, G. Ciliberto, R. Cortese, A. Nicosia, E. Fattori and A. Folgori, submitted for publication) was digested with BglII and XbaI restriction enzymes, and the DNA fragment containing the Kozak sequence and the sequence coding NS3-5A from a BK isolate (39) was cloned into the polypMRKpdelE1 vector and digested with BglII and XbaI, generating the shuttle plasmid shNS3-NS5Akozak. The polypMRKpdelE1 shuttle vector, for recombination into Ad5 and Ad6 backbone, derives from MRKAd5 shuttle vector (45), which has been modified by inserting a polylinker into the unique BglII restriction site of MRKAd5. A mutated version of the NS5B gene was obtained by replacing the Gly-Asp-Asp sequence corresponding to amino acid positions 1711 to 1713 of the complete polyprotein with Ala-Ala-Gly. This polymerase motif is conserved among all positive-stranded RNA viruses, and mutating these three residues inhibits or abolishes the RNA-dependent RNA polymerase activity of purified HCV NS5B and the infectivity of HCV RNA in chimpanzees (17). The mutated NS5B fragment was obtained by assembly PCR and inserted into shNS3-5Akozak vector via homologous recombination, generating polypMRKpdelE1NSmut. In polypMRKpdelE1NSmut the NSmut coding sequence is under the control of human cytomegalovirus early promoter and bovine growth hormone polyadenylation signal.
The expression cassette and the flanking regions that contain adenovirus sequences allowing homologous recombination with both Ad5 and Ad6 were excised by digestion with PacI and Bst1107I restriction enzymes and cotransformed with either pAd5HVO (E1− E3−) (45) or pAd6 (E1− E3−) (7) ClaI-linearized genome plasmids into the bacterial strain BJ5183, to generate pAd5HVONSmut and pAd6E1-E3-NSmut, respectively.
MRKAd5 and MRKAd6 adenovirus rescue and quantification.
Adenovectors were rescued by using the human E1-expressing PER.C6 cell line. PER.C6 cells were passaged in Dulbecco modified Eagle medium ([DMEM] catalog no. 41966-290; GibcoBRL) containing 10% fetal bovine serum (catalog no. 10270-06; GibcoBRL) and 1% penicillin-streptomycin-glutamine. Recombinant MRKAd5 and MRKAd6 viruses were rescued and amplified as previously described (44). Briefly, 5 × 106 PER.C6 cells planted on 6-cm culture dishes were transfected by Lipofectamine (Invitrogen) with 10 μg of cloned viral vector released from plasmid sequences by PacI digestion. Complete cytopathic effect was observed about 6 days posttransfection. Cells and supernatant were harvested, freeze-thawed three times, clarified by spinning at 2,000 rpm for 20 min at room temperature (RT). The resulting lysate was serially passed on PER.C6 cells to increase the titer of the rescued virus. A large prep was obtained by infection of 10 cell factories (catalog no. 167695; Nunc) and purification of the cell lysate on CsCl gradient.
To determine the number of viral particles (VP), the CsCl-purified viruses were diluted 1/10 and 1/100 in 0.1% sodium dodecyl sulfate-phosphate-buffered saline (PBS). As a control, buffer A105 (8) was used. These dilutions were incubated for 10 min at 55°C. After tubes were spun briefly, the optical density at 260 nm was measured. The number of VP was calculated on the basis of the following equation: 1 optical density (at 260 nm) unit = 1.1 × 1012 VP/ml.
In vitro expression in mammalian cells.
HeLa cells were plated at 1.5 × 106 cells/10-cm culture dish in DMEM supplemented by 10% heat-inactivated fetal calf serum (catalog no. 1027106; GibcoBRL). After 24 h, medium was removed and cells were infected with 5 ml of fetal calf serum-free medium containing the virus diluted to different multiplicities of infection (MOIs of 50, 250, and 1,250). The infection was carried out for 1 h at 37°C in the CO2 incubator. Then the virus was removed, 5 ml of DMEM supplemented with 5% horse serum (GibcoBRL catalog no. 16050098) was added, and the cells were kept at 37°C for 48 h.
Cell extracts were prepared in 1% Triton-TEN buffer (10 mM Tris, 1 mM EDTA, 100 mM NaCl). The extracts, normalized according to total protein content, were separated on a 12.5% acrylamide gel and blotted on nitrocellulose. Nonspecific binding sites were blocked with milk buffer (5% nonfat dry milk, 0.05% Tween 20 in PBS) for 1 h at RT. Mouse monoclonal antibodies directed to NS3 and NS5B and rabbit polyclonal antibody against NS5A were diluted in milk buffer and incubated overnight at 4°C. After extensive washes in 0.05% Tween 20-PBS, secondary antibody (anti-mouse horseradish peroxidase conjugate [Sigma A8924, diluted 1/1000]; anti-rabbit horseradish peroxidase conjugate [Pierce 31463, diluted 1/5000]) was added and incubated at RT for 1 h. Filters were washed, developed by using 5 ml/filter of SuperSignal West Pico Chemiluminescent Substrate (Pierce catalog no. 34080), and exposed on Kodak films for a few minutes.
Neutralization assay.
This assay was based on blocking the infection of 293 cells by MRKAd5 and MRKAd6 carrying the gene for secreted alkaline phosphatase (SEAP). 293 cells were seeded in a 96-well plate at 3 × 104 cells/well 2 days before the assay. The test sera were heat inactivated, diluted in five fourfold increments (from 1:18 through 1: 4,608), and preincubated for 1 h at 37°C with a fixed amount of virus (approximately 104 PFU/ml) in 10% fetal bovine serum-DMEM (phenol red free; GibcoBRL catalog no. 31053-028). The mixture of virus plus serum was added to the plate and left for 1 h at 37°C. After the incubation, 200 μl/well of fresh medium was added. At 24 h postinfection SEAP expression was determined on a 50-μl sample by using Phospha-light TM, Chemiluminescent Reporter Assay (TROPIX catalog no. BP300). Neutralization titers were defined as the dilution where a 50% reduction of SEAP activity from serum sample was observed relative to SEAP activity from virus alone.
Animals and immunization.
BALB/c, C3H, CD1 and C57BL/6 mice were purchased from Charles River (Como, Italy). Rhesus macaques (Macaca mulatta) were housed at New Iberia Research Center, New Iberia, La. The animals involved in the studies all met the following criteria: in good health, free of known infectious or immunological disease, and no previous contact with a pathogen related to HCV. All animal care and treatment were in accordance with standards approved by the Institutional Animal Care and Use Committee in conformity with national and international laws and policies (EEC Council Directive 86/609, OJ L 358, 1, 12 December 1987; Italian Legislative Decree 116/92, Gazzetta Ufficiale della Repubblica Italiana no. 40, 18 February 1992).
In a first set of experiments, 6-week-old female mice were immunized with either 107 or 109 MRKAd5-NSmut or MRKAd6-NSmut VP suspended in 100 μl of physiological solution and injected in the quadriceps muscles (50 μl/site). Three weeks after priming, the animals were boosted by means of the same dosage used for priming. Two weeks after boost, mice were euthanized and splenocytes were prepared and tested by enzyme-linked immunospot (ELISPOT) assay. In a second set of experiments, mice were preimmunized twice with 1010 VP of Ad5 expressing an unrelated antigen 2 weeks before immunization. Preimmunized and naïve mice were then immunized with a single injection of MRKAd5-NSmut or MRKAd6-NSmut at 108 VP, and immune response was tested on splenocytes 2 weeks after immunization.
Rhesus macaques were immunized with a suspension of 108, 1010, or 1011 MRKAd5-NSmut or MRKAd6-NSmut VP in 1 ml of buffer A105 injected in the deltoid muscle at two sites (0.5 ml/site). One group of monkeys was immunized with 5 mg of a DNA plasmid encoding the HCV NS region (pV1JnsNSOPTmut) in 1 ml of PBS, injected in the deltoid muscle at two sites (0.5 ml/site). Construction and characterization of the pV1JnsNSOPTmut has been described in detail elsewhere (S. Capone et al., submitted). Monkey groups and the number and timing of injections are detailed in Table 1. At serial time points blood was drawn and serum samples and PBMC were prepared as described (S. Capone et al., submitted) and frozen for future use in neutralization and immunological assays.
TABLE 1.
Rhesus immunization schedule
| Group | Animal no. | Immunogen | Dose | Schedule (wk) |
|---|---|---|---|---|
| A | 99C059 | MRKAd5-NSmut | 1011 VP | 0, 4, 24 |
| 99C060 | ||||
| 97X009 | ||||
| 96069 | ||||
| B | 98C047 | MRKAd6-NSmut | 1011 VP | 0, 4, 24 |
| 98C055 | ||||
| 93G | ||||
| 97X014 | ||||
| C | S201 | MRKAd5-NSmut | 1010 VP | 0, 4, 24 |
| 075Q | ||||
| 137Q | ||||
| D | S207 | MRKAd6-NSmut | 1010 VP | 0, 4, 24 |
| 035Q | ||||
| 057Q | ||||
| 98D209 | ||||
| 106Q | ||||
| 113Q | ||||
| E | 57G | MRKAd5-NSmut | 108 VP | 0, 4, 24 |
| 98C096 | ||||
| 98C099 | ||||
| F | 077Q | MRKAd6-NSmut | 108 VP | 0, 4, 24 |
| S206 | ||||
| 086Q | ||||
| 95116 | ||||
| 138T | ||||
| G | 97X007 | pV1JnsNSOPTmut | 5 mg | 0, 4, 8 |
| 99C071 | MRKAd6-NSmut | 108 VP | 24 | |
| 96075 | ||||
| 21G | ||||
| 99C161 | ||||
| 99C166 |
At the beginning of this study, rhesus autologous B lymphoblastoid cell lines (B-LCLs) were generated by immortalizing peripheral blood mononuclear cells (PBMC) from each animal with herpesvirus papio (culture supernatant of the baboon B-cell line S594) as described (S. Capone, submitted).
Peptides and recombinant vaccinia virus for T-cell assays.
The peptide sequence spanning the NS3-NS5B region reproduced the amino acid sequence of the HCV BK strain (39). Peptides were purchased from Bio-Synthesis Inc., Lewisville, Texas. The peptides, 20 amino acids (aa) in length, overlapping by 10 aa, were synthesized with free N-terminal amine and free C-terminal carboxylate and purified by preparative high-pressure liquid chromatography (HPLC). To facilitate analysis, the NS3-NS5B peptides were combined in six pools covering NS3 protease (NS3p), NS3 helicase (NS3h), NS4, NS5A, and NS5B (split in two pools as NS5B-I and NS5B-II). Stock pools were stored in 100% dimethyl sulfoxide (DMSO) at a concentration of 0.6 mg/ml of each peptide and were used at a final concentration of approximately 4 μg/ml, attained by dilution in culture medium. Peptide G-1480 corresponds to aa 1626 to 1645 of the HCV strain BK polyprotein. DMSO alone was used as a negative control for immunological assays since extensive analysis showed no difference in the level of background response obtained with either unrelated peptide pool or DMSO alone.
The recombinant vaccinia virus encoding the nonstructural proteins NS2, NS3, NS4, and NS5, strain BK 1b (VacHCV-NS), was kindly provided by J. Condra (Merck and Co., Inc.).
Immunological assays.
Antigen-specific IFN-γ production by splenocytes of immunized mice was determined by a standard ELISPOT assay described in detail elsewhere (46). Briefly, cells were plated in duplicate wells at two different densities (250,000 and 500,000 cells per well) and stimulated overnight with NS peptide pools or peptide G-1480. DMSO and concanavalinA ([ConA] C 2010; Sigma-Aldrich) were used, respectively, as negative and positive controls.
An IFN-γ ELISPOT assay with rhesus PBMC was performed as described (S. Capone et al., submitted) by plating 200,000 and 400,000 cells in duplicate per well and stimulating overnight with 20-mer HCV NS peptide pools. DMSO and ConA were used, respectively, as negative and positive controls. The ELISPOT response was considered positive when all of the following conditions were met: IFN-γ production was present in ConA-stimulated wells; there were at least 55 specific spots/million PBMC to at least one HCV peptide pool; the number of spots seen in positive wells was three times the number detected in the mock control wells (DMSO); and responses decreased with dilutions of PBMC.
IFN-γ intracellular staining (ICS) and fluorescence-activated cell sorting analysis in rhesus PBMC were performed as described elsewhere (S. Capone et al., submitted) using the following conjugated antibodies for the cell surface staining: CD3-allophycocyanin (rhesus clone FN-18, custom conjugated) CD4-phycoerythrin (clone L-200; Pharmingen catalog no. 550630), and CD8-peridinin chlorophyll protein (Beckton Dickinson catalog no. 345774). For intracellular staining IFN-γ fluorescein isothiocyanate (Biosource catalog no. AHC4338) antibody was used. PBMC were restimulated overnight using 20-mer HCV NS peptide pools. DMSO and staphylococcal enterotoxin B (Sigma-Aldrich catalog no. S-4881) served, respectively, as negative and positive controls. A threshold for positive response was set at 0.1% IFN-γ+ CD3+ CD8+ or IFN-γ+ CD3+ CD4+ T cells and three times the negative control.
A standard 4-h 51Cr release cytotoxicity assay was performed exactly as described (S. Capone et al., submitted). CTL responses were scored positive when percent specific lysis at the two highest effector-to-target ratios was greater or equal to the percent lysis of control target wells plus 10.
Statistical analysis.
Spearman's correlation coefficient was computed to determine the degree of correlation between NAb titers against Ad5 and Ad6. Student's t test was used to assess differences between distributions. A P value of <0.05 was considered significant.
RESULTS
Ad6 has a lower seroprevalence than Ad5, and anti-Ad5 antibodies do not cross-react with Ad6.
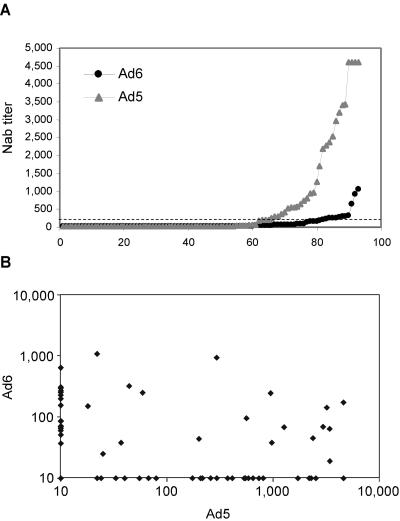
To test whether an Ad vector based on serotype 6 might be a valid alternative to the most common serotype 5 Ad-based vector, we investigated the prevalence of anti-Ad5 and anti-Ad6 NAbs in the human population. The method employed is based on a 96-well format with the use of recombinant Ad vectors carrying SEAP as a reporter gene (3). We screened a panel of sera from 93 European individuals and found that 48% of the tested samples had NAbs to Ad5, while a lower frequency of samples positive for anti-Ad6 NAb was detected (38%). Furthermore, 31 and 11 subjects had NAb titers above 200 against Ad5 and Ad6, respectively (P < 0.005) (Fig. 1A). Interestingly, a similar analysis performed in individuals enrolled in an Ad5-based HIV vaccine early phase I clinical trial suggested that anti-Ad5 titers below 200 have a limited impact on vector immunogenicity (J. W. Shiver and E. A. Emini, personal communication). We also performed a similar survey on 41 sera from Egypt, the country with the highest HCV prevalence worldwide (22%) (34). The results of this experiment revealed a seemingly lower seroprevalence of Ad6 compared to Ad5 (17% versus 25% of sera with no NAb, respectively; data not shown).
FIG. 1.
Prevalence and cross-reactivity of anti-Ad5 and anti-Ad6 neutralizing antibodies. (A) Ad5 and Ad6 seroprevalence. Individual NAb titers against Ad5 or Ad6 present in whole sera from 93 European individuals are shown. The dashed line corresponds to NAb titers of 200. (B) Correlation between neutralization titers measured in the same panel of sera from European individuals with MRKAd5 and MRKAd6 in SEAP neutralization assay. Statistical evaluation was done by Spearman correlation coefficient (rho = 0.12; P = 0.91).
The antibody cross-reactivity between Ad5 or Ad6 serotypes was examined analyzing the NAb titers obtained on the described serum panel from European subjects by Spearman's correlation coefficient (Fig. 1B). The analysis showed no correlation between the two serotypes, indicating that the presence of a high titer for one serotype does not result in a cross-reactive titer for the other serotype.
Because of the lower seroprevalence of Ad6 with respect to Ad5 and the lack of cross-reactivity between the two serotypes, Ad6 was chosen as a vector to be tested in mice and nonhuman primates for the induction of anti-HCV CMI.
MRKAd6-NSmut and MRKAd5-NSmut vectors direct expression of correctly processed NS products.
We generated replication-defective Ad vectors from both Ad5 and Ad6 serotypes which direct expression of the HCV NS region from the BK strain (genotype 1b). The derivation of the vector backbones has been previously reported (7). We decided to use an E3-deleted version of these backbones to increase vector capacity. The inserted NS gene was mutated to create an inactive form of the RNA-dependent RNA polymerase NS5B. As reported (17), an identical mutation in the context of the genetic background of an infectious HCV H77 strain generates a strain [HCV FL(pol−)] that has wild-type-like protein expression and processing but has lost infectivity.
The recombinant viruses were rescued in a PER.C6 cell line, and a complete cytopathic effect was already visible 5 days posttransfection. Although we had reached about the 103% cloning capacity of the vectors, growth rate was satisfactory (approximately 104 VP/cell). Genetic stability is the major concern in scaling up production, so we tested the genetic stability of our recombinant adenovirus vectors after 14 consecutive passages. DNA structure was analyzed by radioactive restriction digestion labeling, and no genetic change was detected (data not shown).
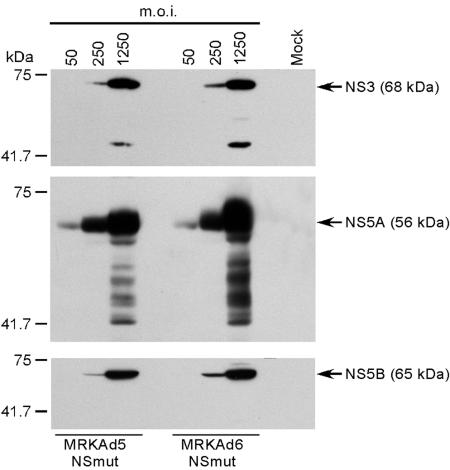
Expression of the inserted transgene was tested by infection of HeLa cells with either MRKAd6-NSmut or MRKAd5-NSmut at MOIs of 50, 250, and 1,250. At 48 h postinfection cell extracts were prepared and tested in Western blotting with antibodies directed at NS3, NS5A, and NS5B. Results showed that the HCV polyprotein was correctly cleaved and that the two adenovirus vectors were perfectly equivalent in terms of NS protein expression levels (Fig. 2). Multiple bands were detected when anti-NS3 and anti-NS5A antibodies were probed against extracts generated with the highest MOI (1,250). At present, it is not clear whether these bands are degradation products or are due to poor antibody specificity.
FIG. 2.
NS polyprotein expression and processing by infection of HeLa cells with MRKAd5-NSmut and MRKAd6-NSmut. Western blotting of whole-cell extracts from HeLa cells infected at different MOIs with MRKAd5-NSmut or MRKAd6-NSmut. Whole-cell extracts were normalized for total protein content before loading on gel. Mature NS3, NS5A, and NS5B products (indicated by arrows) were detected with specific antibodies. Extract from mock-infected cells was used as a negative control (mock). Molecular weight markers are reported on the left side of each panel.
MRKAd6-NSmut shows equivalent immunogenicity to MRKAd5-NSmut in mice and can overcome preexisting anti-Ad5 immunity.
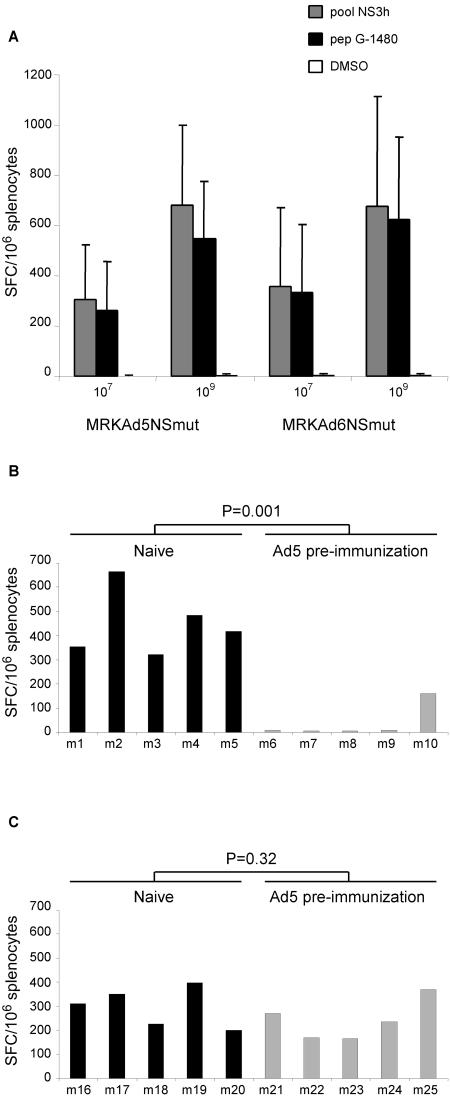
MRKAd5-NSmut was injected in different mice strains to evaluate its potential to elicit anti-HCV T-cell immune responses. Four different strains of mice (BALB/c, n = 10; C57BL/6, n = 9; C3H, n = 5; and CD1, n = 7) were injected intramuscularly with 109 VP of CsCl-purified virus, and each animal received two doses three weeks apart. T cell response was analyzed by the quantitative ELISPOT assay measuring the number of IFN-γ secreting T cells in response to pools of 20-mer peptides encompassing the NS3-NS5B sequence. MRKAd5-NSmut was capable of inducing T-cell responses targeting different NS antigens in all strains of mice but to a different extent presumably due to the different major histocompatibility complex haplotypes of the tested mice strains (data not shown). A potent response against the peptide pool covering the NS3 helicase domain (NS3h) was induced in C57BL/6 mice. This response was narrowed down to a single 20-mer peptide containing a CD8 epitope (peptide G-1480; data not shown) to facilitate quantitative peptide-specific immune assays. Immunogenicity studies were then carried out in C57BL/6 mice to test the MRKAd6-NSmut vector in a head-to-head comparison with MRKAd5-NSmut. Groups of mice were injected with two doses of 107 or 109 VP of CsCl-purified virus 3 weeks apart. T-cell response was analyzed by IFN-γ ELISPOT assay using the peptide pool NS3h and peptide G-1480. As shown in Fig. 3A, MRKAd6-NSmut displays a similar potency to the corresponding serotype 5-based vector. Statistical analysis of the data confirmed that the two vectors induced equivalent T-cell responses (P = 0.67 and P = 0.53 for CMI induced by injection of dose of 107 VP and measured using the NS3h peptide pool and the G-1480 peptide, respectively; P = 0.98 and P = 0.58 for the corresponding data obtained by immunizing mice with 109 VP).
FIG. 3.
Immunogenicity of MRKAd5-NSmut and MRKAd6-NSmut in naïve and in Ad5 preimmune mice. (A) Immunogenicity of MRKAd5-NSmut and MRKAd6-NSmut in naïve mice. IFN-γ ELISPOT responses to the NS3h peptide pool and the CD8+ peptide G-1480 in C57BL/6 mice immunized with MRKAd5-NSmut and MRKAd6-NSmut at doses of 107 and 109 VP (indicated at the bottom). Results are expressed as the number of IFN-γ SFC per 106 splenocytes and are reported on the vertical axis. Each bar represents the average of the response to the NS3h peptide pool (gray bars) and CD8+ G-1480 peptide (black bars) measured in groups of 10 immunized mice. Response to DMSO is represented by the white bars. Average number of spots in DMSO control samples was 2 per 106 splenocytes. (B and C) Immunogenicity of MRKAd5-NSmut and MRKAd6-NSmut in mice with preexisting anti-Ad5 immunity. C57BL/6 mice were preimmunized with 1010 VP of Ad5 expressing an unrelated antigen. Groups (n = 5) of naive mice or mice with anti-Ad5 immunity were immunized with 108 VP of MRKAd5-NSmut or MRKAd6-NSmut, and responses against NS3h peptide pool were assessed by IFN-γ ELISPOT assay. Results are expressed as the number of IFN-γ SFC per 106 splenocytes and are reported on the vertical axis. Each bar represents the individual response of animals immunized with MRKAd5-NSmut (B) or MRKAd6-NSmut (C). Anti-Ad5 neutralization titers were between 200 and 4,000 except in mouse m10 (NAb of 50).
We next determined the impact of anti-Ad5 immunity on cellular responses elicited by adenovirus 5 and 6 serotypes. To model preexisting anti-Ad5 immunity, C57BL/6 mice were preimmunized with 1010 particles of Ad5 expressing an unrelated antigen 2 weeks before immunization with the HCV vectors. Groups of naive and Ad5-preimmunized mice (5 mice/group) were then injected with a single dose of 108 VP of MRKAd5-NSmut or MRKAd6-NSmut. In Fig. 3B and C are shown the data from one representative experiment indicating that anti-Ad5 preexisting immunity completely abrogated HCV-specific cellular immune response elicited by the Ad5-based HCV vector in four out of five animals. The difference between preimmunized and naïve animals was statistically significant (P = 0.001). Interestingly, the single animal that did show a detectable, though reduced CMI, developed only very low anti-Ad5 NAb (mouse m10; titer = 50) (Fig. 3B). In contrast, all preimmunized mice injected with 108 VP of the MRKAd6-NSmut vector developed high Ad5-specific NAb titers (up to 4,000) but no detectable Ad6-specific NAb titers (data not shown), and yet they developed CMI levels comparable to naïve mice (P = 0.32) (Fig. 3C).
MRKAd6-NSmut and MRKAd5-NSmut induce comparable CMI in rhesus macaques.
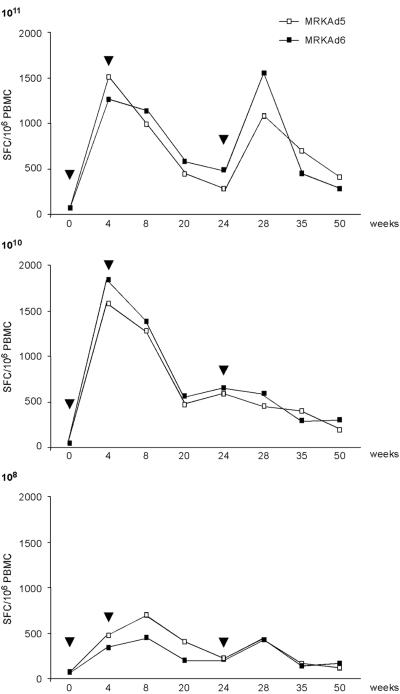
To evaluate the immunological potency of the MRKAd5-NSmut and MRKAd6-NSmut vectors in a primate animal model, groups of three to six rhesus macaques were immunized with escalating doses (108, 1010, and 1011 VP) of the two recombinant viruses (Table 1). An immunization schedule consisting of two injections at 0 and 4 weeks was adopted. CMI was measured at different time points by IFN-γ ELISPOT assay using six pools of 20-mer peptides encompassing the NS3-NS5B sequence. Responses peaked after the first immunization (week 4) in groups of animals immunized with the higher doses (1010 and 1011 VP), while monkeys immunized with the lowest dose of the two vectors (108 VP) reached maximal response after the second injection (week 8). Peak T-cell responses of each individual animal are shown in Tables 2 to 4. T-cell responses as high as 2,600 IFN-γ spot-forming cells (SFC)/million PBMC were detected against several peptide pools in the monkeys that received 1011 VP/dose (groups A and B for MRKAd5-NSmut and MRKAd6-NSmut, respectively), with the highest responses directed against NS3p, NS3h, and NS5A antigens. A potent and multispecific response was induced also in monkeys immunized with 1010 VP/dose (groups C and D for MRKAd5-NSmut and MRKAd6-NSmut, respectively), with numbers of IFN-γ-secreting T cells reaching up to 3,100 SFC/million PBMC. The highest responses were measured against NS3p, NS3h, and NS5A, confirming previous data obtained at 1011 VP/dose. Positive responses against all other pools covering the NS region (NS4, NS5B-I, and NS5B-II) were measured and ranged from 62 to 447 SFC/million PBMC. Injection of 108 VP/dose was slightly less efficient, with three out of three animals (MRKAd5-NSmut immunization group) and four out of five animals (MRKAd6-NSmut immunization group) showing a positive response after the second injection. In both groups high precursor frequencies were detected (up to 2,100 SFC/million PBMC). Overall these data showed that both MRKAd5-NSmut and MRKAd6-NSmut elicited a strong and broad T-cell response in rhesus macaques, with the best responses observed at 1011 and 1010 VP. We prospectively monitored anti-Ad5 and anti-Ad6 NAb titers in the immunized animals. Similar humoral immunity was induced by the two vectors, and titers increased after the second immunization in a dose-dependent manner. According to the human data, anti-Ad5 and anti-Ad6 antibodies did not cross-react (data not shown).
TABLE 2.
ELISPOT response to the individual NS peptide pools in rhesus monkeys at the peak of the immune response after priming with 1011 VPa
| Peptide pool | No. of SFC with 1011 VP of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| MRKAd5-NSmut (group A)
|
MRKAd6-NSmut (group B)
|
|||||||
| 99C059 | 99C060 | 99X009 | 96069 | 98C047 | 98C055 | 93G | 97X014 | |
| NS3p | 28 | 81 | 1,308 | 1,618 | 477 | 25 | 93 | 1,022 |
| NS3h | 2,600 | 161 | 1,008 | 123 | 959 | 398 | 81 | 1,513 |
| NS4 | 31 | 74 | 101 | 40 | 36 | 14 | 99 | 53 |
| NS5A | 181 | 99 | 69 | 96 | 171 | 45 | 1,237 | 98 |
| NS5B-I | 24 | 31 | 40 | 20 | 18 | 32 | 23 | 51 |
| NS5B-II | 11 | 58 | 38 | 164 | 88 | 4 | 13 | 40 |
| DMSO | 6 | 15 | 1 | 16 | 8 | 3 | 1 | 5 |
Results are expressed as IFN-γ SFC per 106 PBMC, and positive responses (as defined in Materials and Methods) are highlighted in bold.
TABLE 3.
ELISPOT response to the individual NS peptide pools in rhesus monkeys at the peak of the immune response after priming with 1010 VPa
| Peptide pool | No. of SFC with 1010 VP of:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| MRKAd5-NSmut (group C)
|
MRKAd6-NSmut (group D)
|
||||||||
| S201 | 075Q | 137Q | S207 | 035Q | 057Q | 98D209 | 106Q | 113Q | |
| NS3p | 928 | 69 | 254 | 363 | 382 | 150 | 3,110 | 263 | 404 |
| NS3h | 317 | 436 | 98 | 180 | 316 | 119 | 2,115 | 642 | 1,008 |
| NS4 | 56 | 101 | 45 | 126 | 113 | 62 | 373 | 72 | 19 |
| NS5A | 1,530 | 1,100 | 413 | 1,780 | 688 | 114 | 103 | 37 | 347 |
| NS5B-I | 149 | 23 | 92 | 447 | 111 | 81 | 149 | 22 | 10 |
| NS5B-II | 398 | 32 | 80 | 153 | 38 | 16 | 314 | 428 | 19 |
| DMSO | 29 | 6 | 29 | 9 | 6 | 9 | 0 | 1 | 3 |
Results are expressed as IFN-γ SFC per 106 PBMC, and positive responses (as defined in Materials and Methods) are highlighted in bold.
TABLE 4.
ELISPOT response to the individual NS peptide pools in rhesus monkeys at the peak of the immune response after priming with 108 VPa
| Peptide pool | No. of SFC with 108 VP of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| MRKAd5-NSmut (group E)
|
MRKAd6-NSmut (group F)
|
|||||||
| 57G | 98C096 | 98C099 | 077Q | S206 | 086Q | 95116 | 138T | |
| NS3p | 67 | 104 | 21 | 64 | 31 | 89 | 77 | 124 |
| NS3h | 461 | 813 | 39 | 430 | 51 | 2,100 | 86 | 1,975 |
| NS4 | 23 | 23 | 45 | 24 | 34 | 36 | 54 | 22 |
| NS5A | 41 | 22 | 734 | 38 | 34 | 91 | 62 | 47 |
| NS5B-I | 40 | 23 | 49 | 28 | 34 | 50 | 58 | 44 |
| NS5B-II | 26 | 25 | 8 | 15 | 17 | 56 | 168 | 138 |
| DMSO | 17 | 6 | 13 | 19 | 18 | 28 | 44 | 7 |
Results are expressed as IFN-γ SFC per 106 PBMC, and positive responses (as defined in Materials and Methods) are highlighted in bold.
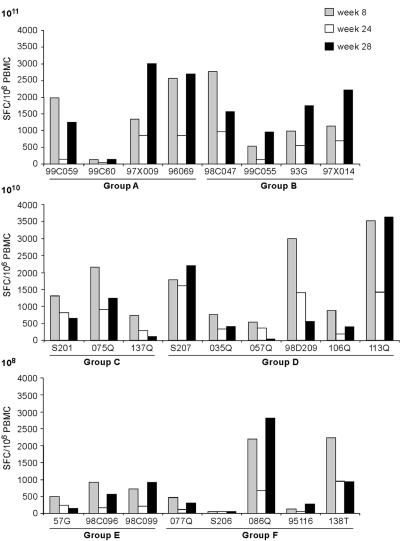
T-cell responses measured by ELISPOT assay slightly declined from week 12 to week 24 consistently with the contraction of effector T cells and with the development of a pool of memory cells. All monkeys were boosted at week 24 with the homologous virus at the same dose used for priming. To analyze the effect of boosting in the different immunization groups, the total response targeting NS antigens measured postprime (time [T] = 8 weeks), before boost (T = 24 weeks), and 4 weeks after boost (T = 28 weeks) was plotted for individual monkeys as shown in Fig. 4. Anamnestic responses were observed following the boost, with peak levels that were comparable or exceeded those observed at week 4 in all the monkeys that received either MRKAd5-NSmut or MRKAd6-NSmut at 1011 VP. Boosting of animals primed with lower doses also effectively induced an anamnestic responses in a subset of animals (four out of nine at 1010 dose and five out of eight at 108).
FIG. 4.
Effect of adenovirus vector boosting injection in rhesus macaques. IFN-γ ELISPOT responses are shown for individual monkeys after priming (T = 8 weeks, gray bars), before boosting (T = 24 weeks, white bars), and after boosting (T = 28 weeks, black bars). Each bar represents the total anti-NS response, calculated by adding up positive responses to individual NS peptide pools and correcting for DMSO background. Results are expressed as the number of IFN-γ SFC per 106 PBMC. Groups A, C, and E were immunized with MRKAd5-NSmut at the indicated doses (top left). Groups B, D, and F were immunized with MRKAd6-NSmut at the indicated doses (top left).
Long-term persistence of the T-cell response induced by both vectors was observed throughout an extended period of time. Figure 5 shows the profile of anti-HCV T-cell responses induced in monkeys by MRKAd5-NSmut and MRKAd6-NSmut at the three different doses. Detectable levels of antigen-specific T cells were found for as long as 1 year in all groups. At T = 50 weeks after the first immunization, the mean ELISPOT values per million PBMC were 401 and 281 in groups A and B, 192 and 310 in groups C and D, and 111 and 147 in groups E and F, respectively. Thus, the potency and longevity of CMI induced in nonhuman primates by the two vectors was comparable.
FIG. 5.
Strength and longevity of the anti-HCV T-cell responses induced in rhesus macaques by MRKAd5-NSmut and MRKAd6-NSmut. The immune response induced by different doses of the two vectors were measured by IFN-γ ELISPOT assay. The geometric mean of the total anti-NS response for the different immunization groups during 50 weeks of follow-up is shown. The total anti-HCV IFN-γ ELISPOT response was calculated for each time point and each animal by adding up positive responses to the individual NS peptide pools and correcting for DMSO background. Numbers on the vertical axis represent the number of SFC per 106 PBMC, and arrowheads indicate immunization dates. MRKAd5, MRKAd5-NSmut; MRKAd6, MRKAd6-NSmut.
MRKAd5-NSmut and MRKAd6-NSmut show equivalent efficiency of induction of HCV-specific CD8+ T cells with cytotoxic activity.
The relative contribution of CD4+ and CD8+ T-cell subsets to the overall anti-HCV NS T-cell response was evaluated by IFN-γ ICS and fluorescence-activated cell sorting analysis. PBMC collected at various time points during the immunization schedule were stimulated with the peptide pools that scored positive by ELISPOT assay, and cytokine production from CD8+ and CD4+ T cells was tested. High levels of HCV-specific CD8+ T cells were observed in each animal, as shown from a representative ICS experiment performed with PBMC collected 4 weeks after the boosting immunization (Table 5). In agreement with the ELISPOT data, MRKAd5-NSmut and MRKAd6-NSmut showed equivalent efficiency of induction of CD8+ T-cell responses. The percentage of IFN-γ+ CD3+ CD8+ cells ranged from 0.14 to 1.92 after priming and from 0.1 to 1.49 after boosting at the two higher doses (Table 5, groups A, B, C and D). At the lowest dose (groups E and F) the percentage of IFN-γ+ CD3+ CD8+ cells was barely above the threshold we set for the assay (0.1%), with the only exception of rhesus 098Q in group F, where frequencies of IFN-γ+ CD3+ CD8+ were 0.53 and 2 after priming and boosting, respectively. HCV-specific IFN-γ-secreting CD4+ T cells were also induced in the immunized animals although at lower levels than CD8+ (range of IFN-γ+ CD3+ CD4+ from 0.11 to 0.15%) (Table 5). This finding is corroborated by our own data indicating that high levels of CD4+ T-cell responses can be measured by the same ICS assay in rhesus macaques immunized by DNA with electroporation (S. Capone et al., submitted). Thus, both adenovirus vectors showed a higher efficiency of induction of CD8+ T-cell responses as previously reported (8).
TABLE 5.
HCV-specific CD4 and CD8 T-cell responses in rhesus monkeys after priming and after boosting by IFN-γ ICSa
| Dose (VP) | % IFN-γ CD3+ CD8+ or CD4+ lymphocytes at indicated time:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MRKAd5-NSmut
|
MRKAd6-NSmut
|
|||||||||
| Group and animal |
T = 8
|
T = 28
|
Group and animal |
T = 8
|
T = 28
|
|||||
| CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | |||
| 1011 | A | B | ||||||||
| 99C059 | 1.14 | 0.04 | 0.62 | 0.09 | 98C047 | 0.35 | 0.04 | 0.52 | 0.01 | |
| 99C060 | 0.01 | 0.11 | 0.01 | 0.02 | 98C055 | 0.19 | 0.06 | 0.37 | 0.01 | |
| 97X009 | 0.65 | 0.12 | 0.80 | 0.02 | 93G | 0.72 | 0.03 | 1.49 | 0.03 | |
| 96069 | 1.30 | 0.01 | 0.90 | 0.02 | 97X014 | 0.40 | 0.13 | 0.65 | 0.10 | |
| Avg | 0.78 | 0.07 | 0.56 | 0.04 | Avg | 0.42 | 0.07 | 0.76 | 0.04 | |
| 1010 | C | D | ||||||||
| S201 | 0.50 | 0.11 | 0.16 | 0.07 | S207 | 0.44 | 0.03 | 0.38 | 0.01 | |
| 075Q | 0.97 | 0.06 | 1.15 | 0.02 | 035Q | 0.44 | 0.04 | 0.12 | 0.01 | |
| 137Q | 0.01 | 0.01 | 0.05 | 0.02 | 057Q | nd | nd | 0.04 | 0.04 | |
| 98D209 | 1.92 | 0.01 | 0.40 | 0.03 | ||||||
| 106Q | 0.14 | 0.03 | 0.10 | 0.01 | ||||||
| 113Q | 1.01 | 0.01 | 1.26 | 0.01 | ||||||
| Avg | 0.49 | 0.06 | 0.45 | 0.04 | Avg | 0.79 | 0.02 | 0.38 | 0.02 | |
| 108 | E | F | ||||||||
| 57G | 0.12 | 0.01 | 0.10 | 0.02 | 077Q | 0.07 | 0.01 | 0.10 | 0.01 | |
| 98C096 | 0.09 | 0.02 | 0.10 | 0.01 | S206 | 0.01 | 0.01 | 0.01 | 0.01 | |
| 98C099 | 0.13 | 0.02 | 0.19 | 0.01 | 086Q | 0.53 | 0.02 | 2.00 | 0.01 | |
| 95116 | 0.01 | 0.01 | 0.01 | 0.04 | ||||||
| 138T | 0.09 | 0.15 | 0.20 | 0.03 | ||||||
| Avg | 0.11 | 0.02 | 0.13 | 0.01 | Avg | 0.14 | 0.04 | 0.46 | 0.02 | |
Each number represents the total anti-NS response calculated by adding up positive responses to individual NS peptide pools and correcting for DMSO background. Positive responses as defined in Materials and Methods are highlighted in bold.
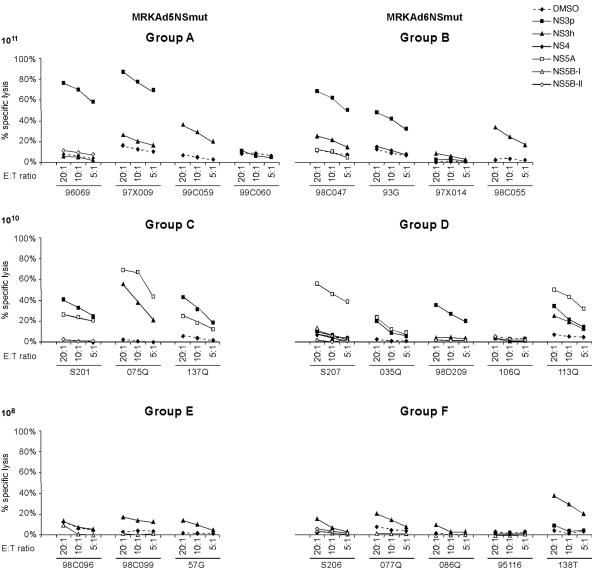
HCV-specific CD8+ T cells induced in immunized animals exhibited functional, cytotoxic activities in CTL assays. The majority of animals immunized with either MRKAd5-NSmut or MRKAd6-NSmut at the higher doses exhibited moderate to strong cytotoxicity against autologous HCV peptide-pulsed B-LCLs as shown in Fig. 6. No major differences in the frequency of responders, number of peptide pools targeted, and levels of specific lysis were detected among Ad5- and Ad6-based vectors. In agreement with the ELISPOT and ICS data, injection of 108 VP of either vector led to a weaker CTL activity. With few exceptions the ability to produce IFN-γ upon antigen stimulation correlated with cytotoxic activity.
FIG. 6.
Bulk cytotoxicity assay in rhesus monkeys immunized with MRKAd5-NSmut and MRKAd6-NSmut. PBMC collected at T = 35 weeks (7 weeks after boosting) were restimulated in vitro with VacHCV-NS and then tested for their ability to kill autologous B-LCLs pulsed with either DMSO alone or with the individual NS peptide pools (NS3p, NS3h, NS4, NS5A, NS5B-I, and NS5B-II). Results are expressed as the percentage of specific lysis at each effector-to-target (E:T) ratio tested.
MRKAd6-NSmut efficiently boosts DNA-primed rhesus monkeys.
As an alternative to the homologous Ad/Ad immunization protocol, we tested a DNA prime/Ad6 boost immunization protocol where MRKAd6-NSmut was injected at a low dose (108 VP is the equivalent of 5 × 106 PFU) to mimic the effect of preexisting anti-adenovirus neutralizing immunity on a dose of 1011 VP (8).
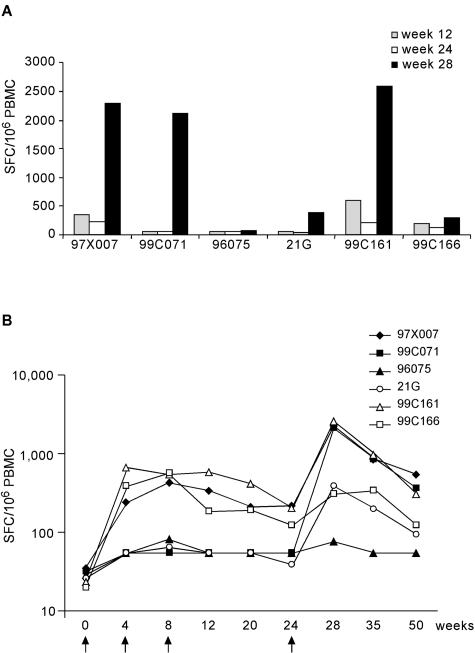
Six rhesus macaques were given priming immunizations of 5 mg of HCV-expressing DNA plasmid (pV1JNSOPTmut) (S. Capone et al. submitted) at weeks 0, 4, and 8 (Table 1). The DNA vector was found to be less immunogenic than the adenoviral vector in rhesus macaques, with only three out of six animals showing significant levels of IFN-γ-secreting cells (Fig. 7A, 97X007, 99C161, and 99C166). MRKAd6-NSmut injection at week 24 increased T-cell responses in two of the DNA-responding animals (97X007 and 99C161), while the third animal (99C166) showed only a modest improvement of T-cell responses compared to the postprime (week 12) and preboost (week 24) CMI. Among the remaining three animals that did not respond to the DNA immunization, two developed T-cell immunity after MRKAd6-NSmut boosting (99C071 and 21G), while the third one did not show any response by IFN-γ ELISPOT (Fig. 7A, 96075). After MRKAd6-NSmut immunization, the CMI response ranged from 73 to 2,533 SFC/106 PBMC at week 28. To verify if DNA priming affected the vigor of postboost responses, we have compared CMI after MRKAd6-NSmut injection (week 28) between animals that showed a DNA-primed T-cell response and those that did not. The results of this analysis suggested that the success in DNA priming (as measured by IFN-γ ELISPOT assay) did not significantly affect the vigor of boosted CMI (P = 0.48).
FIG. 7.
ELISPOT responses in naked DNA-primed, MRKAd6-NSmut-boosted rhesus monkeys. (A) DNA priming and MRKAd6-NSmut (108 viral particles) boosting in rhesus monkeys. ELISPOT responses are shown for individual monkeys after priming (T = 12 weeks), before boost (T = 24 weeks) and after boost (T = 28 weeks). Each bar represents the total anti-NS response, calculated by adding up the positive responses to individual NS peptide pools and correcting for DMSO background. Results are expressed as the number of IFN-γ SFC per 106 PBMC. (B) Strength and longevity of anti-NS ELISPOT responses induced in DNA primed- and /adenovirus-boosted monkeys over time. The line represents the geometric mean of total anti-NS ELISPOT responses of the six animals during a 50 week follow-up. The total anti-NS ELISPOT response was calculated for each time point and for each animal by adding up the positive responses to the individual NS peptide pools and correcting for DMSO background. Numbers on the y axis represent the number of IFN-γ SFC per 106 PBMC. Arrows indicate immunization dates.
The levels of antigen-specific T cells remained significant for as long as 1 year in five animals (Fig. 7B). Even at this time point there was no difference in the vigor of CMI between animals who did respond to DNA and those who did not (P = 0.39). In contrast, higher ELISPOT values were detected at late time points in animals (97X007, 99C071, and 99C161) that developed more potent responses after Ad6 injection (P = 0.04 at week 50).
Characterization of the T-cell response induced by the heterologous prime-boost regimen by IFN-γ ICS and CTL assays showed that HCV-specific IFN-γ+ CD8+ T cells with cytotoxic activity were induced (data not shown).
DISCUSSION
Nonreplicating human serotype 5 adenovirus is an attractive vector for vaccine application as it was shown to induce very potent transgene product-specific antibody and CD8+ T-cell responses in rodents, dogs, and nonhuman primates (8, 40). Although studies comparing the efficacy of adenoviral vectors to that of other subunit vaccines are still incomplete, available data show them to outperform poxvirus vectors, DNA vaccines, and alphavirus vectors with regard to induction of humoral and cellular immune responses (40). Adenoviral vectors induce protective immune responses quickly, and these responses are long lived. The exceptional immunogenicity of replication-defective Ad5 vectors is likely due to a number of properties: (i) the high levels of transgene expression driven in most vectors by the potent cytomegalovirus promoter, (ii) the activation of the innate immune system, (iii) the transduction of immature dendritic cells driving their maturation into antigen-presenting cells, and (iv) a sufficiently long time of antigen presentation due to lack of apoptosis induction of the transduced cells.
A number of Ad5-based HCV vectors have been previously reported to be immunogenic in mice (1, 2, 6, 25, 29, 33). However, preexisting anti-adenovirus immunity and, in particular, neutralizing antibodies which reduce cell uptake of the adenoviral vectors can significantly dampen vaccine responses and represent a major limitation to the successful use of common serotypes of adenovirus, including Ad5 and Ad2, as vaccine vehicles in a human population (13). This could be overcome by increasing vector doses, but this approach is limited by potential toxicity issues and would make production a very difficult task. Thus, a high priority toward developing an effective adenovirus-based vaccine approach would be the generation of novel vectors with lower seroprevalence than Ad5 but equivalent immunological potency.
The data reported in this work confirmed previous findings that anti-Ad6 neutralizing antibodies are rarer or are present at lower titers than those measured against Ad5. This would imply that a vaccine based on the Ad6 serotype may have a significant value if it could overcome anti-Ad5 preexisting immunity. By comparing NAb titers against both Ad5 and Ad6 serotypes in a cohort of 93 European individuals, we determined that there is no cross-reactivity between NAb against the two adenovirus serotypes. We also provided direct evidence for Ad6-based immunization as a potential route to overcome anti-Ad5 preexisting immunity, by demonstrating that an Ad6-based HCV vector elicited potent cellular immune responses in mice that were not suppressed by preexisting anti-Ad5 NAb at levels typically found in humans. We did not investigate whether anti-Ad5 T-cell responses cross-reactive with Ad6 were induced in this model system. However, as previously shown for other adenovirus vectors (4), this antivector CMI, if present, was not capable of reducing the MRKAd6-NSmut immunogenicity. In contrast, the immunological potency of the corresponding Ad5-based vector was strongly blunted by preimmunization with an adenovirus vector belonging to the same serotype.
Vectors based on human non subgroup C adenovirus have been previously reported (4, 36). Among them, adenovirus serotype 35 has been proposed as an alternative to Ad5 for vaccine delivery because of its low seroprevalence. Comparison of Ad5- and Ad35-based vectors in mice and nonhuman primates showed a lower immunological potency of the latter vector (4, 36). Similarly, two other vectors based on the rare serotypes Ad24 and Ad34 induced lower CMI than Ad5 in nonhuman primates (36). These data demonstrate that it would be important to evaluate vaccine candidates for their ability to induce potent and long-lived immune responses in different species and, in particular, in nonhuman primates. In fact, rhesus macaques are a suitable model for preclinical immunogenicity studies because they are phylogenetically and physiologically similar to humans (32) and their well-characterized immune system is comparable to that of humans in terms of cytokine production and regulation (24). Both MRKAd5-NSmut and MRKAd6-NSmut vectors showed equivalent immunological potency in mice at two different doses (107 and 109 VP/dose). Most importantly, the immunogenicity of the MRKAd6-NSmut vector was comparable to that of the corresponding MRKAd5-NSmut vector in terms of potency, longevity, and breadth of HCV-specific T-cell response and over a wide range of doses (108 to 1011 VP/dose) in rhesus macaques. MRKAd6-NSmut induced high levels of HCV-specific T cells in 13 out of 15 immunized monkeys, and this response was sustained for several months. Most of the response was due to the induction of HCV-specific CD8+ T cells with the ability to kill target cells in vitro. Finally, MRKAd6-NSmut induced multispecific T-cell responses against different parts of the NS region. This is a crucial point because a vaccine that elicits responses to multiple epitopes would be required, given the high HCV variability and the heterogeneity of the human major histocompatibility complex alleles. In conclusion, the MRKAd6-NSmut vector described here has the desired properties of low seroprevalence and high immunological potency to be further considered for vaccine development.
Early induction of a strong cytotoxic T-cell response targeting multiple antigens appears to be required for protection against chronic HCV infection (15, 21, 37). By a prospective immunological study on acutely infected HCV individuals, we defined the qualitative and quantitative characteristics of such a protective CMI response (13a, 37). In analyzing the immune response induced by MRKAd6-NSmut in rhesus macaques, we have used the same reagents and assays utilized in the human studies and have observed that the response elicited in monkeys by MRKAd6-NSmut was qualitatively and quantitatively comparable to that measured in acute/resolving HCV-infected subjects. These findings hold the promise for the MRKAd6-NSmut to be an effective candidate vaccine for the prevention of chronic persistent infection induced by HCV in humans. Consistent with this hypothesis, preliminary results obtained in a vaccination and heterologous challenge experiment in the chimpanzee model indicated that the Ad6-based candidate vaccine described in this work is essential for the induction of an effective immunity against HCV (13b).
Several efforts are presently focused on combining Ad vectors with other vaccine carriers in heterologous prime-boost regimens. In fact, the repeated use of adenovirus vectors belonging to the same serotype in general does not afford a significant booster effect, due to the induction of Ad-neutralizing antibodies after the first application of the vector. This limitation can be circumvented by a DNA priming-viral vector boosting approach (8, 43). Here we provide evidence that priming with naked DNA combined with MRKAd6-NSmut as a booster can result in a T-cell response approaching that observed with multiple high doses of an Ad5- or Ad6-based vectors. A recent DNA prime-Ad5 boost immunization study in rhesus macaques with vectors encoding the HCV NS3 protein showed increased CMI after injection of 5 × 109 of PFU of the Ad vector, with IFN-γ ELISPOT peak responses reaching up to 1150 SFC/106 PBMC (31). The MRKAd6-NSmut vector described in this work was capable of inducing higher responses (up to 2,533 SFC/106 PBMC) even if used at a very low dose (108 VP is equivalent to 5 × 106 PFU) that was selected to mimic the effect of preexisting anti-adenovirus neutralizing immunity on a dose of 1011 VP. This higher immunogenicity could be due the larger antigenic information encoded by the present vector, the different HCV genotype sequence used to generate the expression cassette (BK versus J strain), or to the architecture of the vector.
Because naked DNA has a lower efficiency of immunization compared to Ad5- or Ad6-based immunization in rhesus macaques, it is conceivable that the use of two very potent adenoviral vectors from different serotypes would represent a further improvement of the heterologous prime-boost strategy. To our knowledge, the MRKAd6-NSmut described in this work is the first human adenoviral vector from an alternative serotype with lower seroprevalence and equivalent immunological potency to Ad5 and may represent a good candidate for either priming or boosting immunizations. We are currently characterizing novel adenovirus isolates to identify one with similar characteristics to Ad6 to be used in a combined modality regimen with the MRKAd6-NSmut.
Acknowledgments
We acknowledge S. Dubey, D. C. Freed, and W. Trigona for outstanding assistance, T. Strickland for providing sera, M. Emili for help in figure preparation, and all the staff at the New Iberia Research Center for the excellent care of monkeys and samples.
REFERENCES
- 1.Arribillaga, L., A. L. de Cerio, P. Sarobe, N. Casares, M. Gorraiz, A. Vales, O. Bruna-Romero, F. Borras-Cuesta, G. Paranhos-Baccala, J. Prieto, J. Ruiz, and J. J. Lasarte. 2002. Vaccination with an adenoviral vector encoding hepatitis C virus (HCV) NS3 protein protects against infection with HCV-recombinant vaccinia virus. Vaccine 21:202-210. [DOI] [PubMed] [Google Scholar]
- 2.Arribillaga, L., P. Sarobe, A. Arina, M. Gorraiz, F. Borras-Cuesta, J. Ruiz, J. Prieto, L. Chen, I. Melero, and J. J. Lasarte. 2005. Enhancement of CD4 and CD8 immunity by anti-CD137 (4-1BB) monoclonal antibodies during hepatitis C vaccination with recombinant adenovirus. Vaccine 23:3493-3499. [DOI] [PubMed] [Google Scholar]
- 3.Aste-Amezaga, M., A. J. Bett, F. Wang, D. R. Casimiro, J. M. Antonello, D. K. Patel, E. C. Dell, L. L. Franlin, N. M. Dougherty, P. S. Bennett, H. C. Perry, M. E. Davies, J. W. Shiver, P. M. Keller, and M. D. Yeager. 2004. Quantitative adenovirus neutralization assays based on the secreted alkaline phosphatase reporter gene: application in epidemiologic studies and in the design of adenovector vaccines. Hum. Gene Ther. 15:293-304. [DOI] [PubMed] [Google Scholar]
- 4.Barouch, D. H., M. G. Pau, J. H. Custers, W. Koudstaal, S. Kostense, M. J. Havenga, D. M. Truitt, S. M. Sumida, M. G. Kishko, J. C. Arthur, B. Korioth-Schmitz, M. H. Newberg, D. A. Gorgone, M. A. Lifton, D. L. Panicali, G. J. Nabel, N. L. Letvin, and J. Goudsmit. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 172:6290-6297. [DOI] [PubMed] [Google Scholar]
- 5.Bassett, S. E., B. Guerra, K. Brasky, E. Miskovsky, M. Houghton, G. R. Klimpel, and R. E. Lanford. 2001. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology 33:1479-1487. [DOI] [PubMed] [Google Scholar]
- 6.Bruna-Romero, O., J. J. Lasarte, G. Wilkinson, K. Grace, B. Clarke, F. Borras-Cuesta, and J. Prieto. 1997. Induction of cytotoxic T-cell response against hepatitis C virus structural antigens using a defective recombinant adenovirus. Hepatology 25:470-477. [DOI] [PubMed] [Google Scholar]
- 7.Casimiro, D. R., A. J. Bett, T. M. Fu, M. E. Davies, A. Tang, K. A. Wilson, M. Chen, R. Long, T. McKelvey, M. Chastain, S. Gurunathan, J. Tartaglia, E. A. Emini, and J. W. Shiver. 2004. Heterologous human immunodeficiency virus type 1 priming-boosting immunization strategies involving replication-defective adenovirus and poxvirus vaccine vectors. J. Virol. 78:11434-11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chirmule, N., K. J. Propert, S. A. Magosin, Y. Qian, R. Qian, and J. M. Wilson. 1996. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 6:1574-1583. [DOI] [PubMed] [Google Scholar]
- 10.D'Ambrosio, E., N. Del Grosso, A. Chicca, and M. Midulla. 1982. Neutralizing antibodies against 33 human adenoviruses in normal children in Rome. J. Hyg. 89:155-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diepolder, H. M., J. T. Gerlach, R. Zachoval, R. M. Hoffmann, M. C. Jung, E. A. Wierenga, S. Scholz, T. Santantonio, M. Houghton, S. Southwood, A. Sette, and G. R. Pape. 1997. Immunodominant CD4+ T-cell epitope within nonstructural protein 3 in acute hepatitis C virus infection. J. Virol. 71:6011-6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farci, P., H. J. Alter, D. C. Wong, R. H. Miller, S. Govindarajan, R. Engle, M. Shapiro, and R. H. Purcell. 1994. Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization. Proc. Natl. Acad. Sci. USA 91:7792-7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald, J. C., G. P. Gao, A. Reyes-Sandoval, G. N. Pavlakis, Z. Q. Xiang, A. P. Wlazlo, W. Giles-Davis, J. M. Wilson, and H. C. J. Ertl. 2003. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J. Immunol. 170:1416-1422. [DOI] [PubMed] [Google Scholar]
- 13a.Folgori, A., E. Spada, M. Pezzanera, L. Ruggeri, A. Mele, A. R. Garbugua, M. P. Perrone, P. Del Porto, E. Piccolella, R. Cortese, A. Nicosia, and A. Vitelli. Early impairment of HCV-specific T-cell proliferation during acute infection leads to failure of viral clearance. Gut, in press. [DOI] [PMC free article] [PubMed]
- 13b.Folgori, A., S. Capone, L. Ruggeri, A. Meola, E. Sporeno, B. Bruni Ercole, M. Pezzanera, R. Tafi, M. Arcuri, E. Fattori, A. Lahm, A. Luzzago, A. Vitelli, S. Colloca, R. Cortese, and A. Nicosia. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzee. Nat. Med., in press. [DOI] [PubMed]
- 14.Gerlach, J. T., H. M. Diepolder, M. C. Jung, N. H. Gruener, W. W. Schraut, R. Zachoval, R. Hoffmann, C. A. Schirren, T. Santantonio, and G. R. Pape. 1999. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology 117:933-941. [DOI] [PubMed] [Google Scholar]
- 15.Gruner, N. H., T. J. Gerlach, M. C. Jung, H. M. Diepolder, C. A. Schirren, W. W. Schraut, R. Hoffmann, R. Zachoval, T. Santantonio, M. Cucchiarini, A. Cerny, and G. R. Pape. 2000. Association of hepatitis C virus-specific CD8+ T cells with viral clearance in acute hepatitis C. J. Infect. Dis. 181:1528-1536. [DOI] [PubMed] [Google Scholar]
- 16.Inchauspe, G., and S. Feinstone. 2003. Development of a hepatitis C virus vaccine. Clin. Liver Dis. 7:243-259. [DOI] [PubMed] [Google Scholar]
- 17.Kolykhalov, A. A., K. Mihalik, S. M. Feinstone, and C. M. Rice. 2000. Hepatitis C virus-encoded enzymatic activities and conserved RNA elements in the 3′ nontranslated region are essential for virus replication in vivo. J. Virol. 74:2046-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanford, R. E., B. Guerra, D. Chavez, C. Bigger, K. M. Brasky, X. H. Wang, S. C. Ray, and D. L. Thomas. 2004. Cross-genotype immunity to hepatitis C virus. J. Virol. 78:1575-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauer, G. M., E. Barnes, M. Lucas, J. Timm, K. Ouchi, A. Y. Kim, C. L. Day, G. K. Robbins, D. R. Casson, M. Reiser, G. Dusheiko, T. M. Allen, R. T. Chung, B. D. Walker, and P. Klenerman. 2004. High resolution analysis of cellular immune responses in resolved and persistent hepatitis C virus infection. Gastroenterology 127:924-936. [DOI] [PubMed] [Google Scholar]
- 20.Lavanchy, D., and B. McMahon. 2001. Worldwide prevalence and prevention of hepatitis C, p. 185. In H. Liang (ed.), Hepatitis C. Academic Press, San Diego, Calif.
- 21.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lechner, F., N. H. Gruener, S. Urbani, J. Uggeri, T. Santantonio, A. R. Kammer, A. Cerny, R. Phillips, C. Ferrari, G. R. Pape, and P. Klenerman. 2000. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur. J. Immunol. 30:2479-2487. [DOI] [PubMed] [Google Scholar]
- 23.Major, M. E., K. Mihalik, M. Puig, B. Rehermann, M. Nascimbeni, C. M. Rice, and S. M. Feinstone. 2002. Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge. J. Virol. 76:6586-6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makitalo, B., M. Andersson, I. Arestrom, K. Karlen, F. Villinger, A. Ansari, S. Paulie, R. Thorstensson, and N. Ahlborg. 2002. ELISpot and ELISA analysis of spontaneous, mitogen-induced and antigen-specific cytokine production in cynomolgus and rhesus macaques. J. Immunol. Methods 270:85-97. [DOI] [PubMed] [Google Scholar]
- 25.Matsui, M., O. Moriya, and T. Akatsuka. 2003. Enhanced induction of hepatitis C virus-specific cytotoxic T lymphocytes and protective efficacy in mice by DNA vaccination followed by adenovirus boosting in combination with the interleukin-12 expression plasmid. Vaccine 21:1629-1639. [DOI] [PubMed] [Google Scholar]
- 26.Mehta, S. H., A. Cox, D. R. Hoover, X. H. Wang, Q. Mao, S. Ray, S. A. Strathdee, D. Vlahov, and D. L. Thomas. 2002. Protection against persistence of hepatitis C. Lancet 359:1478-1483. [DOI] [PubMed] [Google Scholar]
- 27.Missale, G., R. Bertoni, V. Lamonaca, A. Valli, M. Massari, C. Mori, M. G. Rumi, M. Houghton, F. Fiaccadori, and C. Ferrari. 1996. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J. Clin. Investig. 98:706-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nascimbeni, M., E. Mizukoshi, M. Bosmann, M. E. Major, K. Mihalik, C. M. Rice, S. M. Feinstone, and B. Rehermann. 2003. Kinetics of CD4+ and CD8+ memory T-cell responses during hepatitis C virus rechallenge of previously recovered chimpanzees. J. Virol. 77:4781-4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park, S. H., S. H. Yang, C. G. Lee, J. W. Youn, J. Chang, and Y. C. Sung. 2003. Efficient induction of T helper 1 CD4+ T-cell responses to hepatitis C virus core and E2 by a DNA prime-adenovirus boost. Vaccine 21:4555-4564. [DOI] [PubMed] [Google Scholar]
- 30.Poynard, T., M. F. Yuen, V. Ratziu, and C. L. Lai. 2003. Viral hepatitis C. Lancet 362:2095-2100. [DOI] [PubMed] [Google Scholar]
- 31.Rollier, C., E. J. Verschoor, G. Paranhos-Baccala, J. A. Drexhage, B. E. Verstrepen, J. L. Berland, N. Himoudi, C. Barnfield, P. Liljestrom, J. J. Lasarte, J. Ruiz, G. Inchauspe, and J. L. Heeney. 2005. Modulation of vaccine-induced immune responses to hepatitis C virus in rhesus macaques by altering priming before adenovirus boosting. J. Infect. Dis. 192:920-929. [DOI] [PubMed] [Google Scholar]
- 32.Roth, G. S., J. A. Mattison, M. A. Ottinger, M. E. Chachich, M. A. Lane, and D. K. Ingram. 2004. Aging in rhesus monkeys: relevance to human health interventions. Science 305:1423-1426. [DOI] [PubMed] [Google Scholar]
- 33.Seong, Y. R., S. Choi, J. S. Lim, C. H. Lee, C. K. Lee, and D. S. Im. 2001. Immunogenicity of the E1E2 proteins of hepatitis C virus expressed by recombinant adenoviruses. Vaccine 19:2955-2964. [DOI] [PubMed] [Google Scholar]
- 34.Shepard, C. W., L. Finelli, and M. J. Alter. 2005. Global epidemiology of hepatitis C virus infection. Lancet Infect. Dis. 5:558-567. [DOI] [PubMed] [Google Scholar]
- 35.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 36.Shiver, J. W., and E. A. Emini. 2004. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu. Rev. Med. 55:355-372. [DOI] [PubMed] [Google Scholar]
- 37.Spada, E., A. Mele, A. Berton, L. Ruggeri, L. Ferrigno, A. Garbuglia, M. Perrone, G. Girelli, P. Del Porto, E. Piccolella, M. U. Mondelli, P. Amoroso, R. Cortese, A. Nicosia, A. Vitelli, and A. Folgori. 2004. Multi-specific T-cell response and negative HCV RNA tests during acute HCV infection are early prognostic factors of spontaneous clearance. Gut 53:1673-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sullivan, N. J., T. W. Geisbert, J. B. Geisbert, L. Xu, Z. Y. Yang, M. Roederer, R. A. Koup, P. B. Jahrling, and G. J. Nabel. 2003. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature 424:681-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takamizawa, A., C. Mori, I. Fuke, S. Manabe, S. Murakami, J. Fujita, E. Onishi, T. Andoh, I. Yoshida, and H. Okayama. 1991. Structure and organization of the hepatitis C virus genome isolated from human carriers. J. Virol. 65:1105-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tatsis, N., and H. C. J. Ertl. 2004. Adenoviruses as vaccine vectors. Mol. Ther. 10:4616-4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wedemeyer, H., X. S. He, M. Nascimbeni, A. R. Davis, H. B. Greenberg, J. H. Hoofnagle, T. J. Liang, H. Alter, and B. Rehermann. 2002. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J. Immunol. 169:3447-3458. [DOI] [PubMed] [Google Scholar]
- 43.Yang, Z. Y., L. S. Wyatt, W. P. Kong, Z. Moodie, B. Moss, and G. J. Nabel. 2003. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J. Virol. 77:799-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Youil, R., T. J. Toner, Q. Su, M. Chen, A. Tang, A. J. Bett, and D. R. Casimiro. 2002. Hexon gene switch strategy for the generation of chimeric recombinant adenovirus. Hum. Gene Ther. 13:311-320. [DOI] [PubMed] [Google Scholar]
- 45.Youil, R., T. J. Toner, Q. Su, D. Casimiro, J. W. Shiver, L. Chen, A. J. Bett, B. M Rogers, E. C. Burden, A. Tang, M. Chen, E. A. Emini, D. C. Kaslow, J. G. Aunins, and N. E. Altaras. 2003. Comparative analysis of the effects of packaging signal, transgene orientation, promoters, polyadenylation signals, and E3 region on growth properties of first-generation adenoviruses. Hum. Gene Ther. 14:1017-1034. [DOI] [PubMed] [Google Scholar]
- 46.Zucchelli, S., S. Capone, E. Fattori, A. Folgori, A. Di Marco, D. Casimiro, A. J. Simon, R. Laufer, N. La Monica, R. Cortese, and A. Nicosia. 2000. Enhancing B- and T-cell immune response to a hepatitis C virus E2 DNA vaccine by intramuscular electrical gene transfer. J. Virol. 74:11598-11607. [DOI] [PMC free article] [PubMed] [Google Scholar]