Abstract
The mitogen-activated protein kinase p38 plays a critical role in inflammation, cell cycle progression, differentiation, and apoptosis. The activity of p38 is stimulated by a variety of extracellular stimuli, such as the proinflammatory cytokine tumor necrosis factor alpha (TNF-α), and subjected to regulation by other intracellular signaling pathways, including the cyclic AMP (cAMP) pathway. Yet the underlying mechanism by which cAMP inhibits p38 activation is unknown. Here we show that the induction of dynein light chain (DLC) by cAMP response element-binding protein (CREB) is required for cAMP-mediated inhibition of p38 activation. cAMP inhibits p38 activation via the protein kinase A-CREB pathway. The inhibition is mediated by the CREB target gene Dlc, whose protein product, DLC, interferes with the formation of the MKK3/6-p38 complex, thereby suppressing p38 phosphorylation activation by MKK3/6. The inhibition of p38 activation by cAMP leads to suppression of NF-κB activity and promotion of apoptosis in response to TNF-α. Thus, our results identify DLC as a novel inhibitor of the p38 pathway and provide a molecular mechanism by which cAMP suppresses p38 activation and promotes apoptosis.
p38 is a subfamily of the mitogen-activated protein kinase (MAPK) superfamily (23, 56), with two ubiquitously expressed isoforms, p38α and p38β, and two tissue-specific isoforms, p38γ and p38δ (74). p38 is activated by a variety of extracellular stimuli, from the physical stresses, such as UV and osmotic shock, to proinflammatory cytokines like interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-α) (74). The activation of p38 is mediated by sequential protein phosphorylation through a MAPK module, i.e., MAP3K→MAP2K→MAPK (33, 36). Three MAP2Ks (MKK3, JNKK1/MKK4, and MKK6) and several MAP3Ks (ASK1, DLK/MUK/ZPK, TAK-1, and MEKKs) have been reported to be involved in p38 activation (5, 12, 24, 35, 74). In addition, the enzymatic activity of p38 can be enhanced by TAK1-binding protein 1, which interacts with p38 and stimulates the autophosphorylation of the latter (19). The activation of p38 can also be regulated by cytoskeletal structures. p150Glued, which is a key component of the cytoplasmic dynein-dynactin motor complex, interacts with and is required for sorbitol-induced activation of MKK3 and MKK6 (MKK3/6) (8). Consistently, disruption of microtubules and dynein-dynactin motor complex interferes with the activation of MKK3/6 (8). Furthermore, the activation of p38 is regulated by other intracellular signaling pathways, such as the cyclic AMP (cAMP) pathway (6, 9, 17, 51, 54). Once activated, p38 phosphorylates and regulates the activities of several transcription factors, such as ATF-2, Elk, MEF2A, NF-AT, and Sap1 (74), and nontranscription factors, such as MAPKAPK2, MNK1, PRAK, and MSK1 (74). Recent studies also show that p38 phosphorylates RelA/p65, which is the major transactivating subunit of the transcription factor NF-κB, thereby stimulating its transcriptional activity (43). Enormous evidence shows that p38 plays a central role in regulating many cellular processes, including inflammation, cell cycle progression, differentiation, and apoptosis (74).
The second messenger cAMP is produced from ATP by adenylyl cyclases (AC) and can be degraded to 5′-AMP by phosphodiesterases (27, 63). AC is stimulated by a variety of extracellular stimuli, such as hormones, growth factors, and neurotransmitters through G-protein (Gs)-coupled membrane receptors (63). In addition, AC can be activated by pharmacological agents, such as forskolin (FSK), which is a direct activator of AC (59), isoproterenol, which is a synthetic agonist for the β-adrenergic family of receptors (3), or cholera toxin (CTX), which causes constitutive activation of Gs by stimulating ADP-ribosylation of its α-subunit (41). cAMP regulates many cellular activities, from proliferation to apoptosis, in a cell type-dependent manner (49, 60, 62, 70, 71). The biological functions of cAMP are mediated by its downstream effectors like protein kinase A (PKA) (14, 16) and cAMP-regulated guanine nucleotide exchange factors (cAMP-GEFs) (cAMP-GEF-I and cAMP-GEF-II, also known as Epac, exchange proteins directly activated by cAMP [10, 13, 61]). PKA holoenzymes (PKAI and PKAII) are heterotetramers that are composed of two regulatory subunits and two catalytic subunits (14, 16). PKAI contains regulatory subunit RI and localizes in the cytoplasm (16), whereas PKAII contains regulatory subunit RII and concentrates in juxtamembranes and cellular organelles (16). Increased intracellular cAMP binds to and induces the R subunits to dissociate from the C subunits (14, 16), allowing the latter to phosphorylate nontranscription factors, such as the proapoptotic Bcl-2 family protein BAD, and thereby inhibit its activity (25), or translocate into the nucleus to phosphorylate transcription factors, such as cAMP response element-binding protein (CREB) at Ser133 (45). The phosphorylation of CREB potentiates its transcription activity via recruitment of several transcription coactivators, such as CBP and p300 (2, 45), thereby stimulating expression of target genes that are involved in many cellular activities (30, 76). The newly discovered cAMP-GEFs mediate some of the biological functions of cAMP in a PKA-independent manner (10, 13, 61). The activity of cAMP-GEFs is stimulated upon binding to cAMP, and in turn it activates the small Ras-like GTPase Rap I through promoting the exchange of GDP to GTP (10, 13, 61). GTP-bound, activated Rap I has been shown to be involved in many cellular events, including proliferation, differentiation, T-cell anergy, and platelet activation (10, 13, 61).
The cAMP signaling pathway has been reported to regulate p38 activity in a cell context-dependent manner, being either inhibitory or stimulatory (6, 9, 17, 51, 54). cAMP inhibits p38 activation induced by iron chelators, lipopolysaccharide, or thrombin in HL-60, macrophages, and epithelial cells (9, 17, 51). However, cAMP may also activate the p38 pathway, since activation of p38 and its upstream kinase MKK3 is required for induction of cAMP-target gene uncoupling protein 1 (UCP1) in adipocytes (6, 54). Yet in either case the underlying mechanism is unknown. In this report, we show that, at least in HeLa cells and fibroblasts, cAMP disrupts the formation of the MKK3/6-p38 complex via CREB-induced dynein light chain (DLC) (also known as PIN/LC8), which is a key component of the dynein-dynactin motor and regulates many signaling molecules and enzymes (15, 31, 32, 39, 47, 50, 53, 57, 67), thereby inhibiting p38 activation. The inhibition of p38 by cAMP leads to suppression of NF-κB activation and promotion of apoptosis in response to TNF-α. Thus, our data provide a molecular mechanism by which cAMP inhibits p38 activation for apoptosis.
MATERIALS AND METHODS
Cell culture.
Rat-1, NIH 3T3, and human fibrosarcoma HT-1080 cells were grown in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Murine hematopoietic FL5.12 cells were a gift from Stanley Korsmeyer and were cultured in ISCOVE'S medium, supplemented with 10% Wehi supernatant containing IL-3, 5% fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Reagents.
Antibodies against Flag (M2) tag, dynein intermediate chain, β-actin, forskolin, isoproterenol, cholera toxin, prostaglandin PGE2, epinephrine, kemptide, Hoechst 33258, emetine, actinomycin D, and KG501 were from Sigma. 8-pCPT-2′-O-Me-cAMP and 6-MB-cAMP were from Biolog. Antibodies against CREB, phospho-CREB, p38, phospho-p38, and phospho-MKK3/6 were from Cell Signaling. Antibodies against hemagglutinin (HA), MKK3/6, dynein heavy chain, and dynein light chain were from Santa Cruz. Antibodies against p50 dynamitin and p150Glued were from BD Company. Mouse IL-1β and TNF-α were purchased from R&D Systems. The cell-permeative and myristoylated PKA inhibitor (MyPKI) and SB203580 were from Calbiochem. [γ-32P]ATP (3,000 mCi/nmol) was from Dupont NEN.
Plasmids.
Expression vectors of M2-p38, M2-p38(Y182F), HA-MKK6b, HA-MKK6b(EE), IL-8-LUC, EVX-LUC, GAL4-LUC, GAL4-Elk, and GAL4-CREB have been described elsewhere (2, 24, 28, 35, 40, 48). The rat DLC (rDLC) clone was from Openbiosystems. pcDNA3.1 Xpress-rDLC was generated by addition of an N-terminal Xpress tag to rDLC and confirmed by DNA sequencing. pcDNA3.1 M2-PKAcα was generated by addition of an N-terminal M2 tag to PKAcα and confirmed by DNA sequencing. Transfection was performed by using ExGen500 (MBI Fermentas) according to the manufacturer's protocol. Luciferase assays were performed as described before (35).
RNA interference and adenoviruses.
Small interfering RNAs (siRNAs) that target rat CREB and rDLC mRNAs were designed based on nucleotides 653 to 671 (rat CREB) and 532 to 550 (rDLC) relative to the translation start sites, respectively, and purchased from Dharmacon. Cells were transfected with siRNAs using Lipofectamine 2000 (Invitrogen), according to the manufacturer's protocol. Recombinant adenovirus containing ACREB (kindly provided by Chen-Ming Fan, Carnegie Institution of Washington) was constructed by subcloning ACREB cDNA (1) into pTRACK CMV and then inserting it into pAdeasy-1 by homologous recombination. The viral preparation and purification was carried out as described previously (37, 65). Cells were infected with adenovirus at a final concentration of 3 × 1010 PFU/ml (multiplicity of infection = 300).
In vitro PKA kinase assays.
Cell extracts were incubated with the fluorescence-labeled PKA substrate kemptide in a kinase reaction, according to the manufacturer's protocol. The phosphorylated kemptide was separated from nonphosphorylated kemptide by 0.8% agarose electrophoresis. The fluorescent images were taken with a luminescent image analyzer, LAS-3000 (Fujifilm), and analyzed quantitatively (26).
Immune complex kinase assays, immunoprecipitation, and immunoblotting analysis.
Immune complex kinase assays were performed and quantitated as described previously (35, 75). For coimmunoprecipitation analysis, Rat-1 cells were cotransfected with mammalian expression vectors encoding HA-MKK6b, M2-p38, and Xpress-DLC. After 30 h, cells were harvested and lysed in lysis buffer (20 mM Tris, pH 7.6, 250 mM NaCl, 3 mM EDTA, 1.5 mM EGTA, 10 mM p-nitrophenylphosphate, 1 mM Na3VO4, 1% Nonidet P-40, 1 mM dithiothreitol, and 10 μg/ml aprotinin). After clarification by centrifugation, cell lysates (1 mg) were incubated with anti-M2 monoclonal antibody in the presence of 30 μl (50% [vol/vol]) of protein A-Sepharose beads at 4°C for 4 h. Immunoblotting analysis was done as described previously (64, 66).
Apoptosis assays.
Cells were stained with Hoechst (H33258), and nuclear condensation and DNA fragmentation were visualized by fluorescence microscopy, as described previously (37, 66, 72, 75).
RESULTS
Elevation of cAMP inhibits p38 activity and phosphorylation.
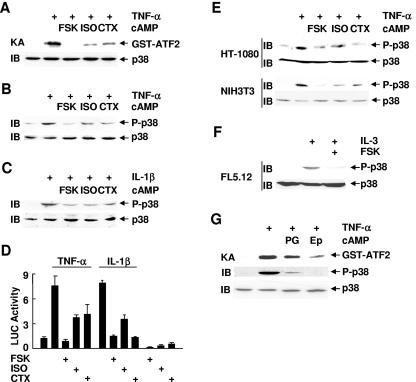
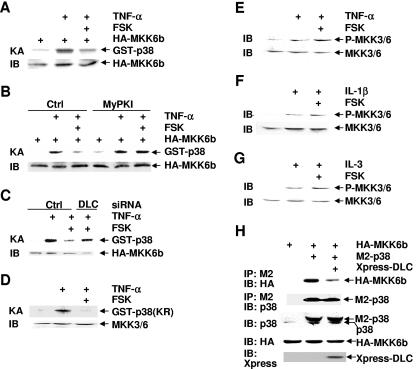
To investigate the regulation of p38 by cAMP, Rat-1 cells were pretreated with cAMP elevation agents (forskolin, isoproterenol, or cholera toxin) for 30 to 60 min, followed by stimulation with or without TNF-α for 15 min. Immune complex kinase assays showed that TNF-α-stimulated p38 activity was profoundly inhibited by forskolin, isoproterenol, or cholera toxin (Fig. 1A). Immunoblotting analysis revealed that TNF-α- or IL-1β-induced phosphorylation of p38 at Thr180 and Tyr182, which is required for p38 activation (5, 74), was also significantly inhibited (Fig. 1B and C), suggesting that cAMP inhibits the activation of p38 by its upstream kinases. Consequently, TNF-α- or IL-1β-stimulated transcriptional activity of Elk, which is regulated by p38, was also inhibited by forskolin, isoproterenol, or cholera toxin (Fig. 1D). TNF-α- or IL-3-induced phosphorylation of p38 was also inhibited by forskolin, isoproterenol, or cholera toxin in several other cell lines, such as HT-1080, NIH 3T3, and FL5.12 cells (Fig. 1E and F). Similar results were obtained when cells were pretreated with physiologically relevant cAMP inducers, such as prostaglandin PGE2 and epinephrine (Fig. 1G). Taken together, these data suggest that cAMP negatively regulates p38 activation by TNF-α, IL-1β, and IL-3 in the cell lines examined.
FIG. 1.
cAMP inhibits p38 activation by TNF-α, IL-1β, and IL-3. (A-C) Rat-1 cells were pretreated with or without forskolin (FSK (20 μM, 30 min), isoproterenol (ISO) (200 μM, 30 min), or cholera toxin (CTX) (100 ng/ml, 60 min), followed by stimulation with or without TNF-α (A and B) or IL-1β (C) (5 ng/ml, 15 min each). The activity of endogenous p38 was measured by immune complex kinase assays (KA) with purified GST-ATF2 (2 μg) as the substrate (A). Phosphorylation and expression of p38 were analyzed by immunoblotting (IB) with anti-phospho-p38 and anti-p38 antibodies, respectively (B and C). (D) Rat-1 cells were cotransfected with Gal4-LUC (0.2 μg) and Gal4-Elk (0.05 μg). After 30 h, cells were pretreated with FSK, isoproterenol, or CTX as described for panel A, followed by stimulation with or without TNF-α or IL-1β (5 ng/ml, 10 h each). LUC activity was determined as described previously (35). (E) Inhibition of TNF-α-induced p38 phosphorylation by cAMP in HT-1080 and NIH 3T3 cells was analyzed as described for panel B. (F) FL5.12 cells were deprived of IL-3 for 2 h, followed by IL-3 readdition for 10 min. Cells were treated with or without FSK (20 μM, 30 min) prior to IL-3 readdition. Phosphorylation and expression of p38 were analyzed by immunoblotting. (G) Rat-1 cells were pretreated with or without prostaglandin PGE2 (PG) (20 μM, 15 min) or epinephrine (Ep) (20 μM, 30 min) before stimulation with or without TNF-α (5 ng/ml, 15 min). The activity and phosphorylation of p38 were analyzed by immune complex kinase assays and immunoblotting, respectively.
PKA is required for cAMP-mediated inhibition of p38 activation.
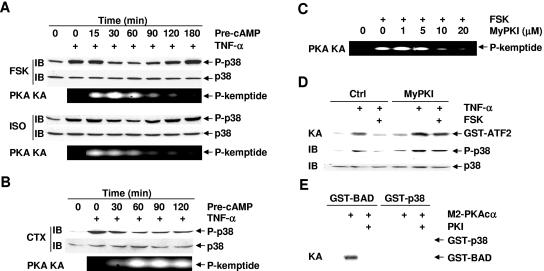
PKA is the most important effector of cAMP action, although other cAMP-binding proteins, such as cAMP-GEFs, can mediate some of the biological functions of cAMP in a PKA-independent manner (10, 13, 14, 16, 61). To determine whether PKA is involved in cAMP-mediated inhibition of p38 activation, Rat-1 cells were pretreated with or without forskolin, isoproterenol, or cholera toxin for various periods of times and then stimulated with TNF-α or left untreated. Immunoblotting analysis revealed that TNF-α-induced p38 phosphorylation was inhibited by forskolin or isoproterenol in a biphasic manner. The inhibition occurred from 30 to 60 min after the pretreatment with forskolin or isoproterenol (Fig. 2A), decreased at 90 min, and completely diminished at 180 min after the pretreatment (Fig. 2A). Under these conditions, PKA was significantly activated by the pretreatment with forskolin or isoproterenol, as measured by phosphorylation of the fluorogenic specific PKA substrate kemptide (Fig. 2A). Activation of PKA occurred at 15 min and lasted up to 60 min after the pretreatment (Fig. 2A), corresponding to the inhibition of TNF-α-induced p38 phosphorylation (Fig. 2A). Similar results were obtained with cholera toxin, which induced much slower but sustained inhibition of p38, which occurred at 60 min and lasted up to 120 min after the pretreatment (Fig. 2B). PKA activation by cholera toxin also had a slower kinetics, which occurred at 30 min after the pretreatment but was sustained up to 120 min, again corresponding to its inhibition of p38 phosphorylation (Fig. 2B).
FIG. 2.
cAMP inhibits p38 activation in a PKA-dependent manner. (A and B) Rat-1 cells were pretreated with or without FSK (20 μM), ISO (200 μM), or CTX (100 ng/ml) for various times as indicated, followed by stimulation with or without TNF-α (5 ng/ml, 15 min). Phosphorylation and expression of p38 were analyzed by immunoblotting, and PKA activity was measured by phosphorylation of the fluorogenic kemptide, respectively. (C) Rat-1 cells were pretreated with various doses of the specific PKA inhibitor MyPKI for 30 min, followed by treatment with or without FSK (20 μM, 30 min). PKA activity was determined as in panel A. (D) Rat-1 cells were pretreated with or without MyPKI (10 μM, 30 min) prior to FSK treatment (20 μM, 30 min). After stimulation with TNF-α (5 ng/ml, 15 min), the activity and phosphorylation of p38 were analyzed by immune complex kinase assays and immunoblotting, respectively. (E) Rat-1 cells were transfected with mammalian expression vector encoding M2-PKAcα (4 μg). After 40 h, the activity of M2-PKAcα was measured by immune complex kinase assays with GST-BAD (2 μg) or GST-p38 (2 μg) as substrates. For PKI inhibition, the kinase reaction mixture was incubated with the synthetic peptide inhibitor PKI (1 μg, 30 min) on ice prior to the addition of the ATP mixture. Crtl, control.
To determine whether PKA is required for cAMP-mediated inhibition of p38, we used MyPKI, which is a specific cell-permeative PKA inhibitor (20, 46). Rat-1 cells were treated with forskolin in the presence or absence of MyPKI. PKA kinase assays showed that activation of PKA by forskolin was inhibited by MyPKI in a dose-dependent manner (Fig. 2C). Treatment of cells with MyPKI reversed forskolin-mediated inhibition on TNF-α-induced p38 phosphorylation and activation (Fig. 2D) and the transcription activity of Elk (data not shown). To test whether PKA directly inhibits p38 via protein phosphorylation, Rat-1 cells were transfected with mammalian expression vector encoding M2-PKAcα, which is the catalytic subunit of PKA and is constitutively active when transfected into cells (14). Immune complex kinase assays showed that M2-PKAcα did not phosphorylate purified glutathione S-transferase (GST)-p38 proteins (Fig. 2E) but readily phosphorylated its known substrate, GST-BAD (Fig. 2E) (25). The inability of M2-PKAcα to phosphorylate GST-p38 was not due to the possibly poor quality of the GST fusion protein, since GST-p38 was efficiently phosphorylated by active HA-MKK6b(EE) (data not shown). These results, in combination with the observations that there was a delay between the activation of PKA and the inhibition of p38 by forskolin, isoproterenol, and cholera toxin (Fig. 2A and B), suggest that PKA is involved in cAMP-mediated inhibition of p38 activation through an indirect mechanism.
The involvement of PKA in the inhibition by cAMP of p38 activation did not exclude the possibility that the inhibition by cAMP may also be mediated by other cAMP effectors, such as cAMP-GEFs. To test this possibility, Rat-1 cells were pretreated with 8-pCPT-2′-O-Me-cAMP, which is a potent specific activator of cAMP-GEFs (10), followed by stimulation with or without TNF-α. Immunoblotting analysis revealed that TNF-α-induced phosphorylation of p38 was not affected by 8-pCPT-2′-O-Me-cAMP but was inhibited by 6-MB-cAMP, a site-selective activator of PKA but a poor activator of cAMP-GEFs (data not shown) (10). Taken together, the inhibition by cAMP on p38 activation is likely mediated by PKA rather than cAMP-GEFs.
The inhibition of p38 activation by cAMP requires de novo protein synthesis and depends on CREB-mediated transcription.
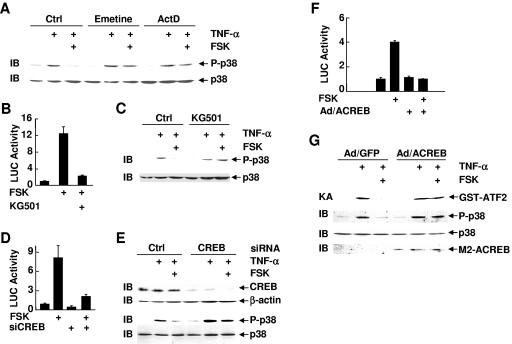
The biphasic nature of cAMP-mediated inhibition of p38 phosphorylation and the significant delay between the inhibition and PKA activation suggest that PKA may indirectly inhibit p38 activation. It is possible that the cAMP-PKA pathway may inhibit p38 activation in a transcription-dependent manner. To test this scenario, Rat-1 cells were pretreated with or without forskolin, followed by treatment with TNF-α in the presence or absence of the protein synthesis inhibitor emetine or the RNA synthesis inhibitor actinomycin D, or left untreated. The inhibition by forskolin of TNF-α-induced p38 phosphorylation was abolished by emetine or actinomycin D (Fig. 3A). Emetine and actinomycin D themselves had no detectable effects on p38 phosphorylation (Fig. 3A). This suggests that de novo protein synthesis is required for the inhibition of p38 by the cAMP-PKA pathway. In support of this notion, KG501, a small-molecule compound that interferes with the binding between transcription factors and cofactors, such as CBP/p300 (2), and has been shown to inhibit the transcriptional activity of PKA downstream target CREB (Fig. 3B, 2), also reversed the inhibition by forskolin of TNF-α-induced p38 phosphorylation (Fig. 3C). KG501 itself did not inhibit p38 phosphorylation (Fig. 3C).
FIG. 3.
CREB transcriptional activity is required for cAMP-mediated inhibition of p38 activation. (A) Rat-1 cells were treated with or without emetine or actinomycin D (ActD) (1 μg/ml each, 30 min) prior to FSK treatment (20 μM, 30 min), followed by stimulation with TNF-α (5 ng/ml, 15 min), or left untreated. Phosphorylation and expression of p38 were determined. (B) Rat-1 cells were cotransfected with Gal4-LUC (0.2 μg) and Gal4-CREB (0.05 μg). After 30 h, cells were treated with the transcription inhibitor KG501 (10 μM, 30 min) prior to stimulation with or without FSK (20 μM, 10 h). LUC activity was determined as described previously (35). (C) Rat-1 cells were treated with or without KG501 (10 μM, 30 min) prior to FSK treatment (20 μM, 30 min), followed by stimulation with or without TNF-α (5 ng/ml, 15 min). Phosphorylation and expression of p38 were determined. (D) Rat-1 cells were cotransfected with CREB siRNA or the control scramble siRNA (200 nM each) and mammalian expression vector encoding the CREB-LUC reporter gene (EVX-LUC) (0.4 μg). After 36 h, cells were treated with or without FSK (20 μM, 10 h). LUC activity was determined as described previously (35). (E) Rat-1 cells were transfected with CREB siRNA or the control scramble siRNA (200 nM each). After 48 h, cells were pretreated with FSK (20 μM, 30 min), followed by stimulation with TNF-α (5 ng/ml, 15 min), or left untreated. Phosphorylation of p38 and expression of CREB, β-actin, and p38 were determined. (F) Rat-1 cells were transfected with the CREB-LUC reporter gene (EVX-LUC, 0.4 μg), followed by infection with adenovirus ACREB (Ad/ACREB) or the control adenovirus green fluorescent protein (Ad/GFP) (multiplicity of infection = 300 each). Cells were treated with or without FSK (20 μM, 10 h). LUC activity was determined as described previously (35). (G) Rat-1 cells were infected with Ad/GFP or Ad/ACREB as described for panel F. After 48 h, cells were treated with FSK (20 μM, 30 min) prior to stimulation with TNF-α (5 ng/ml, 15 min) or left untreated. The activity and phosphorylation of p38 and expression of M2-ACREB were analyzed by immune complex kinase assays and immunoblotting, respectively. Ctrl, control.
The cAMP-PKA pathway activates several transcription factors, including CREB, CREM, and ATF-1 (30, 45, 76). Among them, CREB is the major effector of the cAMP-PKA pathway (30, 45, 76). We used CREB siRNA to test whether CREB mediates the inhibition by the cAMP-PKA pathway of p38 activation. Transfection of Rat-1 cells with CREB siRNA but not the control scramble siRNA abolished forskolin-stimulated CREB transcription activity, as monitored by a CREB-luciferase reporter gene (Fig. 3D). Immunoblotting analysis revealed that CREB siRNA, which specifically inhibited CREB expression (Fig. 3E), but not the control siRNA reversed the inhibitory effect of forskolin on TNF-α-induced p38 phosphorylation (Fig. 3E). This is not the result of changes in p38 expression (Fig. 3E). Consistently, infection of Rat-1 cells with adenoviral vector encoding ACREB (Ad/ACREB), which is a specific CREB inhibitor that utilizes its acidic amphipathic extension to prevent the basic region of CREB from binding to DNA (1), but not green fluorescent protein (Ad/GFP) blocked forskolin-induced activation of CREB transcriptional activity (Fig. 3F). The inhibition by forskolin of TNF-α-induced p38 phosphorylation and activation was also reversed by Ad/ACREB, as measured by immune complex kinase assays and immunoblotting analysis, respectively (Fig. 3G). ACREB had no detectable effect on the inhibition by cAMP of TNF-α-induced ERK activation (data not shown), suggesting the effect of ACREB on p38 activation is specific.
Upregulation of dynein light chain by the cAMP-PKA-CREB pathway is required for inhibiting p38 activation.
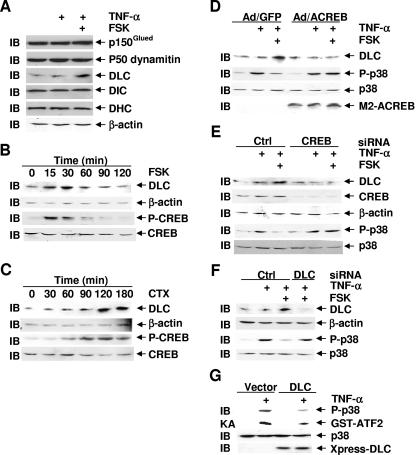
To determine how the cAMP-PKA-CREB pathway inhibits p38 activation, we searched for known CREB target genes that may be involved in regulation of the MKK3/6-p38 pathway. Previously, it was reported that p150Glued, a key component of the dynein-dynactin complex, was required for activation of the MKK3/6-p38 pathway in HeLa cells in response to sorbitol and TNF-α (8). Furthermore, both dynein heavy chain (DHC) and DLC have been reported to be encoded by CREB target genes (30), and DLC is involved in numerous dynein-independent biological processes (15, 31, 32, 39, 47, 50, 53, 57, 67). These observations led us to test whether the components of the dynein-dynactin complex are involved in the inhibition of p38 by the cAMP-PKA-CREB pathway. Immunoblotting analysis revealed that forskolin specifically induced expression of DLC but not other dynein motor proteins examined, including DHC, dynein intermediate chain, p150Glued, and p50 dynamitin (Fig. 4A). Forskolin-induced expression of DLC was rapid, occurring only 15 min after the treatment (Fig. 4B). In contrast, cholera toxin-induced expression of DLC was slower but sustained (Fig. 4C). The different kinetics in induction of DLC expression by forskolin and cholera toxin was correlated with their effects on phosphorylation of CREB at Ser133 (Fig. 4B and C), PKA activation, and inhibition of p38 activation (Fig. 2A and B).
FIG. 4.
Upregulation of dynein light chain by cAMP via CREB is essential for cAMP-mediated inhibition of p38 activation. (A) Rat-1 cells were treated with or without FSK (20 μM, 30 min) prior to stimulation with TNF-α (5 ng/ml, 15 min) or left untreated. Expression of p150Glued, p50 dynamitin, DHC, dynein intermediate chain (DIC), or DLC was analyzed by immunoblotting. (B and C) Rat-1 cells were treated with or without FSK (20 μM) (B) or CTX (100 ng/ml) (C) for various times as indicated. Phosphorylation of CREB and expression of DLC, CREB, and β-actin were analyzed by immunoblotting. (D) Rat-1 cells were infected with Ad/GFP or Ad/ACREB as for Fig. 3F. After 48 h, cells were treated with FSK (20 μM, 30 min) prior to stimulation with TNF-α (5 ng/ml, 15 min) or left untreated. Phosphorylation of p38 and expression of p38, M2ACREB, and DLC were determined. (E) Rat-1 cells were transfected with CREB siRNA or the control (Crtl) scramble siRNA (200 nM each). After 48 h, cells were pretreated with or without FSK (20 μM, 30 min), followed by stimulation with or without TNF-α (5 ng/ml, 15 min). Phosphorylation of p38 and expression of p38, CREB, β-actin, and DLC were determined. (F) Rat-1 cells were transfected with DLC siRNA or the control (Crtl) scramble siRNA (100 nM each). After 48 h, cells were treated with or without FSK (20 μM, 30 min) prior to stimulation with TNF-α (5 ng/ml, 15 min) or left untreated. Phosphorylation of p38 and expression of DLC, β-actin, and p38 were determined. (G) Rat-1 cells were transfected with a mammalian expression vector encoding Xpress-DLC (4 μg). After 24 h, cells were stimulated with or without TNF-α (5 ng/ml, 15 min). The activity and phosphorylation of p38 and expression of Xpress-DLC were analyzed by immune complex kinase assays and immunoblotting, respectively.
To determine whether DLC is upregulated by forskolin via the PKA-CREB pathway under the conditions that forskolin inhibits p38 activation, Rat-1 cells were infected with Ad/ACREB or Ad/GFP, pretreated with or without forskolin and then stimulated TNF-α, or left untreated. Immunoblotting analysis revealed that DLC expression was upregulated by forskolin when TNF-α-induced p38 phosphorylation was inhibited by forskolin (Fig. 4D). Furthermore, the upregulation of DLC by forskolin was inhibited by Ad/ACREB, which also abolished the inhibition of forskolin on TNF-α-induced p38 phosphorylation (Fig. 4D). Similar results were obtained with CREB siRNA (Fig. 4E). These results demonstrated that cAMP via CREB induces expression of DLC, which is well correlated with the inhibition by cAMP of TNF-α-induced p38 activation.
If the cAMP-PKA-CREB pathway inhibits p38 activation via induction of DLC, inhibition of DLC expression should abrogate the inhibition by cAMP of p38 activation. To test this hypothesis, Rat-1 cells were transfected with DLC siRNA or the control scramble siRNA, pretreated with or without forskolin, and then stimulated with TNF-α or left alone. Immunoblotting analysis revealed that forskolin induced DLC expression and inhibited TNF-α-induced p38 phosphorylation (Fig. 4F). Transfection of the cells with DLC siRNA, which significantly inhibited DLC expression (Fig. 4F), abolished the inhibition by forskolin on TNF-α-induced p38 phosphorylation (Fig. 4F). Conversely, ectopic expression of DLC in Rat-1 cells significantly inhibited TNF-α-stimulated p38 activity and its phosphorylation, as measured by immune complex kinase assays and immunoblotting, respectively (Fig. 4G).
The cAMP-PKA-CREB-DLC pathway inhibits p38 activation through interference with the formation of the MKK3/6-p38 complex.
cAMP inhibits p38 activation by TNF-α, IL-1β, and IL-3 (Fig. 1), which utilize different receptor complexes to activate downstream signaling effectors (4, 11, 68). This suggests that the inhibition may occur distally of the receptors and the receptor complexes. In addition, the phosphorylation of p38 at Thr180 and Tyr182 by MKK3/6 was inhibited (Fig. 1). To determine the molecular mechanism by which cAMP inhibits p38 phosphorylation activation, Rat-1 cells were transfected with a mammalian expression vector encoding HA-MKK6, which is one of the major MAP2Ks for p38 (24). After 30 h, cells were pretreated with forskolin for 30 min, followed by stimulation with or without TNF-α. Immune complex kinase assays showed that TNF-α-stimulated HA-MKK6 activity was inhibited by forskolin pretreatment (Fig. 5A). The inhibition was not the result of the difference in expression of HA-MKK6 (Fig. 5A). The inhibition by forskolin on TNF-α-stimulated MKK6 activity was reversed by MyPKI (Fig. 5B) or by DLC siRNA but not by the control siRNA (Fig. 5C), suggesting that the inhibition was indeed mediated by the cAMP-PKA-CREB-DLC pathway. Pretreatment of cells with forskolin also inhibited TNF-α-induced activation of endogenous MKK3/6, as measured by immune complex kinase assays (Fig. 5D). Taken together, these results suggest that cAMP-mediated inhibition of p38 activation may occur at or above the level of MAP2Ks. To distinguish these possibilities, Rat-1 cells were pretreated with forskolin for 30 min, followed by stimulation with TNF-α or IL-1β. Immunoblotting analysis revealed that the phosphorylation of MKK3/6, which are major MAP2Ks for p38 in cells (5, 12, 24), was not affected (Fig. 5E and F). Similar results were obtained with FL5.12 cells, in which pretreatment with forskolin had no effects on IL-3-induced phosphorylation of MKK3/6 (Fig. 5G). PKA also did not phosphorylate MKK3/6 (data not shown). These data suggest that cAMP inhibited p38 activation by interfering with its phosphorylation by MKK3/6, rather than the MAP3Ks that phosphorylate and activate MKK3/6.
FIG. 5.
The cAMP-PKA-CREB-DLC pathway inhibits p38 activation through interference with the formation of the MKK3/6-p38 complex. (A) Rat-1 cells were transfected with a mammalian expression vector encoding HA-MKK6b (0.1 μg). After 48 h, cells were pretreated with or without FSK (20 μM, 30 min), followed by stimulation with TNF-α (5 ng/ml, 5 min), or left untreated. The activity of HA-MKK6b was measured by immune complex kinase assays with GST-p38 (2 μg) as a substrate. (B) Rat-1 cells were transfected with mammalian expression vector encoding HA-MKK6b as described for panel A. Cells were treated with or without MyPKI (10 μM, 30 min) prior to FSK treatment (20 μM, 30 min), followed by stimulation with or without TNF-α (5 ng/ml, 5 min). The kinase activity of MKK6 was measured as for panel A. (C) Rat-1 cells were transfected with a mammalian expression vector encoding HA-MKK6b, along with DLC siRNA or the control scramble siRNA (100 nM each). After 48 h, cells were treated with or without FSK (20 μM, 30 min) prior to stimulation with TNF-α (5 ng/ml, 5 min) or left untreated. The kinase activity of MKK6 was measured as for panel A. (D) Rat-1 cells were pretreated with or without FSK (20 μM, 30 min), followed by stimulation with TNF-α (5 ng/ml, 5 min) or left alone. The endogenous MKK3/6 were isolated, and their activity and expression were determined by immune complex kinase assays and immunoblotting, respectively. (E-G) Rat-1 cells were pretreated with or without FSK (20 μM, 30 min), followed by stimulation with TNF-α (E), IL-1β (F) (5 ng/ml, 5 min each), or left untreated. FL5.12 cells were deprived of IL-3 for 2 h, followed by IL-3 readdition for 5 min. Cells were treated with or without FSK (20 μM, 30 min) prior to IL-3 readdition (G). Phosphorylation and expression of MKK3/6 were analyzed by immunoblotting using anti-phospho-MKK3/6 and anti-MKK3/6 antibodies, respectively. (H) Rat-1 cells were cotransfected with mammalian expression vectors encoding HA-MKK6b, M2-p38, and Xpress-DLC (2 μg each). After 30 h, cells were harvested. The lysates were immunoprecipitated with anti-M2 monoclonal antibody and subjected to immunoblotting analysis with anti-HA, p38, and Xpress antibodies, respectively. Crtl, control.
The above observations that cAMP inhibited p38 phosphorylation and activation by MKK3/6 but not phosphorylation of MKK3/6 by MAP3Ks suggest that the cAMP-PKA-CREB-DLC pathway may inhibit the interaction between p38 and MKK3/6. To test this hypothesis, Rat-1 cells were transfected with mammalian expression vectors encoding HA-MKK6, M2-p38, and Xpress-DLC. Immunoprecipitation in combination with immunoblotting analysis revealed that HA-MKK6 interacted with M2-p38 (Fig. 5H), consistent with previous reports (8, 73). The interaction between HA-MKK6 and M2-p38 was significantly inhibited by cotransfected Xpress-DLC (Fig. 5H). The inhibition by Xpress-DLC was not the result of the difference in immunoprecipitated M2-p38 in the MKK6-p38 complexes or the difference in expression levels of HA-MKK6 and M2-p38 (Fig. 5H). Thus, elevation of cAMP may via DLC disrupt the MKK3/6-p38 complex, thereby inhibiting p38 phosphorylation and activation.
Inhibition of p38 activation by cAMP suppresses NF-κB activation, thereby contributing to TNF-α-induced apoptosis.
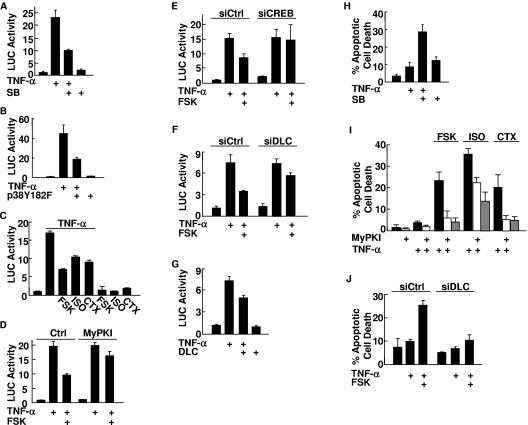
Once activated, p38 can translocate into the nucleus, where it phosphorylates and activates several transcription factors, including NF-κB, which is able to suppress TNF-α-induced apoptosis (7, 18, 22, 42, 55, 69). It has also been reported that cAMP can promote TNF-α-induced apoptosis in a cell-type-dependent manner (49, 70, 71). The observation that TNF-α-induced p38 activation was inhibited by the cAMP pathway promoted us to test whether the inhibition leads to suppressing NF-κB activation, thereby promoting TNF-α-induced apoptosis. To test this hypothesis, we first determined whether the inhibition of p38 activation by the cAMP pathway suppresses NF-κB activation. Treatment with TNF-α stimulated NF-κB transcriptional activity in Rat-1 cells, and the stimulation was inhibited by the p38 inhibitor SB203580 (Fig. 6A) or cotransfected kinase-deficient p38(Y182F) mutant (Fig. 6B), as measured by NF-κB-luciferase reporter gene assays. These results suggest that p38 contributed to TNF-α-induced NF-κB activation in Rat-1 cells, as previously reported for other cell types (7, 18, 22, 69). Pretreatment with forskolin, isoproterenol, or cholera toxin also significantly suppressed TNF-α-induced NF-κB transcriptional activity (Fig. 6C). Similar results were obtained with TNF-α-treated HT1080 cells or with IL-1β-treated Rat-1 cells (data not shown). The inhibition was reversed by the cell-permeative PKA inhibitor MyPKI (Fig. 6D) or by CREB siRNA but not the control siRNA (Fig. 6E), suggesting that the PKA-CREB pathway was involved in the inhibition of NF-κB by cAMP. The inhibition by forskolin of TNF-α-induced NF-κB transcriptional activity was also reversed by DLC siRNA, which released the inhibition of cAMP on p38 activation (Fig. 4F), but not the control siRNA (Fig. 6F). Conversely, TNF-α-induced NF-κB transcriptional activity was inhibited by ectopic expression of DLC (Fig. 6G). By contrast, the cAMP-PKA-CREB-DLC pathway has no significantly inhibitory effect on activation of the IKK complex, IκBα phosphorylation and proteosome-mediated degradation, and NF-κB nuclear translocation (data not shown). These data suggest that the cAMP-PKA-CREB-DLC pathway suppressed TNF-α-induced NF-κB activation through, at least in part, inhibition of p38 activation.
FIG. 6.
Inhibition of p38 by cAMP suppresses NF-κB activation, thereby contributing to TNF-α-induced apoptosis. (A) Rat-1 cells were transfected with a mammalian expression vector encoding the NF-κB reporter gene (IL-8-LUC) (0.2 μg). After 30 h, cells were treated with SB203580 (SB) (20 μM, 30 min) prior to the stimulation with or without TNF-α (5 ng/ml) for 10 h. LUC activity was determined as described previously (35). (B) Rat-1 cells were cotransfected with IL-8-LUC (0.2 μg) and an expression vector encoding p38(Y182F) (0.5 μg). LUC activity was determined after cells were stimulated with or without TNF-α (5 ng/ml) for 10 h. (C) Rat-1 cells were transfected with IL-8-LUC (0.2 μg). After 30 h, cells were treated with FSK (20 μM, 30 min), isoproterenol (ISO) (200 μM, 30 min), or CTX (100 ng/ml, 60 min) prior to stimulation with or without TNF-α (5 ng/ml) for 10 h. LUC activity was determined. (D) LUC activity was determined as for panel C except that Rat-1 cells were treated with or without MyPKI (10 μM, 30 min) prior to FSK treatment. Ctrl, control. (E) Rat-1 cells were cotransfected with IL-8-LUC (0.2 μg), along with CREB siRNA (siCREB) or the control scramble siRNA (siCtrl) (200 nM each). After 36 h, cells were treated with or without FSK (20 μM, 30 min) prior to the stimulation with or without TNF-α (5 ng/ml) for 10 h. LUC activity was determined as described previously (35). (F) Rat-1 cells were cotransfected with IL-8-LUC (0.2 μg), along with DLC siRNA (siDLC) or the control scramble siRNA (siCtrl) (100 nM each). LUC activity was determined as described for panel E. (G) Rat-1 cells were cotransfected with a mammalian expression vector encoding Xpress-DLC or empty vector (0.5 μg each), along with IL-8-LUC (0.2 μg). LUC activity was determined after cells were stimulated with or without TNF-α (5 ng/ml) for 10 h. (H) After pretreatment with SB (20 μM, 30 min), serum-starved Rat-1 cells were incubated with TNF-α for 20 h. The apoptotic cell death was monitored by Hoechst (H33258) staining (37, 66, 72, 75). (I) Rat-1 cells were pretreated with or without MyPKI (10 μM, 30 min) prior to treatment with FSK, isoproterenol, or CTX as for Fig. 1A. Serum-starved Rat-1 cells were incubated with TNF-α (5 ng/ml) for 20 h, and apoptotic cell death was monitored by Hoechst (H33258) staining. (J) Rat-1 cells were transfected with DLC siRNA (siDLC) or the control scramble siRNA (siCtrl) (100 nM each). After 40 h, serum-starved cells were pretreated with FSK (20 μM, 30 min) and treated with TNF-α (5 ng/ml) for 20 h. Apoptotic cell death was monitored by Hoechst (H33258) staining.
Next, we determined whether inhibition of p38 by the cAMP-PKA-CREB-DLC pathway contributes to TNF-α-induced apoptosis. TNF-α significantly induced apoptosis in Rat-1 cells that had been pretreated with the p38 inhibitor SB203580 but not in control cells (Fig. 6H), as reported for other cell types (18, 22, 42, 55, 69). Pretreatment with forskolin, isoproterenol, or cholera toxin also sensitized Rat-1 cells to TNF-α-induced apoptosis (Fig. 6I). The apoptosis induced by TNF-α/cAMP was abrogated by the cell-permeative PKA inhibitor MyPKI (Fig. 6I). Inhibition of DLC expression by DLC siRNA, which released the inhibition by cAMP of p38 activation (Fig. 4F), also abolished the promotion by forskolin of TNF-α-induced apoptosis (Fig. 6J). Taken together, cAMP promotes TNF-α-induced apoptosis through CREB-induced DLC, which inhibits p38 and thereby suppresses NF-κB activation.
DISCUSSION
The MAPK p38 regulates many cellular events, from cell cycle progression to apoptosis, and the activity of p38 itself is tightly controlled by other intracellular signaling pathways, such as the cAMP pathway (6, 8, 9, 17, 19, 51, 54, 74). The regulation of p38 activation by cAMP has been reported to be stimulatory or inhibitory, depending on the cell context and the stimuli (6, 9, 17, 51, 54). However, the underlying mechanism is unknown. In this report we demonstrate that cAMP via CREB-induced DLC disrupts the complex formation between MKK3/6 and p38, thereby suppressing p38 activation and promoting apoptosis. This conclusion is based on the following evidence.
First, elevation of intracellular cAMP concentrations by the physiologically relevant cAMP inducers PGE2 and epinephrine and other reagents like forskolin, isoproterenol, and cholera toxin inhibited p38 activation by TNF-α, IL-1β, and IL-3 in various cell lines (Fig. 1). Second, the PKA-CREB pathway, but not cAMP-GEFs, was required for cAMP-mediated inhibition of p38 activation (Fig. 2 and 3; also data not shown). Third, the CREB target gene DLC was essential for cAMP to inhibit p38 activation (Fig. 4). Fourth, the cAMP-PKA-CREB-DLC pathway inhibited p38 activation by disrupting the complex formation between p38 and its upstream kinases MKK3/6 (Fig. 5). Fifth, the inhibition of p38 activation by cAMP suppressed TNF-α-induced NF-κB activation, thereby promoting apoptosis (Fig. 6). Taken together, our results revealed the molecular mechanism underlying the cross talk between the cAMP and p38 pathways and the importance of this cross talk in TNF-α-induced apoptosis.
The inhibition by cAMP of p38 activation is mediated by the PKA-CREB-DLC pathway. The biological functions of cAMP are mainly mediated by PKA (14, 16) and the newly discovered cAMP binding proteins the cAMP-GEFs, which are GEFs of the small GTPase Rap I (10, 13, 61). Our results show that PKA activation by cAMP inducing agents was well correlated with and required for the inhibition of p38 activation (Fig. 2), whereas activation of cAMP-GEFs did not inhibit p38 activation (data not shown). PKA appeared to inhibit p38 activation indirectly, since the inhibition of p38 activation by cAMP was delayed compared to PKA activation (Fig. 2A and B). In support of this notion, PKA was unable to directly phosphorylate p38 or its upstream kinase MKK3/6 (Fig. 2E; also data not shown). Furthermore, the inhibition by cAMP was abolished by the protein synthesis inhibitor emetine, transcription inhibitor actinomycin D, or KG501, which interferes with the interaction between transcription factors and cofactors (Fig. 3A, B, and C). This suggests that the inhibition by the cAMP-PKA pathway may depend on upregulation of gene expression. Indeed, among the transcription factors that are regulated by PKA, including CREB, CREM, and ATF-1, inactivation of CREB but not CREM or ATF-1 abolished the inhibition by cAMP of TNF-α-induced p38 activation (Fig. 3D, E, F, and G; also data not shown). Consistently, our results show that upregulation of CREB-target gene DLC expression was essential for the inhibition by cAMP of p38 activation (Fig. 4). Thus, cAMP inhibits p38 activation through the PKA-CREB-DLC pathway.
How does the cAMP-PKA-CREB-DLC pathway inhibit the activation of p38? MKK3/6 are major MAP2Ks for p38 activation in vitro and in vivo (5, 12, 24). Genetic disruption of MKK3/6 alleles in mice abolishes p38 activation by a variety of extracellular stimuli, demonstrating that phosphorylation of p38 by MKK3/6 at Thr180 and Tyr182 in the phosphorylation activation T loop is essential for activation of p38 (5, 74). Our results show that in addition to inhibition of p38 phosphorylation activation, forskolin also inhibited the enzymatic activity of MKK3/6 through the PKA-CREB-DLC pathway (Fig. 5A to D). However, the phosphorylation of MKK3/6 by upstream MAP3Ks was not affected (Fig. 5E, F, and G). This excluded MAP3Ks as the targets of cAMP-mediated inhibition. Furthermore, ectopic expression of DLC resulted in disruption of the MKK3/6-p38 complex (Fig. 5H). This suggests that cAMP inhibits TNF-α-induced p38 activation by interfering with the formation of the MKK3/6-p38 complex.
How does DLC disrupt the formation of the MKK3/6-p38 complex? In addition to its role as a key component in the dynein-dynactin motor, DLC regulates a number of cellular activities via binding to many signaling molecules or enzymes, including nitric oxide synthase, the NF-κB inhibitor IκBα, the proapoptotic Bcl-2 family protein Bim, p53-binding protein 1, the neuronal scaffold protein GKAP, the transcriptional repressor TRPS1, and estrogen receptor (15, 21, 31, 32, 39, 47, 50, 53, 57, 67). It has been reported that DLC binds to a conserved K/RXTQT motif in its target proteins (38). Our results show that DLC siRNA released the inhibition by cAMP of the enzymatic activity of MKK6 (Fig. 5C), and ectopic expression of DLC interfered with the formation of MKK3/6-p38 complex (Fig. 5H). However, there was no direct interaction between DLC and MKK3/6 or p38 in vitro and in vivo (data not shown). In support of this notion, there is no K/RXTQT motif in MKK3/6 or p38. Thus, DLC may inhibit the complex formation between MKK3/6 and p38 indirectly. It is possible that the dynein-dynactin motor complex, in which both DLC and p150Glued are key components, is involved in the formation of the MKK3/6-p38 complex. Upregulation of certain components in the dynein-dynactin motor complex resulted in malfunction of the dynein motor (44, 58). In analogy, upregulation of DLC by the cAMP-PKA-CREB pathway may also cause malfunction of the dynein motor complex, thereby interfering with the interaction between MKK3/6 and p38. Another possibility is that DLC may interfere with the interaction between MKK3/6 and p38 via its regulation of other signaling pathways that are involved in regulation of p38 activation. Future studies are needed to test these possibilities.
Does the cAMP-PKA-CREB-DLC pathway selectively inhibit p38 but not other MAP kinases? cAMP is a well-known regulator of MAP kinases in a cell type- and stimulus-dependent manner. Elevation of cAMP can inhibit or activate ERK activity in a cell context-dependent manner (62). cAMP also inhibits JNK activation with unknown mechanisms (29, 52). Our results show that cAMP inhibited p38 activation via the PKA-CREB-DLC pathway (Fig. 1 to 5). Interestingly, the de novo protein synthesis was required for cAMP-mediated inhibition of TNF-α-induced JNK activation but not for the inhibition of TNF-α-induced ERK activation in Rat-1 cells (data not shown). Furthermore, the inhibition of JNK by cAMP also required CREB (data not shown), similar to the inhibition of p38 by cAMP (Fig. 3). However, DLC was not required for the inhibition of JNK by cAMP (data not shown), suggesting that another CREB target gene(s) is involved. Thus, cAMP inhibits MAP kinases through different mechanisms.
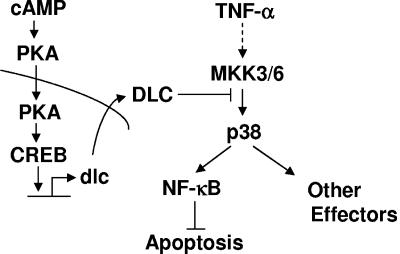
Does the inhibition of p38 activation by the cAMP pathway affect TNF-α-induced NF-κB activation and apoptosis? Previous studies have shown that cAMP can negatively regulate NF-κB activity, thereby contributing to TNF-α-induced apoptosis in certain types of cells with no known mechanisms (49, 70, 71). It is known that TNF-α activates NF-κB via, at least, two mechanisms: activation of the IKK complex that controls the nuclear translocation of NF-κB (34) and activation of p38 that can phosphorylate and further activate NF-κB in the nucleus (43). Our results show that the cAMP pathway had no significant effects on the activity of IKK complex, phosphorylation of IκBα, or NF-κB nuclear translocation (data not shown). Instead, the cAMP pathway negatively regulated NF-κB activity through, at least in part, DLC-mediated inhibition of p38 activation, thereby contributing to TNF-α-induced apoptosis (Fig. 6). Thus, regulation of p38 activity by the cAMP pathway may be the key determinant that decides whether cAMP contributes to apoptosis induced by TNF-α and other death stimuli (Fig. 7).
FIG. 7.
A schematic presentation of the molecular mechanism by which cAMP inhibits p38 activation via CREB-induced DLC.
Acknowledgments
We are grateful to Chen-Ming Fan, Jiahuai Han, Stanley Korsmeyer, Marc Montminy, Wei-Jen Tang, and Zhenguo Wu for providing reagents that make this work possible and Zhenguo Wu for helpful discussion.
This work was partially supported by National Institutes of Health grants CA92650 and CA100460 (to A.L.).
REFERENCES
- 1.Ahn, S., M. Olive, S. Aggarwal, D. Krylov, D. D. Ginty, and C. Vinson. 1998. A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol. Cell. Biol. 18:967-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Best, J. L., C. A. Amezcua, B. Mayr, L. Flechner, C. M. Murawsky, B. Emerson, T. Zor, K. H. Gardner, and M. Montminy. 2004. Identification of small-molecule antagonists that inhibit an activator: coactivator interaction. Proc. Natl. Acad. Sci. USA 101:17622-17627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birnbaumer, L. 1992. Receptor-to-effector signaling through G proteins: roles for βγ dimers as well as α-subunits. Cell 71:1069-1072. [DOI] [PubMed] [Google Scholar]
- 4.Braddock, M., and A. Quinn. 2004. Targeting IL-1 in inflammatory disease: new opportunities for therapeutic intervention. Nat. Rev. Drug Discov. 3:330-339. [DOI] [PubMed] [Google Scholar]
- 5.Brancho, D., N. Tanaka, A. Jaeschke, J. J. Ventura, N. Kelkar, Y. Tanaka, M. Kyuuma, T. Takeshita, R. A. Flavell, R. J. Davis. 2003. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 17:1969-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao, W., K. W. Daniel, J. Robidoux, P. Puigserver, A. V. Medvedev, X. Bai, L. M. Floering, B. M. Spiegelman, and S. Collins. 2004. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol. Cell. Biol. 24:3057-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter, A. B., K. L. Knudston, M. M. Monick, and G. W. Hunninghake. 1999. The p38 mitogen-activated protein kinase is required for NF-κB-dependent gene expression: the role of TATA binding protein (TBP). J. Biol. Chem. 274:30858-30863. [DOI] [PubMed] [Google Scholar]
- 8.Cheung, P. Y., Y. Zhang, J. Long, S. Lin, M. Zhang, Y. Jiang, and Z. Wu. 2004. p150Glued, Dynein, and microtubules are specifically required for activation of MKK3/6 and p38 MAPKs. J. Biol. Chem. 279:45308-45311. [DOI] [PubMed] [Google Scholar]
- 9.Choi, S. C., B. S. Kim, M. Y. Song, E. Y. Choi, H. M. Oh, J. H. Lyou, W. C. Han, H. B. Moon, T. H. Kim, J. M. Oh, H. T. Chung, and C. D. Jun. 2003. Downregulation of p38 kinase pathway by cAMP response element-binding protein protects HL-60 cells from iron chelator-induced apoptosis. Free Radic. Biol. Med. 35:1171-1184. [DOI] [PubMed] [Google Scholar]
- 10.Christensen, A. E., F. Selheim, J. de Rooij, S. Dremier, F. Schwede, K. K. Dao, A. Martinez, C. Maenhaut, J. L. Bos, H. G. Genieser, and S. O. Doskeland. 2003. cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J. Biol. Chem. 278:35394-35402. [DOI] [PubMed] [Google Scholar]
- 11.de Groot, R. P., P. J. Coffer, and L. Koenderman. 1998. Regulation of proliferation, differentiation and survival by the IL-3/IL-5/GM-CSF receptor family. Cell Signal. 10:619-628. [DOI] [PubMed] [Google Scholar]
- 12.Derijard, B., J. Raingeaud, T. Barrett, I. H. Wu, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 267:682-685. [DOI] [PubMed] [Google Scholar]
- 13.de Rooij, J., F. J. Zwartkruis, M. H. Verheijen, R. H. Cool, S. M. Nijman, A. Wittinghofer, and J. L. Bos. 1998. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396:474-477. [DOI] [PubMed] [Google Scholar]
- 14.Doskeland, S. O., E. Maronde, and B. T. Gjertsen. 1993. The genetic subtypes of cAMP-dependent protein kinase—functionally different or redundant? Biochim. Biophys. Acta 1178:249-258. [DOI] [PubMed] [Google Scholar]
- 15.Epstein, E., A. Sela-Brown, I. Ringel, R. Kilav, S. M. King, S. E. Benashski, J. K. Yisraeli, J. Silver, and T. Naveh-Many. 2000. Dynein light chain binding to a 3′-untranslated sequence mediates parathyroid hormone mRNA association with microtubules. J. Clin. Investig. 105:505-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feliciello, A., M. E. Gottesman, and E. V. Avvedimento. 2005. cAMP-PKA signaling to the mitochondria: protein scaffolds, mRNA and phosphatases. Cell Signal. 17:279-287. [DOI] [PubMed] [Google Scholar]
- 17.Feng, W. G., Y. B. Wang, J. S. Zhang, X. Y. Wang, C. L. Li, and Z. L. Chang. 2002. cAMP elevators inhibit LPS-induced IL-12 p40 expression by interfering with phosphorylation of p38 MAPK in murine peritoneal macrophages. Cell Res. 12:331-337. [DOI] [PubMed] [Google Scholar]
- 18.Franco, D. L., M. Nojek, I. L. Molinero, O. A. Coso, and M. A. Costas. 2002. Osmotic stress sensitizes naturally resistant cells to TNF-α-induced apoptosis. Cell Death Differ. 9:1090-1098. [DOI] [PubMed] [Google Scholar]
- 19.Ge, B., H. Gram, F. Di Padova, B. Huang, L. New, R. J. Ulevith, Y. Luo, and J. Han. 2002. MAPKK-independent activation of p38α mediated by TAB1-dependent autophosphorylation of p38α. Science 295:1291-1294. [DOI] [PubMed] [Google Scholar]
- 20.Glass, D. B., H. C. Cheng, L. Mende-Mueller, J. Reed, and D. A. Walsh. 1989. Primary structural determinants essential for potent inhibition of cAMP-dependent protein kinase by inhibitory peptides corresponding to the active portion of the heat-stable inhibitor protein. J. Biol. Chem. 264:8802-8810. [PubMed] [Google Scholar]
- 21.Guan, Z., S. Y. Buckman, L. D. Springer, and A. R. Morrison. 1999. Both p38αMAPK and JNK/SAPK pathways are important for induction of nitric-oxide synthase by interleukin-1β in rat glomerular mesangial cells. J. Biol. Chem. 274:36200-36206. [DOI] [PubMed] [Google Scholar]
- 22.Guo, Y. L., B. Kang, J. Han, and J. R. Williamson. 2001. p38β MAP kinase protects rat mesangial cells from TNF-α-induced apoptosis. J. Cell. Biochem. 82:556-565. [DOI] [PubMed] [Google Scholar]
- 23.Han, J., J. D. Lee, L. Bibbs, and R. J. Ulevitch. 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808-811. [DOI] [PubMed] [Google Scholar]
- 24.Han, J., J. D. Lee, Y. Jiang, Z. Li, L. Feng, and R. J. Ulevitch. 1996. Characterization of the structure and function of a novel MAP kinase kinase (MKK6). J. Biol. Chem. 271:2886-2891. [DOI] [PubMed] [Google Scholar]
- 25.Harada, H., B. Becknell, M. Wilm, M. Mann, L. J. Huang, S. S. Taylor, J. D. Scott, and S. J. Korsmeyer. 1999. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol. Cell 3:413-422. [DOI] [PubMed] [Google Scholar]
- 26.Hogarth, D. K., N. Sandbo, S. Taurin, V. Kolenko, J. M. Miano, and N. O. Dulin. 2004. Dual role of PKA in phenotypic modulation of vascular smooth muscle cells by extracellular ATP. Am. J. Physiol. Cell Physiol. 287:C449-CC456. [DOI] [PubMed] [Google Scholar]
- 27.Houslay, M. D., and D. R. Adams. 2003. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem. J. 370:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang, S., Y. Jiang, Z. Li, E. Nishida, P. Mathias, S. Lin, R. J. Ulevitch, G. R. Nemerow, and J. Han. 1997. Apoptosis signaling pathway in T cells is composed of ICE/Ced-3 family proteases and MAP kinase kinase 6b. Immunity 6:739-749. [DOI] [PubMed] [Google Scholar]
- 29.Hsueh, Y. P., and M. Z. Lai. 1995. c-Jun N-terminal kinase but not mitogen-activated protein kinase is sensitive to cAMP inhibition in T lymphocytes. J. Biol. Chem. 270:18094-18098. [DOI] [PubMed] [Google Scholar]
- 30.Impey, S., S. R. McCorkle, H. Cha-Molstad, J. M. Dwyer, G. S. Yochum, J. M. Boss, S. McWeeney, J. J. Dunn, G. Mandel, and R. H. Goodman. 2004. Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell 119:1041-1054. [DOI] [PubMed] [Google Scholar]
- 31.Jaffrey, S. R., and S. H. Snyder. 1996. PIN: an associated protein inhibitor of neuronal nitric oxide synthase. Science 274:774-777. [DOI] [PubMed] [Google Scholar]
- 32.Kaiser, F. J., K. Tavassoli, G. J. Van den Bemd, G. T. Chang, B. Horsthemke, T. Moroy, and H. J. Ludecke. 2003. Nuclear interaction of the dynein light chain LC8a with the TRPS1 transcription factor suppresses the transcriptional repression activity of TRPS1. Hum. Mol. Genet. 12:1349-1358. [DOI] [PubMed] [Google Scholar]
- 33.Karin, M. 1995. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 270:16483-16486. [DOI] [PubMed] [Google Scholar]
- 34.Karin, M., and A. Lin. 2002. NF-κB at the crossroads of life and death. Nat. Immunol. 3:221-227. [DOI] [PubMed] [Google Scholar]
- 35.Lin, A., A. Minden, H. Martinetto, F. X. Claret, C. Lange-Carter, F. Mercurio, G. L. Johnson, and M. Karin. 1995. Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science 268:286-290. [DOI] [PubMed] [Google Scholar]
- 36.Lin, A. 2003. Activation of the JNK signaling pathway: breaking the brake on apoptosis. Bioessays 25:1-8. [DOI] [PubMed] [Google Scholar]
- 37.Liu, J., Y. Minemoto, and A. Lin. 2004. c-Jun N-terminal protein kinase 1 (JNK1), but not JNK2, is essential for tumor necrosis factor alpha-induced c-Jun kinase activation and apoptosis. Mol. Cell. Biol. 24:10844-10856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo, K. W., S. Naisbitt, J. S. Fan, M. Sheng, and M. Zhang. 2001. The 8-kDa dynein light chain binds to its targets via a conserved (K/R)XTQT motif. J. Biol. Chem. 276:14059-14066. [DOI] [PubMed] [Google Scholar]
- 39.Lo, K. W., H. M. Kan, L. N. Chan, W. G. Xu, K. P. Wang, Z. Wu, M. Sheng, and M. Zhang. 2005. The 8-kDa dynein light chain binds to p53-binding protein 1 and mediates DNA damage-induced p53 nuclear accumulation. J. Biol. Chem. 280:8172-8179. [DOI] [PubMed] [Google Scholar]
- 40.Lu, X., S. Nemoto, and A. Lin. 1997. Identification of c-Jun NH2-terminal protein kinase (JNK)-activating kinase 2 as an activator of JNK but not p38. J. Biol. Chem. 272:24751-24754. [DOI] [PubMed] [Google Scholar]
- 41.Lubran, M. M. 1988. Bacterial toxins. Ann. Clin. Lab. Sci. 18:58-71. [PubMed] [Google Scholar]
- 42.Luschen, S., G. Scherer, S. Ussat, H. Ungefroren, S., and Adam-Klages. 2004. Inhibition of p38 mitogen-activated protein kinase reduces TNF-induced activation of NF-κB, elicits caspase activity, and enhances cytotoxicity. Exp. Cell Res. 293:196-206. [DOI] [PubMed] [Google Scholar]
- 43.Madrid, L. V., M. W. Mayo, J. Y. Reuther, and A. S. Baldwin, Jr. 2001. Akt stimulates the transactivation potential of the RelA/p65 subunit of NF-κB through utilization of the IκB kinase and activation of the mitogen-activated protein kinase p38. J. Biol. Chem. 276:18934-18940. [DOI] [PubMed] [Google Scholar]
- 44.Mallik, R., and S. P. Gross. 2004. Molecular motors: strategies to get along. Curr. Biol. 14:R971-R982. [DOI] [PubMed] [Google Scholar]
- 45.Mayr, B., and M. Montminy. 2001. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2:599-609. [DOI] [PubMed] [Google Scholar]
- 46.Muñiz, M., M. E. Martín, J. Hidalgo, and A. Velasco. 1997. Protein kinase A activity is required for the budding of constitutive transport vesicles from the trans-Golgi network. Proc. Natl. Acad. Sci. USA 94:14461-14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Navarro, C., H. Puthalakath, J. M. Adams, A. Strasser, and R. Lehmann. 2004. Egalitarian binds dynein light chain to establish oocyte polarity and maintain oocyte fate. Nat. Cell Biol. 6:427-435. [DOI] [PubMed] [Google Scholar]
- 48.Nemoto, S., Z. Sheng, and A. Lin. 1998. Opposing effects of Jun kinase and p38 mitogen-activated protein kinases on cardiomyocyte hypertrophy. Mol. Cell. Biol. 18:3518-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patino, J. A. G., V. N. Ivanov, E. Lacy, K. B. Elkon, M. W. Marino, and J. Nikolic-Zugic. 2000. TNF-α is the critical mediator of the cyclic AMP-induced apoptosis of CD8+4+ double-positive thymocytes. J. Immunol. 164:1689-1694. [DOI] [PubMed] [Google Scholar]
- 50.Puthalakath, H., D. C. Huang, L. A. O'Reilly, S. M. King, and A. Strasser. 1999. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell 3:287-296. [DOI] [PubMed] [Google Scholar]
- 51.Rahman, A., K. N. Anwar, M. Minhajuddin, K. M. Bijli, K. Javai, A. L. True, and A. B. Malik. 2004. cAMP targeting of p38 MAP kinase inhibits thrombin-induced NF-κB activation and ICAM-1 expression in endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 287:L1017-L1024. [DOI] [PubMed] [Google Scholar]
- 52.Rao, G. N., and M. S. Runge. 1996. Cyclic AMP inhibition of thrombin-induced growth in vascular smooth muscle cells correlates with decreased JNK1 activity and c-Jun expression. J. Biol. Chem. 271:20805-20810. [DOI] [PubMed] [Google Scholar]
- 53.Rayala, S. K., P. den Hollander, S. Balasenthil, Z. Yang, R. R. Broaddus, and R. Kumar. 2005. Functional regulation of oestrogen receptor pathway by the dynein light chain 1. EMBO Rep. 6:538-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robidoux, J., W. Cao, H. Quan, K. W. Daniel, F. Moukdar, X. Bai, L. M. Floering, and S. Collins. 2005. Selective activation of mitogen-activated protein (MAP) kinase kinase 3 and p38α MAP kinase is essential for cyclic AMP-dependent UCP1 expression in adipocytes. Mol. Cell. Biol. 25:5466-5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roulston, A., C. Reinhard, P. Amiri, and L. T. Williams. 1998. Early activation of c-Jun N-terminal kinase and p38 kinase regulate cell survival in response to tumor necrosis factor α. J. Biol. Chem. 273:10232-10239. [DOI] [PubMed] [Google Scholar]
- 56.Rouse, J., P. Cohen, S. Trigon, M. Morange, A. Alonso-Llamazares, D. Zamanillo, T. Hunt, and A. R. Nebreda. 1994. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 78:1027-1037. [DOI] [PubMed] [Google Scholar]
- 57.Schnorrer, F., K. Bohmann, and C. Nusslein-Volhard. 2000. The molecular motor dynein is involved in targeting swallow and bicoid RNA to the anterior pole of Drosophila oocytes. Nat. Cell Biol. 2:185-190. [DOI] [PubMed] [Google Scholar]
- 58.Schroer, T. A. 2004. Dynactin. Annu. Rev. Cell Dev. Biol. 20:759-779. [DOI] [PubMed] [Google Scholar]
- 59.Seamon, K. B., W. Padgett, and J. W. Daly. 1981. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc. Natl. Acad. Sci. USA 78:3363-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith, P. G., F. Wang, K. N. Wilkinson, K. J. Savage, U. Klein, D. S. Neuberg, G. Bollag, M. A. Shipp, and R. C. Aguiar. 2005. The phosphodiesterase PDE4B limits cAMP-associated PI3K/AKT-dependent apoptosis in diffuse large B-cell lymphoma. Blood 105:308-316. [DOI] [PubMed] [Google Scholar]
- 61.Springett, G. M., H. Kawasaki, and D. R. Spriggs. 2004. Non-kinase second-messenger signaling: new pathways with new promise. Bioessays 26:730-738. [DOI] [PubMed] [Google Scholar]
- 62.Stork, P. J., and J. M. Schmitt. 2002. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 12:258-266. [DOI] [PubMed] [Google Scholar]
- 63.Sunahara, R. K., and R. Taussig. 2002. Isoforms of mammalian adenylyl cyclase: multiplicities of signaling. Mol. Interv. 2:168-184. [DOI] [PubMed] [Google Scholar]
- 64.Tang, F., G. Tang, J. Xiang, Q. Dai, M. R. Rosner, and A. Lin. 2002. Absence of NF-κB-mediated inhibition of c-Jun N-terminal kinase activation contributes to tumor necrosis factor alpha-induced apoptosis. Mol. Cell. Biol. 22:8571-8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang, G., Y. Minemoto, B. Dibling, N. H. Purcell, Z. Li, M. Karin, and A. Lin. 2001. Inhibition of JNK activation through NF-κB target genes. Nature 414:313-317. [DOI] [PubMed] [Google Scholar]
- 66.Tang, G., J. Yang, Y. Minemoto, and A. Lin. 2001. Blocking caspase-3-mediated proteolysis of IKKβ suppresses TNF-α-induced apoptosis. Mol. Cell 8:1005-1016. [DOI] [PubMed] [Google Scholar]
- 67.Vadlamudi, R. K., R. Bagheri-Yarmand, Z. Yang, S. Balasenthil, D. Nguyen, A. A. Sahin, P. den Hollander, and R. Kumar. 2004. Dynein light chain 1, a p21-activated kinase 1-interacting substrate, promotes cancerous phenotypes. Cancer Cell 5:575-585. [DOI] [PubMed] [Google Scholar]
- 68.Varfolomeev, E. E., and A. Ashkenazi. 2004. Tumor necrosis factor: an apoptosis JuNKie? Cell 116:491-497. [DOI] [PubMed] [Google Scholar]
- 69.Varghese, J., S. Chattopadhaya, and A. Sarin. 2001. Inhibition of p38 kinase reveals a TNF-α-mediated, caspase-dependent, apoptotic death pathway in a human myelomonocyte cell line. J. Immunol. 166:6570-6577. [DOI] [PubMed] [Google Scholar]
- 70.Yin, Y., P. D. Allen, L. Jia, M. G. MacEy, S. M. Kelsey, and A. C. Newland. 2000. Constitutive levels of cAMP-dependent protein kinase activity determine sensitivity of human multidrug-resistant leukaemic cell lines to growth inhibition and apoptosis by forskolin and tumour necrosis factor alpha. Br. J. Haematol. 108:565-573. [DOI] [PubMed] [Google Scholar]
- 71.Yin, Y., P. D. Allen, L. Jia, S. M. Kelsey, and A. C. Newland. 2001. 8-Cl-adenosine mediated cytotoxicity and sensitization of T-lymphoblastic leukemia cells to TNFα-induced apoptosis is via inactivation of NF-κB. Leuk. Res. 25:423-431. [DOI] [PubMed] [Google Scholar]
- 72.Yu, C., Y. Minemoto, J. Zhang, J. Liu, F. Tang, T. N. Bui, J. Xiang, and A. Lin. 2004. JNK suppresses apoptosis via phosphorylation of the proapoptotic Bcl-2 family protein BAD. Mol. Cell 13:329-340. [DOI] [PubMed] [Google Scholar]
- 73.Zanke, B. R., E. A. Rubie, E. Winnet, J. Chan, S. Randall, M. Parsons, K. Boudreau, M. McInns, M. Yan, D. J. Templeton, and J. R. Woodgett. 1996. Mammalian mitogen-activated protein kinase pathways are regulated through formation of specific kinase-activator complexes. J. Biol. Chem. 271:29876-29881. [DOI] [PubMed] [Google Scholar]
- 74.Zarubin, T., and J. Han. 2005. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 15:11-18. [DOI] [PubMed] [Google Scholar]
- 75.Zhang, J., J. Liu, C. Yu, and A. Lin. 2005. BAD Ser128 is not phosphorylated by c-Jun NH2-terminal kinase for promoting apoptosis. Cancer Res. 65:8372-8378. [DOI] [PubMed] [Google Scholar]
- 76.Zhang, X., D. T. Odom, S. H. Koo, M. D. Conkright, G. Canettieri, J. Best, H. Chen, R. Jenner, E. Herbolsheimer, E. Jacobsen, S. Kadam, J. R. Ecker, B. Emerson, J. B. Hogenesch, T. Unterman, R. A. Young, and M. Montminy. 2005. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc. Natl. Acad. Sci. USA 102:4459-4464. [DOI] [PMC free article] [PubMed] [Google Scholar]