Abstract
Quorum-sensing systems that have been widely identified in bacteria play important roles in the regulation of bacterial multicellular behavior by which bacteria sense population density to control various biological functions, including virulence. One characteristic of the luxIR quorum-sensing genes is their diverse and discontinuous distribution among proteobacteria. Here we report that the spnIR quorum-sensing system identified in the enterobacterium Serratia marcescens strain SS-1 is carried in a transposon, TnTIR, which has common characteristics of Tn3 family transposons and is mobile between chromosomes and plasmids of different enterobacterial hosts. SpnIR functions in the new host and was shown to negatively regulate the TnTIR transposition frequency. This finding may help reveal the horizontal transfer and evolutionary mechanism of quorum-sensing genes and alter the way that we perceive regulation of bacterial multicellular behavior.
Bacteria use chemical signals to coordinate their population cell density-dependent behavior, which is called quorum sensing (8). Quorum sensing is known to be a common mechanism for regulation of a diverse range of bacterial physiological processes, including virulence factor expression, secondary metabolite production, symbiosis, biofilm formation, and conjugal plasmid transfer, as well as individual survival strategies, such as induction of stationary-phase responses and initiation of colonial surface migration (9, 37). There are two general types of bacterial quorum-sensing systems: gram-negative LuxIR circuits and gram-positive oligopeptide two-component circuits (32). A LuxIR-type quorum-sensing system was initially discovered in Vibrio fischeri and then widely identified in proteobacteria (3). The LuxI-type proteins catalyze the formation of acyl-homoserine lactone (AHL) as a signal by ligating a specific acyl moiety from an acyl-acyl carrier protein to the homocysteine moiety of S-adenosylmethionine (24, 29). The LuxR-type proteins bind their cognate AHL signals and control transcription of target genes. Currently, over 70 LuxIR quorum-sensing systems have been identified (37), and one characteristic of the genes is their discontinuous distribution (22). A comprehensive phylogenetic comparison of LuxI-LuxR family members highlighted the possibility of lateral gene transfer of quorum-sensing systems (3, 20).
In Serratia, a number of different LuxIR-type systems have been described (35), including SwrIR (Serratia liquefaciens MG1) (7), SmaIR (Serratia sp. strain ATCC 39006) (33), SprIR (Serratia proteamaculans) (5), and SpnIR (Serratia marcescens SS-1) (15). The SpnIR quorum-sensing system regulates flagellum-independent population surface migration (sliding) and synthesis of biosurfactant, prodigiosin, and nuclease in S. marcescens SS-1 (15). SpnI synthesizes at least four N-acyl-homoserine lactones, which were identified using high-resolution mass spectrometry and chemical synthesis as N-3-oxohexanoyl- homoserine lactone (3-oxo-C6-HSL), N-hexanoyl-homoserine lactone, N-heptanoyl-homoserine lactone, and N-octanoyl-homoserine lactone. In contrast to most other LuxR homologues, SpnR acts as a negative regulator and is derepressed by 3-oxo-C6-HSL. A gene designated spnT, which is located upstream of spnI, was recently characterized (Wei and Lai, unpublished data). Overexpression of spnT results in inhibition of cell division, chromosomal DNA segregation, biosurfactant production, and the ability to slide independent of spnIR in S. marcescens (Wei and Lai, unpublished). In proximity to the spnTIR genes, we previously identified several transposon remnants and a potential tnpR resolvase gene flanking the spnTIR locus (Fig. 1). While examining the DNA composition around the spnTIR region, we found that the G+C content of spnTIR (38.6%) is obviously different from that of the whole genome of S. marcescens (59.51%) (http://www.sanger.ac.uk/Projects/S_marcescens/). These observations strongly suggested that spnIR quorum-sensing genes in S. marcescens SS-1 might be located in a mobile DNA region and might have been transferred from another organism.
FIG. 1.
Restriction and physical map of the 13-kb spnTIR locus in S. marcescens SS-1. NI, NsiI; EI, EcoRI; EV, EcoRV; BI, BamHI; KI, KpnI; SI, SmaI; PI, PstI. The dashed arrow indicates the disrupted transposase gene. The gray box represents the DNA fragment identified in a previous study (15). The double-headed arrow indicates the region of TnTIR. Scale bar, 1 kb.
In this report, we confirm that the spnIR quorum-sensing genes are carried by a Tn3 family transposon, TnTIR. TnTIR was shown to be mobile between plasmids and chromosomes in S. marcescens and Escherichia coli hosts. Further studies showed that SpnR negatively regulates the transposition frequency of TnTIR. As far as we can tell, this is the first report which provides direct evidence of a luxIR-type quorum-sensing system that is located in a mobile transposon and is involved in regulation of the transposition frequency.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are described in Table 1. E. coli was cultured at 37°C, and S. marcescens and Chromobacterium violaceum CV026 (21) were grown at 30°C in Luria-Bertani (LB) (27) medium. A swarming assay was performed on LB medium solidified with 0.8% Eiken agar (Eiken) by inoculating 5 μl of an overnight broth culture onto the center of an agar plate (18). The CV026 assay was performed as previously described (21) by T-streaking a test strain(s) against C. violaceum CV026 on LB agar plates, followed by observation of purple pigment production after overnight incubation.
TABLE 1.
Strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Relevant characteristics | Reference or source |
|---|---|---|
| Serratia marcescens strains | ||
| CH-1 | Clinical isolate; does not contain spnTIR; swarming positive with flagellum | 18 |
| SS-1 | Environmental isolate; contains spnTIR; swarming negative without flagellum; sliding positive | 31 |
| SS-1ΔR | SS-1 spnR::Smr | 15 |
| Jun-1 | Isolated from repeated subcultures of SS-1; two TnTIR copies are in the chromosome | This study |
| Escherichia coli strains | ||
| K-12 MG1655 | Wild type | American Type Culture Collection (ATCC 47076) |
| CC118 | λ-pir lysogen [Δ(ara-leu) araD ΔlacX74 galE galK phoA20 thi-1 rpsE rpoB argE(Am) recA1]; permissive host for suicide plasmids requiring Pir protein | 14 |
| S17-1 | λ-pir lysogen [thi pro hsdR hsdM+recA RP4 2-Tc::Mu-Km::Tn7 (Tpr Smr)]; permissive host able to transfer suicide plasmid pDM4 to recipient cells via conjugation | 14 |
| Chromobacterium violaceum CV026 | Double mini-Tn5 mutant derived from C. violaceum; AHL biosensor Hgr; cvil::Tn5xyIE; Kanr; spontaneous Smr; non-AHL producer; nonpigmented unless exogenous AHL provided | 21 |
| Plasmids | ||
| pBAD18-Kan | pBAD18, arabinose regulation; Kanr | 13 |
| pDM4-230 | Derived from pDM4(23); contains sacBR genes, Cmr gene, and a 230-bp random sequence; requires Pir protein for replication | This study |
| pUT-Sm | Suicide plasmid containing mini-Tn5 (Smr); requires Pir protein for replication | 6 |
| pKOT101 | Derived from pUT; contains partial spnT coding region; Smr Kanr | This study |
| pKOT102 | Derived from pUT; contains araC and partial spnT coding region; PBAD promoter; Smr | This study |
| pKOT103 | Derived from pUT; contains araC and partial spnT coding region; PBAD promoter; Smr | This study |
| pJR203 | pBAD18-Kan::spnR | This study |
| Primers | ||
| TnP1 | 5′-GGGGCATCCGCTTCGCTTTCAGCAGCGTGAATGA-3′ | This study |
| TnP2 | 5′-GGGGTAATGGGAGCGAAGCTACAATATGACGACA-3′ | This study |
| XSF | 5′-GCCCACGCGCTCCCCGA-3′ | This study |
| TII | 5′-AGCGAGTGCATCATCGG-3′ | This study |
| XgeF | 5′-CCCGGTGTTTCATACAGCCAGCCC-3′ | This study |
| PsiI | 5′-GCATGCCATCGTGTATGGTCAGGGGC-3′ | This study |
| pBADF | 5′-CAAACCCTATGCTACTCCGTCAA-3′ | This study |
| pBADR | 5′-AATTCGCTAGCCCAAAAAAACG-3′ | This study |
| B6 | 5′-CTGCTGACCGGGCCTTCCACT-3′ | This study |
| FR-1 | 5′-CGGCCCTTGTCGGTCATAC-3′ | This study |
Recombinant DNA techniques.
Unless indicated otherwise, standard protocols were used for isolation of plasmid and chromosomal DNA, transformation, electroporation, DNA manipulation (27), and conjugation (6). Southern blot hybridization analysis was performed using the DIG labeling and detection system according to the recommendations of the manufacturer (Roche). DNA sequencing and analysis were performed using a Perkin-Elmer model 377 autosequencer with a Taq DyeDeoxy terminator cycle sequencing kit (Applied Biosystems).
Construction of plasmids pJR203, pJR205, and pYT100.
To construct pJR203, the spnR gene was EcoRI/KpnI digested from pSC200 (15) before insertion into the same restriction site in pBAD18-Kan (13) under PBAD promoter control. To construct pJR205, the spnR gene and its native promoter were PstI digested from pYT100 and then inserted into the same restriction site in pACYC177 (New England Biolabs). pYT100 was a pZero (Invitrogen)-based plasmid that contained a 4-kb NsiI-digested chromosomal DNA fragment which contained complete tnpR and the spnTIR region.
Elimination of tnpA from TnTIR.
Recombinant plasmid pDM4-Tn8 was digested with XhoI, after which three fragments (14 kb, 2.3 kb, and 1.4 kb) were obtained. Ligation of the 14-kb and 2.3-kb fragments with the correct DNA direction generated plasmid pDM4-Tn8dA, a pDM4-based plasmid containing TnTIR::Sm with truncated tnpA.
Transposition assay.
The transposition assay was modified from assays described previously (26). Plasmid pBAD18-Kan, pDM4-230, pACYC177, or pJR205, used as the transposition target for TnTIR::Sm, was transformed into bacterial strains which contained TnTIR::Sm in the chromosome or in a recombinant plasmid. Purified plasmids were transformed into the recipient strains, and this was followed by spreading onto LB agar plates containing appropriate antibiotics. Confirmation of AHL synthesis was obtained by a CV026 assay (21). Plasmids expected to carry TnTIR::Sm were confirmed by restriction enzyme digestion and sequencing with primers B6 and FR-1 (Table 1) designed in TnTIR. The transposition efficiency was determined by dividing the number of streptomycin-resistant colonies by the number of kanamycin-resistant colonies. For conjugational transfer of TnTIR::Sm into the chromosome of E. coli K-12 strain MG1655 or S. marcescens CH-1, ori-R6K-based plasmid pDM4-Tn8, which could not replicate without the Pir protein, was used. Chromosomal insertion was confirmed by Southern blot hybridization.
RESULTS
Duplication of spnTIR region.
The physical map of the spnTIR region in S. marcescens SS-1 is shown in Fig. 1. While spnT is cotranscribed with spnI and functions as a negative regulator of spnR upon sliding and pigment production (15), no known functional domain was identified in SpnT. We therefore tried to explore the underlying mechanism of spnT function. Three different strategies were used to construct a spnT-depleted mutant (data available upon request). Recombinant plasmids constructed in order to knock out spnT were transferred into S. marcescens SS-1 by conjugation, and this was followed by selection of transconjugants with proper antibiotic resistance phenotypes. We then characterized the genotypes of these potential mutants by restriction enzyme digestion and Southern blot hybridization using spnT as the probe. Besides the expected bands from mutated spnT, extra bands were always observed (data not shown). Using tnpR as an alternative probe, a second copy of the wild-type-size spnTIR region was also found in all mutant strains (Fig. 2A). Furthermore, by daily repeated subculture of S. marcescens SS-1 on LB agar plates at 30°C for more than 10 days, we also isolated an S. marcescens strain designated Jun-1 (Table 1) containing two copies of the spnTIR locus (see below). Taken together, these results strongly suggested that the spnTIR locus has the ability to naturally duplicate itself.
FIG. 2.
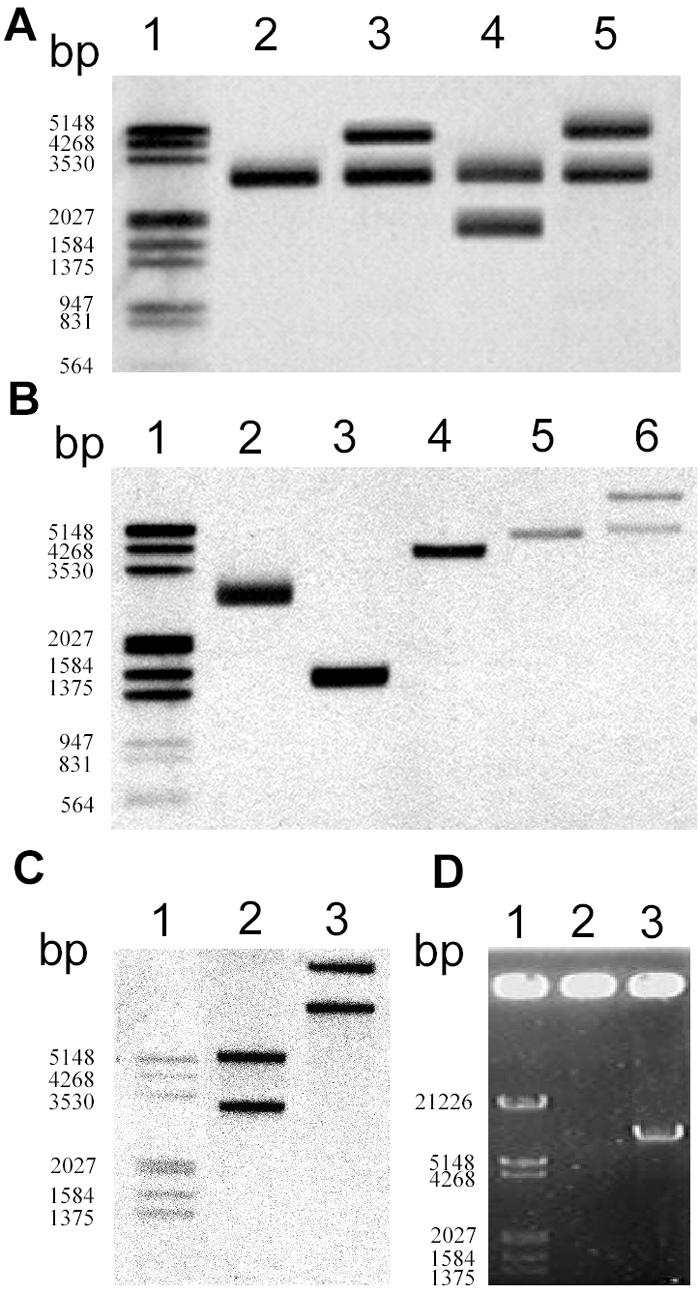
spnTIR region is mobile in S. marcescens SS-1. (A) Attempt to knock out spnT in SS-1 that led to the discovery of the duplication of the spnTIR region. Southern blot hybridization using tnpR as the probe after PstI digestion was performed to determine the restriction patterns of the chromosomal spnTIR region in mutants with potential spnT deletions. Lane 2, SS-1; lane 3, SS-1 KOT101; lane 4, SS-1 KOT102; lane 5, SS-1 KOT103. (B and C) Duplication of spnTIR in S. marcescens Jun-1. Chromosomal DNA of S. marcescens Jun-1 was digested by different restriction enzymes and probed with tnpR (B) or spnR (C). (B) Lane 2, PstI; lane 3, EcoRI; lane 4, NsiI; lane 5, EcoRV; lane 6, BamHI. (C) Lane 2, EcoRV; lane 3, BamHI. (D) Amplification of the potential transposon by single-primer PCR using S. marcescens SS-1 chromosomal DNA as the template and either TnP1 (lane 2) or TnP2 (lane 3) as the primer. Lane 1 contained a DNA marker. The numbers on the left indicate the sizes of markers.
Defining the region of the mobile spnTIR unit.
To examine the potential complete mobile spnTIR DNA fragment in S. marcescens Jun-1, a series of Southern blot hybridization analyses combined with different restriction enzyme digestions was first performed. Based on the supposition that there is a defined duplicated DNA region, the results of the Southern blot hybridization using optimal probes were expected to show either one band (double cuts in the duplicated region by the restriction enzyme) or at least two bands (single cut in the duplicated region by the restriction enzyme). Since the results shown in Fig. 1 and 2A demonstrated that the replicated region should contain at least tnpR, spnT, spnI, and spnR, we used tnpR and spnR as probes in order to try to determine the 5′ and 3′ ends of the mobile DNA unit, respectively. Using tnpR as the probe, two bands for sequences that were about 5 kb long and longer were identified from BamHI digestion but not from EcoRV digestion (Fig. 2B, lanes 5 and 6). Thus, the 5′ end of the mobile spnTIR region was determined to be between BamHI-EcoRV sites, about 3.9 to 4.1 kb upstream of spnT. Using spnR as the probe, two bands were observed after either BamHI or EcoRV digestion (Fig. 2C). In contrast, NsiI digestion resulted in only a single band (Fig. 2B, lane 4), indicating that NsiI cut within the mobile spnTIR unit. Thus, the 3′ end of the mobile spnTIR region was determined to be between NsiI-EcoRV sites, about 390 to 1,340 bp upstream of spnR. Southern blot analyses using other restriction enzymes and DNA fragments (such as spnT or spnI) as probes further confirmed the region of this potential mobile unit (data not shown). As Tn3 family transposons duplicate during transposition, we suggest that the mobile spnTIR unit might be a Tn3 family transposon.
Identification and phylogenetic analysis of the TnTIR transposon.
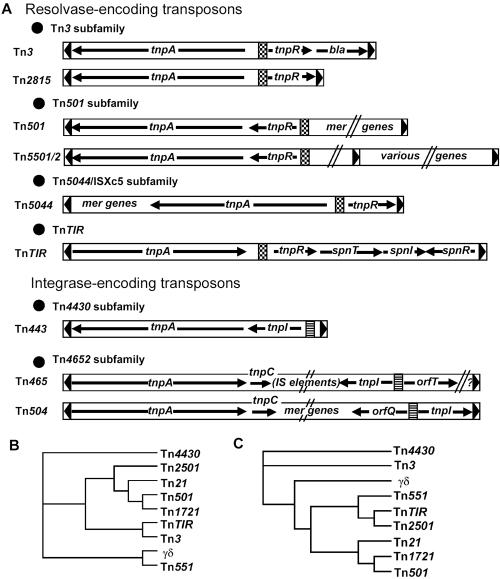
The “single-primer PCR” technique, which uses only one primer designed from the conserved inverted repeat DNA sequences, has been successfully used previously to amplify potential transposons (16). Since Tn3 family transposons bear a 38-bp inverted repeat with the conserved external bases 5′-GGGG-3′ at the ends (12), we tried to clone the potential transposon based on this characteristic. Two 5′-GGGG-3′ sequences were identified in the 840-bp NsiI-EcoRV DNA sequence, from which two individual primers, TnP1 and TnP2 (Table 1), were designed. A single PCR product (about 7 kb) (Fig. 2D) was subsequently amplified with TnP2. Further cloning and sequence analysis of the amplified DNA fragment revealed a complete 7,212-bp transposon in which a transposase gene (tnpA) transcribed in the same direction as tnpR was identified. Thus, the potential Tn3 family transposon comprised five genes, including (5′ to 3′) the typical transposase gene tnpA, the Ser-type resolvase gene tnpR flanked by a res site (12), and spnTIR (Fig. 1). We designated this transposon TnTIR. The genetic organization of TnTIR was obviously unique compared with other Tn3 family transposon subgroups (12) (Fig. 3A), indicating that TnTIR did not belong to previously described subgroups. Phylogenetic analyses of TnTIR by comparison of either TnpA or TnpR with other members of Tn3 family transposons (Fig. 3B and C) revealed different phylogenetic relationships. The TnpA of TnTIR is similar to that of Tn3, while the TnpR is most similar to the TnpR of Tn2501. These data supported the hypothesis that extensive recombination had occurred between different transposon genes in natural environments (12).
FIG. 3.
Analyses of TnTIR. (A) Comparison of genetic organizations of TnTIR and other Tn3 family transposons. Modified from reference 12. The cross-hatched boxes are res sites; the striped boxes are TnpI recombination sites. (B and C) Phylogenetic analyses of TnpA (B) and TnpR (C) in Tn3 family transposons were performed with BioEdit and were viewed with TreeView.
TnTIR transposition.
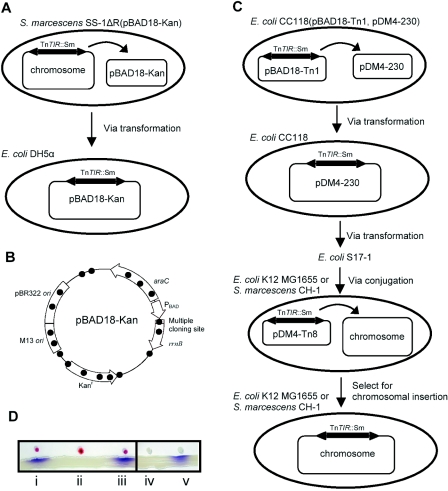
To verify the transposition capability of TnTIR, we used plasmid pBAD18-Kan as a transposition target (Fig. 4A). For ease of screening, pBAD18-Kan was transformed into S. marcescens SS-1ΔR (15), in which the spnR in TnTIR was inserted with a streptomycin-resistant gene cassette (Sm) used as a transposition marker. Plasmids purified from S. marcescens SS-1ΔR(pBAD18-Kan) were subsequently transformed into E. coli DH5α, and this was followed by screening for streptomycin-resistant but not kanamycin-resistant transformants (Fig. 4A). A total of 17 colonies were isolated from about 50,000 transformants; the transposition frequency was about 3.4 × 10−4, which is similar to that of other Tn3 family transposons (2). Restriction analysis showed that the size of the recombinant plasmids was about 14 kb, which equaled the length of the original pBAD18-Kan plasmid plus the length of the TnTIR transposon containing Sm (data not shown). Further DNA sequencing analyses confirmed that there was transposition of TnTIR::Sm into various sites of pBAD18-Kan, producing 5-bp AT-rich direct repeat target sequences, suggesting that random transpositions had occurred (Fig. 4B and Table 2). Among the 17 colonies examined, one clone did not exhibit direct repeat target sequences; the sequence at the left end was 5′-AAGCT-3′, and the sequence at the right end was 5′-CTGAT-3′. This might have resulted from intramolecular transposition (19).
FIG. 4.
Transposition of TnTIR::Sm and production of AHL signals. (A) Strategy used to identify jumping of TnTIR::Sm from the S. marcescens SS-1ΔR chromosome to plasmid pBAD18-Kan. (B) Insertion sites (solid circles) of TnTIR::Sm that jumped into pBAD18-Kan. (C) Strategy used to identify transposition of TnTIR::Sm between pBAD18-Kan (pBAD18-Tn1) and pDM4-230 and from pDM4-230 (pDM4-Tn8) to the chromosome of E. coli K-12 strain MG1655 or S. marcescens CH-1. (D) Confirmation of AHL production by the CV026 assay. Lane i, S. marcescens SS-1; lane ii, S. marcescens CH-1; lane iii, S. marcescens CH-1(TnTIR::Sm); lane iv, E. coli K-12 strain MG1655; lane v, E. coli K-12 strain MG1655(TnTIR::Sm).
TABLE 2.
TnTIR transposition sites and sequences in target plasmids pBAD18-Kan and pDM4
| Plasmid | Region | Sequence (5′-3′) |
|---|---|---|
| pBAD18-Tn1 | araC | GAATA |
| pBAD18-Tn2 | araC | TATTT |
| pBAD18-Tn3 | araC | AAATA |
| pBAD18-Tn4 | rrnB | GAGTA |
| pBAD18-Tn5 | M13 origin | TTATA |
| pBAD18-Tn6 | M13 origin | TTAAT |
| pBAD18-Tn7 | M13 origin | GATTT |
| pBAD18-Tn8 | M13 origin | AGCTT |
| pBAD18-Tn9 | pBR322 origin | ATCTA |
| pBAD18-Tn10 | pBR322 origin | AAGCT |
| pBAD18-Tn11 | PBAD promoter | GGTAT |
| pBAD18-Tn12 | Kanr | TTTTA |
| pBAD18-Tn13 | Kanr | TGATA |
| pBAD18-Tn14 | Kanr | AACAC |
| pBAD18-Tn15 | Kanr | ATGAG |
| pBAD18-Tn16 | Kanr | TATTA |
| pBAD18-Tn17(L) | Multiple cloning site | CTGAT |
| pBAD18-Tn17(R) | rrnB | AAGCT |
| pDM4-Tn1 | RP4 mob region | TATAT |
| pDM4-Tn2 | RP4 mob region | GACCA |
| pDM4-Tn3 | RP4 mob region | AAGCA |
| pDM4-Tn4 | RP4 mob region | TATAA |
| pDM4-Tn5 | RP4 mob region | AAATA |
| pDM4-Tn6 | RP4 mob region | TTATT |
| pDM4-Tn7 | RP4 mob region | TGTAT |
| pDM4-Tn8 | Inserted sequence | TGCAA |
To determine whether TnTIR could jump between plasmids, plasmid pDM4-230 [chloramphenicol resistant; pDM4 (23) containing a 230-bp random DNA fragment inserted into the multiple cloning site] was used as the transposition target for TnTIR::Sm (Fig. 4C). E. coli CC118(pDM4-230) was transformed with pBAD-Tn1, in which TnTIR::Sm was inserted into the araC region (Fig. 4B and Table 2). Plasmids that were purified were transformed into E. coli CC118 for screening colonies which were streptomycin and chloramphenicol resistant but kanamycin sensitive. A total of eight colonies were selected, and their abilities to synthesize AHL were confirmed by the CV026 assay (21) before plasmid purification for sequence analysis. Seven colonies were subsequently found to contain TnTIR::Sm inserted into the mob region, and the other colony (designated pDM4-Tn8) contained TnTIR::Sm in the 230-bp inserted fragment. None of the colonies was found to contain TnTIR::Sm inserted into the sacBR region. Briefly, these results showed that TnTIR::Sm could jump between plasmids.
As lateral gene transfer is now recognized as a major force in genome evolution (3), we asked whether TnTIR can jump from a plasmid to the chromosome of another LuxIR-negative bacterium, such as E. coli K-12 strain MG1655 or S. marcescens CH-1 (18). TnTIR::Sm was transferred into these bacterial host strains through conjugation with pDM4-Tn8, which cannot replicate without the Pir protein (14). Several colonies in which TnTIR::Sm was inserted into the chromosome of each bacterial strain were obtained. These colonies were confirmed by Southern blot analysis (data not shown) and the CV026 assay (Fig. 4D). Thus, TnTIR was proven to be able to transpose from a plasmid to the chromosome of another bacterium and produce AHL normally. We previously had been unable to successfully transfer TnTIR::Sm into some gram-positive bacteria, such as Staphylococcus spp. and Streptococcus spp., suggesting that AHL might be toxic to these organisms (17).
Elimination of tnpA inactivates TnTIR transposition.
It has been reported previously that the TnpA transposase activity determines transposition of Tn3 family transposons (4). In this situation, eliminating the tnpA gene from TnTIR should inactivate TnTIR transposition. The 1,383-bp internal XhoI tnpA fragment was deleted from tnpA in pDM4-Tn8, and this was followed by self-ligation to generate pDM4-Tn8dA, a pDM4-based plasmid containing TnTIR::Sm with a truncated tnpA gene. Using pACYC177 (25) as the transposition target, the efficiencies of transposition for pDM4-Tn8 and pDM4-Tn8dA were then compared. pDM4-Tn8 had a transposition efficiency of about 2.27 × 10−4, whereas the transposition efficiency of pDM4-Tn8dA was less than 10−7. The results indicated that TnpA is required for transposition of TnTIR.
SpnR negatively regulates TnTIR transposition.
Whether the SpnIR quorum-sensing system was involved in regulation of the TnTIR transposition was also examined. Given that expression of the tnpA transposase gene is regulated by TnpR in Tn3 transposons (4), the level of expression of tnpR or tnpA might be regulated by SpnR, leading to up- or down-regulation of the TnTIR transposition frequency. Genes controlled by LuxR homologues usually contain a conserved lux box in their promoter regions (30). However, no potential SpnR-recognized lux box sequences were identified in the promoter regions of tnpA or tnpR, although a lux box is present in the promoter region of spnR, whose expression is autoregulated (15).
To determine the effect of SpnR on the transposition frequency of TnTIR, plasmid pACYC177 or pJR205 (pACYC177 contained spnR under control of its native promoter) was used as the transposition target. These two plasmids were separately transformed into E. coli CC118(pDM4-Tn8), and this was followed by purification for the transposition assay. The transposition frequency for TnTIR::Sm jumping into plasmid pACYC177 was calculated to be 2.27 × 10−4, compared with 2.26 × 10−5 for jumping into plasmid pJR205, which was about 10-fold lower. These results suggested that although no putative lux box was identified in promoters of tnpA or tnpR, SpnR negatively affects the transposition frequency of TnTIR.
SpnIR functions in LuxIR-negative S. marcescens strain CH-1.
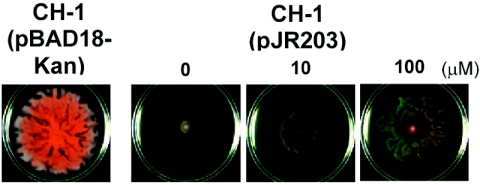
To determine whether SpnIR functions normally after transposition into other luxIR bacterial strains, we evaluated the effect of SpnIR in S. marcescens strain CH-1 (18, 36). Our previous report showed that overexpression of spnR in S. marcescens SS-1 leads to inhibition of sliding and production of nuclease, biosurfactant, and prodigiosin pigment. These effects were alleviated by 3-oxo-C6-HSL, one of the signals synthesized by SpnI (15). The same assays were used to examine the potential function of SpnIR in S. marcescens CH-1. Overexpression of spnR in S. marcescens CH-1 was achieved by transforming CH-1 with plasmid pJR203 (pBAD18-Kan-based plasmid containing spnR under tight control of the PBAD promoter). In contrast to S. marcescens CH-1(pBAD18-Kan), swarming and pigment synthesis by S. marcescens CH-1(pJR203) were completely inhibited at 30°C with 0.2% arabinose (Fig. 5). This effect was alleviated by 3-oxo-C6-HSL in a dose-dependent manner (Fig. 5). Thus, while S. marcescens CH-1 is LuxIR negative, an exogenous SpnIR quorum-sensing system functions in this new host strain.
FIG. 5.
Swarming and pigment synthesis by the LuxIR-negative strain S. marcescens CH-1 are regulated by an SpnIR quorum-sensing system. Recombinant plasmid pJR203 was transformed into S. marcescens CH-1, and this was followed by a swarming assay performed at 30°C on a 0.2% arabinose swarming plate. 3-Oxo-C6 HSL, one of the AHL signals synthesized by SpnI, was added to the swarming plates at a concentration of either 10 or 100 μM to evaluate its effect on S. marcescens CH-1(pJR203) swarming.
DISCUSSION
The luxIR quorum-sensing genes are characterized by their discontinuous and diverse distribution among gram-negative bacteria, and to our knowledge, all of the luxIR quorum-sensing genes have been identified in proteobacteria (3). Comparatively, while the luxR- and luxI-type genes track rRNA gene phylogenies fairly well, considerable strain-dependent variation has also been noted (22). Although at least four luxIR quorum-sensing gene pairs have been identified in Serratia spp., many S. marcescens strains do not contain the luxIR-type quorum-sensing genes. For example, besides the clinical isolate S. marcescens CH-1 from Cambridge, United Kingdom, two-thirds of the 200 S. marcescens clinical isolates from National Taiwan University Hospital were also classified as luxIR negative based on detection results from PCR, CV026, and luminescent bacterial bioreporter assays (31) (Soo and Lai, unpublished data). While as far as we know this is the first evidence which directly shows that luxIR-type quorum-sensing genes are located in a mobile transposon, there have been other reports which indicated that quorum-sensing genes are colocalized with potentially mobile DNA fragments. In Serratia sp. strain ATCC 39006, the smaIR quorum-sensing genes are also flanked by transposon remnants (33). If a mobile quorum-sensing unit is also conserved in other gram-negative bacteria, then it would be interesting to see where and how the quorum-sensing genes were derived during evolution. As more genome sequencing data are accumulated, more quorum-sensing genes are expected to be identified in different bacterial host strains. Based on our experimental results for construction of spnIR-positive E. coli strains in conjugation studies, it might not be surprising if a luxIR-type quorum-sensing-positive E. coli or Salmonella sp. is isolated in the near future. Our findings thus strongly suggest that like transfer of antibiotic resistance genes, lateral gene transfer may play an important role in the transfer of quorum-sensing units between different bacterial genera and species.
Although the luxIR-type systems are distributed unevenly, our current knowledge suggests that multicellularity under regulation of quorum-sensing genes is advantageous for bacterial cells, at least in terms of coordinated regulation of virulence gene expression for survival in adverse environments (8). Thus, the quorum-sensing-positive bacteria are more efficient in cell-to-cell communication and subsequently have a better chance of survival in competition with other organisms. Besides the LuxIR type, other quorum-sensing regulatory systems, either identified or unidentified, may also be involved in coordinating bacterial cell density-dependent behavior, forming a complex regulatory network.
The finding that so far luxIR-type quorum-sensing genes are restricted to proteobacteria is intriguing. As TnTIR showed characteristics typical of Tn3 family transposons which are widely distributed among gram-positive and gram-negative bacteria, TnTIR was also expected to integrate into chromosomes of gram-positive bacteria. However, after repeated tests, we failed to transfer the TnTIR transposon into gram-positive bacteria, such as Staphylococcus spp. and Streptococcus spp. One of the possible reasons for this is toxicity to gram-positive bacteria caused by AHLs (17). Interestingly, none of the eight pDM4 plasmid derivatives into which TnTIR::Sm jumped contained TnTIR::Sm inserted into the sacBR gene derived from the gram-positive bacterium Bacillus subtilis (10). As the underlying mechanisms remain mostly undetermined, we cannot completely rule out the possibility of identifying luxIR-type genes from gram-positive bacteria.
The transposition frequency of a Tn3 family transposon is basically determined by the transposase TnpA, whose gene expression is regulated by the resolvase TnpR (12). Although no conserved lux box sequence was identified in the promoter region of tnpA or tnpR, evidence did suggest that LuxIR-type quorum-sensing systems are involved in regulation of downstream genes without conserved lux box sequences (11). Based on our experimental results, although the underlying molecular mechanisms remain to be determined, SpnR did have a negative effect on the transposition frequency of TnTIR. Besides TnTIR, there are other reports indicating that the quorum-sensing system regulates horizontal gene transfer (28) or the mobility of a mobile genetic element (1). Schaefer et al. provided evidence that long-chain acyl-homoserine lactone could regulate gene transfer in Rhodobacter capsulatus (28). In B. subtilis, Auchtung et al. showed that excision and transfer of ICEBs1 are regulated by a Phr peptide, the quorum-sensing signal encoded by the same transposon (1). These workers showed that RapI activates ICEBs1 gene expression, excision, and transfer and that the PhrI pentapeptides antagonize the activity of RapI. Global DNA damage (SOS) also activates ICEBs1 excision and transfer, independent of RapI and PhrI (1). Twiss et al. used a nice design to identify a large group of host factors which affect IS903 transposition (34). Since quorum sensing regulates abundant cellular metabolism in bacterial cells, SpnR may either directly repress the expression of tnpR or tnpA or alternatively regulate the expression of other genes which influence TnTIR transposition. As more data are accumulated, the effects and mechanisms of quorum-sensing systems on regulation of transposition frequency should be characterized further.
The spnT gene is another gene that has been identified in TnTIR. In fact, identification of TnTIR originated from depletion of spnT in S. marcescens SS-1 (Wei and Lai, unpublished). Although spnT overexpression had a pleiotropic effect on the physiology of S. marcescens SS-1, currently there is no evidence to support the hypothesis that SpnT is involved in the regulation of TnTIR transposition (Wei and Lai, unpublished). In conclusion, while antibiotic resistance genes are widely identified in transposons, including Tn3 family transposons (12), our findings highlight the potential of horizontal transfer of quorum-sensing genes among different hosts. The impact of mobile quorum-sensing genes on regulation of cell density-dependent physiological behavior in various bacterial species should be emphasized after identification of mobile TnTIR. This should help us understand more about the lateral gene transfer and evolution of quorum-sensing genes.
Acknowledgments
We thank Chieh-Chen Huang for providing “single-primer PCR” information.
This work was supported by the Taiwan National Science Council (grants NSC-92-2314-B-002-356, NSC-93-2314-B-002-281, and NSC-94-2320-b-002-078), the Technology Development Program for Academia, the Ministry of Economical Affairs (grant 91-EC-17-A-10-S1-0013), the Medical Research Council (UK), and a joint Royal Society UK/National Science Council of Taiwan project grant, which are gratefully acknowledged.
REFERENCES
- 1.Auchtung, J. M., C. A. Lee, R. E. Monson, A. P. Lehman, and A. D. Grossman. 2005. Regulation of a Bacillus subtilis mobile genetic element by intercellular signaling and the global DNA damage response. Proc. Natl. Acad. Sci. USA 102:12554-12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baum, J. A. 1994. Tn5401, a new class II transposable element from Bacillus thuringiensis. J. Bacteriol. 176:2835-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boucher, Y., C. J. Douady, R. T. Papke, D. A. Walsh, M. E. Boudreau, C. L. Nesbo, R. J. Case, and W. F. Doolittle. 2003. Lateral gene transfer and the origins of prokaryotic groups. Annu. Rev. Genet. 37:283-328. [DOI] [PubMed] [Google Scholar]
- 4.Casadaban, M. J., J. Chou, P. Lemaux, C. P. Tu, and S. N. Cohen. 1981. Tn3: transposition and control. Cold Spring Harbor Symp. Quant. Biol. 45:269-273. [DOI] [PubMed] [Google Scholar]
- 5.Christensen, A. B., K. Riedel, L. Eberl, L. R. Flodgaard, S. Molin, L. Gram, and M. Givskov. 2003. Quorum-sensing-directed protein expression in Serratia proteamaculans B5a. Microbiology 149:471-483. [DOI] [PubMed] [Google Scholar]
- 6.de Lorenzo, V., and K. N. Timmis. 1994. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 235:386-405. [DOI] [PubMed] [Google Scholar]
- 7.Eberl, L., M. K. Winson, C. Sternberg, G. S. Stewart, G. Christiansen, S. R. Chhabra, B. Bycroft, P. Williams, S. Molin, and M. Givskov. 1996. Involvement of N-acyl-l-hormoserine lactone autoinducers in controlling the multicellular behaviour of Serratia liquefaciens. Mol. Microbiol. 20:127-136. [DOI] [PubMed] [Google Scholar]
- 8.Fuqua, C., and E. P. Greenberg. 2002. Listening in on bacteria: acyl-homoserine lactone signalling. Nat. Rev. Mol. Cell Biol. 3:685-695. [DOI] [PubMed] [Google Scholar]
- 9.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35:439-468. [DOI] [PubMed] [Google Scholar]
- 10.Gay, P., D. Le Coq, M. Steinmetz, T. Berkelman, and C. I. Kado. 1985. Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J. Bacteriol. 164:918-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez, J. E., and M. M. Marketon. 2003. Quorum sensing in nitrogen-fixing rhizobia. Microbiol. Mol. Biol. Rev. 67:574-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grindley, N. D. 2002. The movement of Tn3-like elements: transposition and cointegrate resolution, p. 272-302. In Mobile DNA II. ASM Press, Washington, D.C.
- 13.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horng, Y. T., S. C. Deng, M. Daykin, P. C. Soo, J. R. Wei, K. T. Luh, S. W. Ho, S. Swift, H. C. Lai, and P. Williams. 2002. The LuxR family protein SpnR functions as a negative regulator of N-acylhomoserine lactone-dependent quorum sensing in Serratia marcescens. Mol. Microbiol. 45:1655-1671. [DOI] [PubMed] [Google Scholar]
- 16.Karlyshev, A. V., M. J. Pallen, and B. W. Wren. 2000. Single-primer PCR procedure for rapid identification of transposon insertion sites. BioTechniques 28:1078, 1080, 1082. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann, G. F., R. Sartorio, S. H. Lee, C. J. Rogers, M. M. Meijler, J. A. Moss, B. Clapham, A. P. Brogan, T. J. Dickerson, and K. D. Janda. 2005. Revisiting quorum sensing: discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc. Natl. Acad. Sci. USA 102:309-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai, H. C., P. C. Soo, J. R. Wei, W. C. Yi, S. J. Liaw, Y. T. Horng, S. M. Lin, S. W. Ho, S. Swift, and P. Williams. 2005. The RssAB two-component signal transduction system in Serratia marcescens regulates swarming motility and cell envelope architecture in response to exogenous saturated fatty acids. J. Bacteriol. 187:3407-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lebrun, M., A. Audurier, and P. Cossart. 1994. Plasmid-borne cadmium resistance genes in Listeria monocytogenes are present on Tn5422, a novel transposon closely related to Tn917. J. Bacteriol. 176:3049-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lerat, E., and N. A. Moran. 2004. The evolutionary history of quorum-sensing systems in bacteria. Mol. Biol. Evol. 21:903-913. [DOI] [PubMed] [Google Scholar]
- 21.McClean, K. H., M. K. Winson, L. Fish, A. Taylor, S. R. Chhabra, M. Camara, M. Daykin, J. H. Lamb, S. Swift, B. W. Bycroft, G. S. Stewart, and P. Williams. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703-3711. [DOI] [PubMed] [Google Scholar]
- 22.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 23.Milton, D. L., R. O'Toole, P. Horstedt, and H. Wolf-Watz. 1996. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 178:1310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.More, M. I., L. D. Finger, J. L. Stryker, C. Fuqua, A. Eberhard, and S. C. Winans. 1996. Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science 272:1655-1658. [DOI] [PubMed] [Google Scholar]
- 25.Rose, R. E. 1988. The nucleotide sequence of pACYC177. Nucleic Acids Res. 16:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin, E. J., B. J. Akerley, V. N. Novik, D. J. Lampe, R. N. Husson, and J. J. Mekalanos. 1999. In vivo transposition of mariner-based elements in enteric bacteria and mycobacteria. Proc. Natl. Acad. Sci. USA 96:1645-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook, J., T. Maniatis, and E. F. Fritsch. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 28.Schaefer, A. L., T. A. Taylor, J. T. Beatty, and E. P. Greenberg. 2002. Long-chain acyl-homoserine lactone quorum-sensing regulation of Rhodobacter capsulatus gene transfer agent production. J. Bacteriol. 184:6515-6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaefer, A. L., D. L. Val, B. L. Hanzelka, J. E. Cronan, Jr., and E. P. Greenberg. 1996. Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc. Natl. Acad. Sci. USA 93:9505-9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens, A. M., K. M. Dolan, and E. P. Greenberg. 1994. Synergistic binding of the Vibrio fischeri LuxR transcriptional activator domain and RNA polymerase to the lux promoter region. Proc. Natl. Acad. Sci. USA 91:12619-12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swift, S., M. K. Winson, P. F. Chan, N. J. Bainton, M. Birdsall, P. J. Reeves, C. E. Rees, S. R. Chhabra, P. J. Hill, and J. P. Throup, and. 1993. A novel strategy for the isolation of luxI homologues: evidence for the widespread distribution of a LuxR:LuxI superfamily in enteric bacteria. Mol. Microbiol. 10:511-520. [DOI] [PubMed] [Google Scholar]
- 32.Taga, M. E., and B. L. Bassler. 2003. Chemical communication among bacteria. Proc. Natl. Acad. Sci. USA 100(Suppl. 2):14549-14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomson, N. R., M. A. Crow, S. J. McGowan, A. Cox, and G. P. Salmond. 2000. Biosynthesis of carbapenem antibiotic and prodigiosin pigment in Serratia is under quorum sensing control. Mol. Microbiol. 36:539-556. [DOI] [PubMed] [Google Scholar]
- 34.Twiss, E., A. M. Coros, N. P. Tavakoli, and K. M. Derbyshire. 2005. Transposition is modulated by a diverse set of host factors in Escherichia coli and is stimulated by nutritional stress. Mol. Microbiol. 57:1593-1607. [DOI] [PubMed] [Google Scholar]
- 35.Wei, J. R., and H. C. Lai. Quorum sensing regulation in Serratia. Int. J. Med. Microbiol., in press.
- 36.Wei, J. R., Y. H. Tsai, P. C. Soo, Y. T. Horng, S. C. Hsieh, S. W. Ho, and H. C. Lai. 2005. Biochemical characterization of RssA-RssB, a two-component signal transduction system regulating swarming behavior in Serratia marcescens. J. Bacteriol. 187:5683-5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Withers, H., S. Swift, and P. Williams. 2001. Quorum sensing as an integral component of gene regulatory networks in Gram-negative bacteria. Curr. Opin. Microbiol. 4:186-193. [DOI] [PubMed] [Google Scholar]