Abstract
Corynebacterium glutamicum recently has been shown to possess pyruvate:quinone oxidoreductase (PQO), catalyzing the oxidative decarboxylation of pyruvate to acetate and CO2 with a quinone as the electron acceptor. Here, we analyze the expression of the C. glutamicum pqo gene, investigate the relevance of the PQO enzyme for growth and amino acid production, and perform phylogenetic studies. Expression analyses revealed that transcription of pqo is initiated 45 bp upstream of the translational start site and that it is organized in an operon together with genes encoding a putative metal-activated pyridoxal enzyme and a putative activator protein. Inactivation of the chromosomal pqo gene led to the absence of PQO activity; however, growth and amino acid production were not affected under either condition tested. Introduction of plasmid-bound pqo into a pyruvate dehydrogenase complex-negative C. glutamicum strain partially relieved the growth phenotype of this mutant, indicating that high PQO activity can compensate for the function of the pyruvate dehydrogenase complex. To investigate the distribution of PQO enzymes in prokaryotes and to clarify the relationship between PQO, pyruvate oxidase (POX), and acetohydroxy acid synthase enzymes, we compiled and analyzed the phylogeny of respective proteins deposited in public databases. The analyses revealed a wide distribution of PQOs among prokaryotes, corroborated the hypothesis of a common ancestry of the three enzymes, and led us to propose that the POX enzymes of Lactobacillales were derived from a PQO.
Pyruvate:quinone oxidoreductase (PQO) (EC 1.2.2.2) catalyzes the oxidative decarboxylation of pyruvate to acetate and CO2 with a quinone as the physiological electron acceptor. The Escherichia coli PQO enzyme, also designated pyruvate oxidase (POX), is a nonessential, peripheral membrane protein consisting of four identical subunits, each containing tightly bound flavin adenine dinucleotide (FAD) and loosely bound thiamine pyrophosphate (TPP) and Mg2+ (4, 22, 37, 38, 75). This enzyme has been shown to be strongly activated by low concentrations of phospholipids and detergents (5, 8, 15, 16, 28), and the activation has been shown to be accompanied by conformational changes and alteration of various properties of the enzyme (11, 12, 74). Aside from extensive biochemical characterization of the E. coli PQO, the respective gene (poxB) and its expression have been studied in detail (7, 9, 25). Expression of the poxB gene was dependent on sigma factor RpoS and thus induced in the stationary growth phase (7). Accordingly, the authors speculated that the enzyme might be responsible for oxidative pyruvate decarboxylation during the transition between the exponential aerobic growth phase and the stationary growth phase when cultures become anaerobic. Further studies with poxB inactivation mutants and with poxB-overexpressing strains of E. coli indicated that PQO activity contributes to the aerobic growth efficiency and that a high PQO activity, together with acetyl-coenzyme A (CoA) synthetase, can compensate for the pyruvate dehydrogenase complex function (1, 25).
Very recently, we showed for the first time the presence of PQO activity in a prokaryotic organism apart from E. coli and described the isolation, purification, and biochemical analysis of the PQO enzyme from Corynebacterium glutamicum (59). This bacterium is an aerobic, “high-GC gram-positive” organism that grows on a variety of sugars and organic acids and is widely used in the industrial production of amino acids, particularly l-glutamate and l-lysine (35). Due to its importance for the distribution of the carbon flux within the metabolism and for the precursor supply for amino acid synthesis, the phosphoenolpyruvate-pyruvate node of this organism has been intensively studied (reviewed in reference 19), and much attention has been focused on pyruvate-converting enzymes, such as pyruvate carboxylase (34, 49, 50, 51), pyruvate dehydrogenase complex (14, 60, 66), and PQO (59). Like the E. coli enzyme, the C. glutamicum PQO is a homotetrameric flavoprotein containing TPP, and it is activated by Triton X-100, phosphatidylglycerol and dipalmitoyl-phosphatidylglycerol. In contrast, it does not reduce ferricyanide, which is routinely used for assaying the E. coli enzyme (1, 9, 75), and it probably uses a menaquinone instead of ubiquinone as the physiological electron acceptor (59). The C. glutamicum PQO activity was highest in cells grown on complex medium and about threefold lower when glucose was added to the complex medium or when the cells were grown on minimal medium containing different carbon sources, suggesting that the enzyme is regulated by the carbon source in the growth medium.
According to a high degree of identity (>43%) of deduced amino acid sequences in public databases, E. coli- and C. glutamicum-type PQO enzymes seem to be present in other bacteria, too (59). Another type of pyruvate oxidoreductase, also being a flavoprotein with tightly bound FAD, TPP, and a divalent metal ion (64), has been found in and characterized from lactic acid bacteria, such as Lactobacillus delbrueckii (27), Lactobacillus plantarum (40, 41, 63, 64, 70), Streptococcus pyogenes (67), and Streptococcus pneumoniae (48). This type of enzyme catalyzes in a phosphate- and oxygen-dependent reaction the formation of acetyl-phosphate, CO2, and hydrogen peroxide and thus represents a true POX (EC 1.2.3.3). Alignment studies with gene-deduced amino acid sequences revealed that the common and also the different biochemical properties of the PQO and POX enzymes are clearly reflected by communities and differences at the amino acid level (59). However, the evolutionary development of both enzyme types and their phylogenetic relationship so far remained unclear.
In the present communication we report on the sequence, chromosomal organization, and expression analysis of the C. glutamicum PQO gene (pqo), on its inactivation and overexpression, and on possible functions of the PQO enzyme for growth and amino acid production. Furthermore, we investigated the distribution of PQO and POX enzymes in prokaryotes and analyzed the phylogeny of these proteins together with acetohydroxy acid synthase (AHAS), which catalyzes early steps in the synthesis of branched-chain amino acids and previously has been proposed to be evolutionary and functionally related to the E. coli PQO enzyme (10) and to L. plantarum POX (13).
MATERIALS AND METHODS
Bacteria, plasmids, oligonucleotides, and culture conditions.
All bacterial strains and plasmids and their relevant characteristics and sources are given in Table 1. The oligonucleotides used and their sequences are also listed in Table 1. The minimal medium used for C. glutamicum was described previously (20) and contained 1% (wt/vol) acetate, lactate, pyruvate, ribose, maltose, or glucose or 2% (wt/vol) glucose as carbon and energy source. For growth of C. glutamicum MH20-22B and its derivatives, 2 mM leucine was added to the medium. TY medium (57) was used as a complex medium for C. glutamicum and for E. coli. When appropriate, kanamycin (50 μg ml−1) was added to the medium. If not stated otherwise, C. glutamicum was grown aerobically at 30°C and E. coli at 37°C as 60 ml-cultures in 500-ml baffled Erlenmeyer flasks on a rotary shaker at 120 rpm. In glutamate fermentation experiments, Tween 60 (Sigma-Aldrich), prewarmed to 50°C and at a concentration of 25 mg/ml, was added to the culture medium 4 h after inoculation.
TABLE 1.
Strains, plasmids and oligonucleotides used in this study
| Strain, plasmid or oligonucleotide | Relevant characteristic(s) or sequence | Source, reference, or purpose |
|---|---|---|
| E. coli DH5α | supE44 hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 29 |
| C. glutamicum | ||
| WT | Wild-type strain ATCC 13032 | American Type Culture Collection |
| WT Δpqo | pqo deletion mutant of WT strain | This work |
| WT Δpqo(pEKEx2) | pqo deletion mutant of WT strain carrying plasmid pEKEx2 | This work |
| WT Δpqo(pEKEx-pqo) | pqo deletion mutant carrying pqo on plasmid pEKEx2 | This work |
| WT ΔaceE | aceE deletion mutant | 60 |
| WT ΔaceEΔpqo (pEKEx2) | aceE and pqo deletion mutant of WT strain carrying plasmid pEKEx2 | This work |
| WT ΔaceEΔpqo (pEKEx-pqo) | aceE and pqo deletion mutant carrying pqo on plasmid pEKEx2 | This work |
| WT (pET-Ppqo) | WT strain carrying the pqo promoter in plasmid pET2 | This work |
| MH20-22B | Lysine-producing strain | 62 |
| MH20-22B(PEKEx-pqo) | strain MH20-22B carrying pqo on plasmid pEKEx2 | This work |
| MH20-22B Δpqo | pqo deletion mutant of strain MH20-22B | This work |
| Plasmids | ||
| pK19mobsacB | Kmr, mobilizable (oriT), oriV | 58 |
| pK19mutpox | pK19mobsacB carrying a pqo gene with internal 353-bp deletion | This work |
| pK19mutaceE | pK19mobsacB carrying a aceE gene with internal 2,078-bp deletion | 60 |
| pET2 | Promoter probe vector carrying the promoterless cat gene, Kmr | 72 |
| pET-Ppqo | pET2 containing a 372-bp insert of pqo promoter region | This work |
| pEKEx2 | Expression vector for use in E. coli and C. glutamicum | 21 |
| pEKEx-pqo | pEKEx2 carrying the structural pqo gene with ribosomal binding site | 59 |
| Oligonucleotides | ||
| pqodel1 | 5′-AAGGAATTCGTTTTCGAGGCGACCAGACAG-3′ | Primer for deletion in pqo, EcoRI site |
| pqodel2 | 5′-TGTTTAAGTTTAGTGGATGGGCACCGCAGAACAAAGTGACAG-3′ | Primer for deletion in pqo, crossover overlap |
| pqodel3 | 5′-CCCATCCACTAAACTTAAACACGTTCCTTCCTTGATCGGATG-3′ | Primer for deletion in pqo, crossover overlap |
| pqodel4 | 5′-TGGCACAAGCTTGTTAAGCGCTCGCGGTCAATG-3′ | Primer for deletion in pqo, HindIII site |
| pqoprom1 | 5′-ACGCGTCGACGGAAATCGTAGGGGACTGTC-3′ | Primer for amplification of pqo promotor region, SalI site |
| pqoprom2 | 5′-CGCGGATCCTGAACTCCTCAACGTTATGGC-3′ | Primer for amplification of pqo promotor region, BamHI site, and for primer extension experiment with C. glutamicum WT |
| CM4 | 5′-GAAAATCTCGTCGAAGCTCG-3′ | Primer for primer extension experiment with RNA from C. glutamicum WT(pET-Ppqo); 73 |
| pqomap1 | 5′-TACGCATACCCGATCCGAAG-3′ | Primer for analysis of transcriptional organization of pqo |
| maptip2 | 5′-CAAGCACCTCCGCAATATCC-3′ | Primer for analysis of transcriptional organization of pqo |
DNA preparation and transformation.
The isolation of chromosomal DNA and plasmids from C. glutamicum was performed as described previously (21), and plasmid isolation from E. coli was carried out according to the method of Birnboim (3). DNA transfer into C. glutamicum was performed by electroporation, and the recombinant strains were selected on LB-BHIS agar plates containing kanamycin (15 μg ml−1) (71). Electroporation of E. coli was performed with competent cells according to the method of Dower et al. (18).
PCR techniques, DNA manipulation, and Southern hybridization.
PCR experiments were performed in a Biometra personal cycler (Biotron). Amplification of DNA was carried out with Vent polymerase (New England Biolabs). Deoxynucleoside triphosphates were purchased from MBI-Fermentas and oligonucleotides (primers) obtained from MWG-Biotech. PCR conditions were chosen according to fragment length and primer constitution. PCR products were purified from agarose gels using the Nucleospin extract kit (Macherey & Nagel).
Restrictions enzymes, T4 DNA ligase, calf intestinal phosphatase, RNase A, proteinase K, and Taq polymerase were purchased from MBI-Fermentas and used according to the instructions of the manufacturer. After restriction digests, DNA was separated on agarose gels and purified with the Nucleospin extract kit (Macherey & Nagel).
DNA hybridization experiments were performed as described previously (21). A pqo-specific 802-bp DNA fragment was amplified from chromosomal DNA of the C. glutamicum wild type (WT) by PCR with the primers pqodel3 and pqodel4 and used as a probe. Labeling, hybridization, washing, and detection were conducted using a nonradioactive DNA labeling and detection kit and the instructions from Roche Diagnostics.
Cloning of the pqo promoter.
The pqo promoter fragment was amplified from chromosomal DNA of C. glutamicum WT by PCR with the primers pqoprom1 and pqoprom2. The PCR product was digested with SalI and BamHI, ligated into a SalI/BamHI-restricted plasmid, pET2, and transformed into E. coli. The recombinant plasmid, pET-Ppqo, was then isolated from E. coli and introduced into C. glutamicum by electroporation. The nucleotide sequence of the promoter fragment in plasmid pET-Ppqo was verified by DNA sequence analysis.
Construction of C. glutamicum pqo deletion mutants.
Inactivation of the chromosomal pqo gene in C. glutamicum was performed as described previously (44), using crossover PCR and the suicide vector pK19mobsacB. pqo-specific DNA fragments were generated using the primer pairs pqodel1/pqodel2 and pqodel3/pqodel4. Fragment 1 covers 271 bp upstream of pqo and 628 bp of the 5′ end of pqo, fragment 2 the 3′ end of pqo from bp 741 and 49 bp downstream of the pqo stop codon (see Fig. 1). The two fragments were purified, mixed in equal amounts, and subjected to crossover PCR using primers pqodel1 and pqodel4. The resulting fusion product (containing the pqo gene with a deletion of 353 bp) was digested with EcoRI/HindIII, ligated into the EcoRI/HindIII-restricted plasmid pK19mobsacB, and transformed into E. coli. The recombinant plasmid was isolated from E. coli, and the nucleotide sequence of the insert was confirmed by DNA sequencing. Then, the plasmid was electroporated into C. glutamicum WT, WT ΔaceE, and MH20-22B. Applying the method described by Schäfer et al. (58), the intact chromosomal pqo genes in C. glutamicum WT and MH20-22B were replaced by the truncated pqo gene via homologous recombination (double crossover). The screening of the pqo mutants was done on LB agar plates containing 0.5% (wt/vol) glucose and 10% (wt/vol) sucrose. The replacement at the chromosomal locus was verified by PCR using primers pqodel1 and pqodel4 (data not shown) and by Southern blot analysis. For the latter, a labeled pqo probe was hybridized to SalI-restricted and size-fractionated chromosomal DNA from C. glutamicum WT, WT ΔaceE, MH20-22B, and candidate mutant strains. The pqo replacement mutants were designated C. glutamicum WT Δpqo, WT ΔpqoΔaceE, and MH20-22B Δpqo.
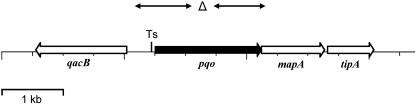
FIG. 1.
Genomic locus of the pqo gene in C. glutamicum. The arrows represent the computer-predicted coding regions of pqo and the adjacent genes, designated qacB, mapA, and tipA and putatively encoding a multidrug efflux protein, a metal-activated pyridoxal enzyme, and a thiostreptone-induced transcriptional activator, respectively. The Δ symbol indicates the deleted region in C. glutamicum WT Δpqo, WT ΔaceEΔpqo, and MH20-22B. The transcriptional start site is indicated as Ts. The double-headed arrows indicate the fragments used for Southern blot hybridizations and for the construction of the pqo deletion mutant.
Overexpression of pqo in C. glutamicum.
For overexpression of the pqo gene, plasmid pEKEx-pqo (59) was introduced into C. glutamicum WT Δpqo and into the lysine producer MH20-22B by electroporation. Expression of the plasmid-borne gene was induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside.
RNA techniques.
Total RNA from exponentially growing cells of C. glutamicum (optical density at 600 nm [OD600] of about 3) was isolated as described recently by Schreiner et al. (60). Aliquots of RNA were stored at −70°C.
For analysis of the transcriptional organization of pqo, mapA, and tipA in C. glutamicum, 1 μg of total RNA from C. glutamicum WT was used for reverse-transcriptase (RT) PCR with primers pqomap1 and maptip2. RT-PCR was performed using the OneStep RT-PCR kit (QIAGEN), following the instructions of the manufacturer. RNA samples were checked for DNA contamination by PCR without prior reverse transcription. Since no amplicon was obtained without reverse transcription, DNA contaminations could be excluded.
Primer extension reactions were carried out as described previously (32) with the IRD800-labeled primer pqoprom2 using RNA from C. glutamicum WT and with the IRD800-labeled primer CM4 using RNA from C. glutamicum (pET-Ppqo). The IRD800-labeled primers were obtained from MWG Biotech. Primer CM4 is homologous to a region 47 to 67 bp downstream of the BamHI site of the multiple cloning site in plasmid pET2. Signals were analyzed with an automatic sequencer (LI-COR 4000L; Licor, Inc.) using a 6% (wt/vol) polyacrylamide gel at 1,500 V and 50°C. For exact localization of the transcriptional start site, sequencing reactions using plasmid pET-Ppqo and the same oligonucleotide used for the respective primer extension reaction were coelectrophoresed.
Enzyme assays.
For the determination of PQO enzyme activity, cell extracts of C. glutamicum were prepared as described recently by Schreiner and Eikmanns (59). The protein concentration of the extracts was determined with a bicinchoninic acid protein assay reagent kit from Pierce with bovine serum albumin as the standard.
PQO activity was assayed photometrically by monitoring the reduction of 2,6-dichloroindophenol (DCPIP) upon addition of pyruvate essentially as described by Cunningham and Hager (16). The reaction mixture contained 100 mM morpholineethanesulfonic acid, pH 6.0, 10 mM MgCl2, 0.1 mM TPP, 1% (wt/vol) Triton X-100, 10% (vol/vol) glycerol, 300 μM (or 1 mM, when indicated) DCPIP, 100 mM potassium pyruvate, and appropriate amounts of cell extract (10 to 100 μl extract of WT cells; 1 to 10 μl extract of pqo-overexpressing cells). After preincubation of the reaction mixture at 40°C for 10 min, the reaction was started by addition of pyruvate, and the reduction of DCPIP was followed by measuring the decrease of absorbance at 600 nm at 40°C. The assay showed linearity with protein concentrations up to 0.4 mg/ml. PQO activity is expressed in units per mg of protein, and 1 unit corresponds to 1 μmol DCPIP reduced per min (ɛ600 = 22 mM−1 cm−1).
For the determination of pyruvate dehydrogenase complex (PDHC) activity, crude extracts of C. glutamicum were prepared as described previously (59), except that cells were washed and resuspended in 100 mM Tris-HCl, pH 7.2, 3 mM l-cysteine, 10 mM MgCl2, and the final ultracentrifugation step was omitted. Enzyme activities were determined photometrically according to the method described by Guest and Creaghan (26). One unit of activity was defined as 1 μmol NADH formed per min.
For the determination of chloramphenicol acetyltransferase (CAT) activity, crude extracts were prepared as described previously (59), except that cells were washed twice in 50 mM Tris-HCl, pH 7, and resuspended in the same buffer containing 10 mM MgCl2, 1 mM EDTA, and 30% (vol/vol) glycerol. The final ultracentrifugation step was omitted. CAT activity was assayed photometrically at 412 nm as described by Shaw (65) in 1 ml 100 mM Tris-HCl, pH 7.8, 1 mM 5,5′-dithiobis-2-nitrobenzoic acid, 0.1 mM acetyl-CoA, and 0.25 mM chloramphenicol. One unit of CAT activity was defined as 1 μmol chloramphenicol acetylated per min at 37°C.
Amino acid analysis.
For analysis of glutamate and lysine accumulation in culture fluid, aliquots were withdrawn and the cells were removed by centrifugation (5 min at 13,000 × g). Amino acids were analyzed as ortho-phthaldialdehyde derivatives by reverse-phase chromatography as described previously (61).
Computational analysis.
Databank searches and alignments were carried out by using BLAST and CLUSTALW (2, 69). Phylogenetic trees were generated using the neighbor-joining method (56), including 100 bootstrap replicates. The accession numbers for all protein sequences used for the phylogenetic analysis were as follows: for PQO/POX enzymes, Shigella flexneri, NP_706752; Escherichia coli, NP_415392; Salmonella enterica serovar Typhimurium, AAL19871; Yersinia pseudotuberculosis, YP_069915; Pseudomonas syringae, ZP_00123951; Burkholderia mallei, YP_106222; Xanthomonas oryzae, YP_202839; Bradyrhizobium japonicum, NP_773926; Corynebacterium glutamicum, NP_601811; Streptomyces avermitilis, BAC69790; Propionibacterium acnes, YP_056174; Bacteroides fragilis, CAH08912; Cytophaga hutchinsonii, ZP_00309672; Acinetobacter sp. strain ADP1, YP_047867; Ralstonia metallidurans, ZP_00277033; Ferroplasma acidarmanus, ZP_00306018; Picrophilus torridus, YP_024280; Sulfolobus solfataricus, NP_344275; Thermoplasma acidophilum, NP_394277; Lactococcus lactis, NP_268201; Oenococcus oeni, ZP_00318607; Pediococcus pentosaceus, ZP_00322361; Enterococcus faecium, ZP_00286254; Streptococcus pneumoniae, AAP87108; Leuconostoc mesenteroides, ZP_00062558; Lactobacillus plantarum, AAS10156; Bacillus subtilis, NP_388315; Oceanobacillus iheyensis, NP_694331; Listeria monocytogenes, ZP_00232899; and Staphylococcus aureus, AAW37329; for AHAS large subunit proteins, Escherichia coli, NP_418127; Mycobacterium avium, Q59498; Streptomyces cinnamonensis, AAV52901; Corynebacterium glutamicum, P42463; Caulobacter vibrioides, AAA23047; Saccharomyces cerevisiae, CAA26400; Geobacillus stearothermophilus, AAL99356; Lactococcus lactis, Q02137; and Methanococcus aeolicus, AAB53488.
RESULTS
Analysis of the PQO gene and its chromosomal organization.
The C. glutamicum WT pqo gene was sequenced in the course of the determination of the genome sequence (NC_003450 and BX927147) (31, 68). Due to the similarity of the deduced polypeptide to the E. coli “pyruvate oxidase,” the respective gene has been designated the poxB gene (cg2891 in reference 31; AX253251). However, according to the biochemical characterization of the enzyme (59), we proved the respective C. glutamicum enzyme to be a PQO, and therefore, we here rename the C. glutamicum gene the pqo gene.
The C. glutamicum pqo gene has a length of 1,740 bp, and the predicted gene product (protein identification no. NP_601811) consists of 579 amino acids with a predicted molecular mass of 61,951 Da, which corresponds well with the experimentally determined mass of the purified protein monomer (59). The pqo gene is preceded by a typical ribosomal binding site (GAGGAG) and directly followed by two genes designated mapA and tipA. The former gene codes for a protein with 30% identity to a so-called “metal-activated pyridoxal enzyme” (BAA31547; possibly a d-threonine aldolase) from Arthrobacter sp. strain DK-38, the latter for a protein with 36% identity to a “thiostrepton-induced transcriptional activator” (SwissProt data bank no. P32184) from Streptomyces lividans. The open reading frames for pqo and mapA overlap by 1 bp, and mapA and tipA are separated by 47 bp. Further inspection of the C. glutamicum genomic locus of pqo revealed that it is located upstream of and in opposite orientation to a gene (qacB) coding for a polypeptide with 36% identity to a component of a multidrug efflux protein (AAQ10697) of Staphylococcus aureus. The genomic organization of the C. glutamicum pqo gene is depicted in Fig. 1.
Transcriptional analysis of the pqo gene.
RT-PCR experiments were performed to analyze the size and organization of the C. glutamicum pqo transcript. RT-PCR using primers covering the 3′ end of pqo and the 5′ end of tipA and total RNA of C. glutamicum WT resulted in an amplification product of about 1,500 bp in size (data not shown), indicating that pqo, mapA, and tipA are organized as an operon.
To confirm a promoter in front of the C. glutamicum pqo gene and to investigate transcriptional regulation of this gene, a fusion between the putative pqo promoter region and the promoterless CAT gene was constructed in the promoter probe vector pET2. The resulting plasmid, pET-Ppqo, was transformed into C. glutamicum WT, and CAT activity was determined for the plasmid-carrying strain during growth in different media. Whereas C. glutamicum carrying the host plasmid pET2 showed no CAT activity (<0.01 U [mg protein]−1) on either medium, C. glutamicum WT(pET-Ppqo) displayed significant activities. As was the case with PQO activity of C. glutamicum WT (Table 2), the CAT activities of C. glutamicum WT(pET-Ppqo) were lower when the cells were grown on TY medium plus glucose (0.41 units/mg protein) or glucose-minimal medium (0.56 units/mg protein) instead of TY medium without any additional carbon source (0.79 unit/mg protein). In the course of growth, all these activities did not change significantly. These results confirmed the presence of a promoter immediately upstream of the pqo gene and argue for transcriptional control as a reason for the different PQO activities observed on different media (59) (Table 2).
TABLE 2.
Specific activities of PQO and of the PDHC in cell extracts of different C. glutamicum strains grown in complex medium (TY) or minimal medium (MM) containing 1% (wt/vol) glucose (Glc) as a carbon source and harvested in early stationary phase
| C. glutamicum straina | Medium | Sp act (U/mg of protein)c
|
|
|---|---|---|---|
| PQO | PDHC | ||
| WT | TY | 0.058 | 0.098 |
| TY + Glc | 0.024 | ND | |
| MM + Glc | 0.030 | ND | |
| WT Δpqo | TY | <0.001 | 0.095 |
| TY + Glc | <0.001 | ND | |
| MM + Glc | <0.001 | ND | |
| WT Δpqo(pEKEx2) | TY | <0.001 | ND |
| WT Δpqo(pEKEx-pqo) | TY | 0.960 | ND |
| WT ΔaceE | TYb | 0.026 | <0.001 |
| WT ΔaceE Δpqo (pEKEx2) | TYb | <0.001 | <0.001 |
| WT ΔaceE Δpqo (pEKEx-pqo) | TYb | 1.205 | <0.001 |
WT, wild-type strain ATCC 13032.
For optimal growth of C. glutamicum WT ΔaceE and WT ΔaceE Δpqo, the medium was supplemented with 0.5% (wt/vol) potassium acetate.
The values are means obtained from at least two independent cultivations and two determinations per experiment. The standard deviations were in all cases below 10%. ND, not determined.
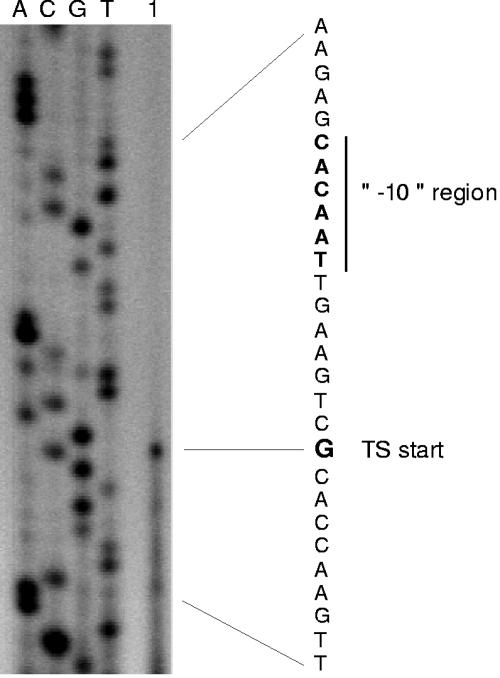
To identify the transcriptional initiation site of the C. glutamicum pqo gene, primer extension experiments were performed with two different primers and total RNA of C. glutamicum WT and C. glutamicum WT(pET-Ppqo). With both RNAs, signals were obtained corresponding to the G residue 45 bp upstream of the translational start codon. The result of the experiment with RNA from C. glutamicum WT and primer pqoprom2 is shown in Fig. 2. Analysis of the DNA sequence upstream of the transcriptional initiation site of the pqo gene revealed, in appropriate distances (12 to 7 bp and 36 to 31 bp, respectively) upstream of the transcriptional start site, sequences CACAAT and GTGGCA with similarity to typical −10 and −35 regions of C. glutamicum vegetative promoters (TAT/CAAT and TTGCCA, respectively) (45, 46).
FIG. 2.
Primer extension analysis of the transcriptional start site of the C. glutamicum pqo gene. The primer extension product is shown in lane 1. Lanes A, C, G, and T represent the products of sequencing reactions with the same primer used for the primer extension reaction. The relevant DNA sequence (coding strand) is shown on the right; the transcriptional start site and the predicted −10 region are indicated. Note that the sequence given to the right represents the noncoding strand and thus is complementary to the sequence of the sequencing reactions.
Inactivation of the chromosomal pqo gene and pqo overexpression.
To study whether C. glutamicum requires the pqo gene for growth and for PQO activity, the chromosomal pqo gene was replaced by a truncated pqo gene. The resulting strain, C. glutamicum WT Δpqo, and C. glutamicum WT Δpqo(pEKEx-pqo), a derivative carrying the pqo gene on the expression vector pEKEx2, were tested for growth on different media and for their PQO activities.
C. glutamicum WT, WT Δpqo, and WT Δpqo(pEKEx-pqo) grew equally well (doubling time and final optical density) on TY complex medium and on minimal medium containing glucose, maltose, ribose, lactate, pyruvate, or acetate (not shown). There were also no differences in the growth characteristics of the three strains when the cells were grown under oxygen limitation, i.e., when the cultures were incubated without agitation, or when the cells were incubated under phosphate limitation. Neither C. glutamicum WT nor the Δpqo strain showed acetate formation during growth under any condition. Plate count experiments with stationary-phase cells (24 h, 48 h, and 168 h after inoculation) of C. glutamicum WT and C. glutamicum WT Δpqo revealed no obvious difference in viability.
As shown in Table 2, the Δpqo mutant and the Δpqo mutant carrying the empty plasmid pEKEx2 were devoid of any detectable PQO activity, whereas C. glutamicum WT Δpqo(pEKEx-pqo) showed about 15-fold-higher specific PQO activities than did the original WT host strain. These results indicate that PQO is not essential for growth of C. glutamicum under the conditions tested here and that pqo overexpression has neither a positive nor a negative effect on the growth of this organism. Moreover, the results corroborate that the pqo gene in C. glutamicum in fact encodes a protein with PQO activity.
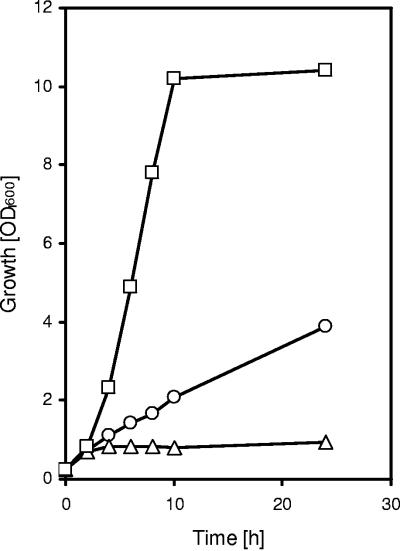
Since C. glutamicum constitutively possesses acetate kinase and phosphotransacetylase and these enzymes catalyze acetate activation to acetyl-CoA (52), we previously speculated that PQO activity and the two acetate-activating enzymes catalyze a bypass of the PDHC reaction, i.e., the oxidative decarboxylation of pyruvate with acetyl-CoA and CO2 as products (59). The defined PDHC-negative mutant C. glutamicum WT ΔaceE, however, showed negligible growth in TY medium and in minimal medium containing glucose, indicating that the PQO activity in the WT strain cannot compensate for the PDHC activity under the conditions tested (60). To test for the possibility that a high PQO activity can substitute for PDHC activity, we constructed the double mutant C. glutamicum WT ΔaceEΔpqo, transformed it with pEKEx-pqo or pEKEx2 (as a control), and assayed for the growth of the strains on different media. The double mutant C. glutamicum WT ΔaceEΔpqo carrying pEKEx2 showed no growth on TY complex medium (Fig. 3) and was also unable to grow on minimal glucose medium unless supplemented with >0.1% acetate (not shown). However, overexpression of the pqo gene and thus high specific PQO activity in the double mutant C. glutamicum WT ΔaceEΔpqo(pEKEx-pqo) (Table 2) led to linear growth on TY complex medium (Fig. 3). Whereas C. glutamicum WT ΔaceEΔpqo carrying pEKEx2 showed maximal final OD600s of 0.8 to 1.0, C. glutamicum WT ΔaceEΔpqo carrying vector pEKEx-pqo reproducibly grew to a final OD600 of about 4. These results indicate that a high PQO activity can compensate at least partially for PDHC activity in C. glutamicum.
FIG. 3.
Growth of C. glutamicum WT (open squares), WT ΔpqoΔaceE (pEKEx-pqo) (open circles), and C. glutamicum WT ΔpqoΔaceE (pEKEx2) (open triangles) on TY complex medium.
Significance of PQO for amino acid production.
Standard glutamate fermentations were performed with C. glutamicum WT, WT Δpqo, and WT Δpqo(pEKEx-pqo). All three strains showed the same growth and accumulated about 8 mM glutamate in the culture medium within 24 h of incubation. These results show that the capacity of C. glutamicum to produce glutamate is not influenced by the absence or by the level of PQO activity.
To analyze the significance of PQO activity for l-lysine production by C. glutamicum, we constructed a pqo overexpresser and a PQO-negative derivative of the lysine producer C. glutamicum MH20-22B. In minimal medium containing 4% (wt/vol) glucose, the mutant showed about the same growth behavior as the parental strain; however, the overexpresser MH20-22B(pEKEx-pqo) grew to a significantly lower final optical density (about 10 versus about 30). Amino acid analysis of the culture supernatants after 24 h of incubation revealed that the mutant MH20-22B Δpqo and the parental strain MH20-22B both accumulated about 30 mM lysine, whereas the pqo overexpresser produced between 8 and 10 mM lysine. These results indicate that neither the absence nor the overproduction of PQO significantly affects lysine production of C. glutamicum MH20-22B.
Sequence comparisons and phylogenetic analyses.
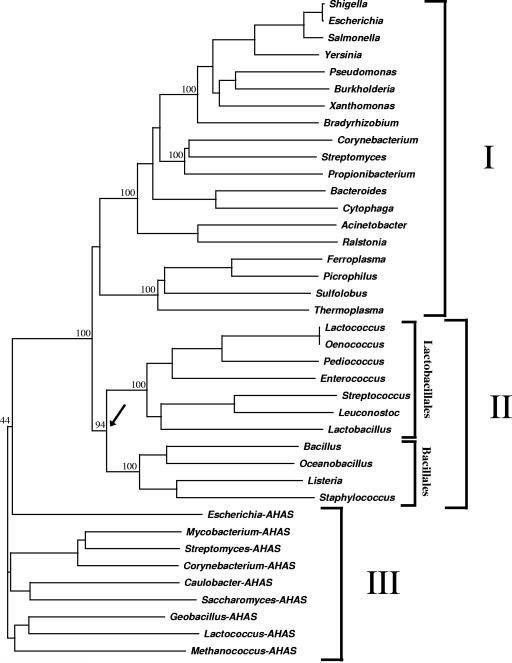
NCBI database searches with the C. glutamicum PQO sequence revealed between 52% and 70% identity to putative POX or PQO enzymes from “high-GC gram positives”, between 34% and 44% to putative POXs/PQOs from gram-negative bacteria, and between 31% and 36% to those from archaea. The identity of the C. glutamicum PQO to putative POXs from “low-GC gram positives” (e.g., lactic acid bacteria and bacilli) was between 26% and 32%. Amino acid sequence comparisons in earlier studies (10, 13) revealed that both the E. coli PQO enzyme and the L. plantarum POX have significant identity (about 30%) to the large subunit of AHAS. The relatively high degree of amino acid sequence identity in these protein families suggests a common ancestry of the PQO, POX, and AHAS enzymes. To elucidate the phylogeny of these protein families, the PQO and POX enzymes and the large subunits of functionally proven AHAS enzymes from various organisms were aligned and phylogenetic trees were constructed according to the neighbor-joining method. As shown in Fig. 4, the AHAS sequences group together (cluster III) and are separated from all (putative and experimentally proven) PQO and POX protein sequences. The phylogenetic tree of the PQO and POX protein sequences displays a dichotomy dividing the proteins into two discrete clusters. Cluster I contains the functionally analyzed PQO enzymes of E. coli and C. glutamicum as well as various sequences from other proteobacteria, “high-GC gram-positive” bacteria (Actinobacteria), and from archaea, whereas cluster II exclusively entails sequences of the “low-GC gram-positive” bacteria, including the known POX enzymes of the lactic acid bacteria L. plantarum and S. pneumoniae (36, 67). It is noteworthy that cluster II also contains a PQO protein just recently identified in S. aureus (47). In combination, the results of the sequence comparisons and of the phylogenetic analyses indicate a wide distribution of the PQO and/or POX enzymes among prokaryotes and show the relationship between these enzymes and the large subunits of AHAS.
FIG. 4.
Phylogenetic tree of PQO, POX, and AHAS enzymes deposited in public databases. Clusters I and II represent PQO/POX enzymes, cluster III AHAS enzymes. Numbers at the nodes give the bootstrap values. The arrow indicates the proposed separation of the PQO and POX enzymes. Organisms are listed in Materials and Methods.
DISCUSSION
The transcriptional data presented here show that in contrast to the proven and functionally characterized PQO genes in other organisms (i.e., in E. coli and in S. aureus [8, 25, 47]), C. glutamicum pqo is expressed independently of the growth phase. In E. coli, PQO activity and PQO gene expression have been shown to be maximal in the early stationary phase and to be completely dependent on sigma factor RpoS (7). In S. aureus, the respective cidC gene, which is cotranscribed in an operon with two further genes (see below), is expressed in a sigma factor B-dependent manner, and expression was highest in the early exponential growth phase and induced by growth in the presence of glucose (53, 54, 55). The different mode of regulation of the PQO genes in E. coli, S. aureus, and C. glutamicum may be linked to the genomic context of the respective genes, and/or it may be related to different functions of PQO activity within the metabolism of the three organisms. In E. coli, the poxB gene is monocistronic (7), and thus, expression control might aim solely at the advantage (or disadvantage) the organism has in the presence (or the absence) of PQO activity. PQO activity has been proposed to be advantageous for survival during the transition between exponential and stationary growth phases and for growth of E. coli under microaerobic conditions, i.e., when the PDHC and pyruvate formate lyase activities are low (7). Abdel-Hamid et al. (1) showed that PQO activity also is advantageous for optimal aerobic growth efficiency in glucose minimal medium, and Moreau (39) recently proposed that PQO activity may protect phosphate-starved, nongrowing E. coli cells against oxidative stress by diverting a part of the carbon flux from the PDHC and thus by decreasing the production of NADH. In S. aureus, the PQO gene is organized in an operon together with genes encoding a murein hydrolase regulator (55), and here the PQO has been shown to be involved in the generation of acetic acid that contributes to cell death and lysis in high-glucose stationary-phase cultures (47). In C. glutamicum, the PQO gene also is organized in an operon, together with genes coding for enzymes with no obvious relation to PQO. It is conceivable that the (weak) regulation of the operon and thus of the PQO gene in C. glutamicum is adapted to the physiological function of the proteins encoded by the other genes of the operon.
In comparison to the parental C. glutamicum strain, neither the pqo-overexpressing strain nor the PQO-deficient mutant showed any difference in growth rate and yield on either medium or under any conditions tested. The inactivation of the pqo gene had no effect on survival in stationary phase and under phosphate starvation, and acetate was secreted neither by the parental strain nor by the mutant strain. Long-term competitive growth experiments with C. glutamicum WT and C. glutamicum WT Δpqo did not reveal any (dis)advantage for one or the other strain (data not shown). Also, amino acid (glutamate and l-lysine) production was not affected by the absence of PQO activity. In summary, the PQO-negative mutant did not show any phenotype under all conditions tested, and further attempts are necessary to clarify the relevance of PQO in C. glutamicum. However, it is clear that the metabolic function of the PQO in C. glutamicum is different from those proposed for E. coli or S. aureus.
According to our results, the function of PQO in C. glutamicum should also be different from that described for the POX enzymes in the facultative heterofermentative lactic acid bacterium L. plantarum. In this organism, POX plays a key role in the conversion of lactate to acetate, which takes place under aerobic conditions and glucose limitation (23, 24, 42, 43). Accordingly, Lorquet et al. (36) recently showed a strong induction of the POX gene in L. plantarum by aeration and a repression by glucose, and these authors corroborated the predominant role of POX in the control of acetate production under aerobic conditions.
We previously showed that the PQO activity in C. glutamicum WT, together with the activities of the acetate activating enzymes acetate kinase and phosphotransacetylase, is not able to compensate for PDHC activity (60). By overexpression of the pqo gene, we here demonstrate that PQO, when present in large amounts, can substitute at least partially for the PDHC in C. glutamicum. On the one hand, this result suggests that there might be conditions when PQO activity becomes essential for survival, e.g., when PDHC synthesis is down-regulated or switched off. On the other hand, the result indicates that even under such high-level PQO conditions the carbon flux through the PQO reaction is still severely restricted in the cell. This restriction might be due to the low affinities of the C. glutamicum PQO for pyruvate (Km = 30 mM (59) and of the acetate kinase for acetate (Km = 7.9 mM (52). However, it is conceivable that in nature or under different culture conditions, there are conditions when the carbon flux through PQO is less restricted or even decontrolled.
Earlier studies by Chang and Cronan (10) have already reported substantial evidence that the AHAS enzymes are related to PQO proteins. These authors showed that the PQO of E. coli possesses AHAS activity and proposed that the requirement for flavin binding and the ubiquinone-binding site of the AHAS enzymes are vestigial remains that reflect the common ancestry of the PQO and AHAS enzymes. Our phylogenetic analyses revealed that the PQO and POX enzymes cluster together and are separated from the AHAS large subunits, indicating that the former two enzymes are more closely related to each other than to the AHAS proteins (Fig. 4). The fact that the PQO enzymes functionally have been shown to be present in proteobacteria, in “high-GC gram-positive” bacteria, and in “low-GC gram-positive” bacteria suggests that the POX proteins, which have so far been found only in lactic acid bacteria, are derived from PQO enzymes. All the findings might indicate that PQO proteins represent the progenitors of the POX as well as AHAS enzymes.
The fact that AHAS proteins as well as putative PQO enzymes have been found in archaea (6) indicates that the AHAS and PQO proteins separated early in the evolution of prokaryotes. It has been suggested that amino acid biosynthetic pathways and thus AHAS enzymes evolved when early environments became more depleted of amino acids (10). Thus, it is likely that AHAS enzymes are derived from a PQO protein via an early gene duplication event, probably prior to the diversification of bacteria. The fact that POX proteins are exclusively present in lactic acid bacteria (Lactobacillales) indicates that these enzymes trace back to developments that occurred late during the evolution of bacteria. The recent finding that S. aureus possesses PQO activity and thus a PQO protein (47) suggests that the PQO and POX enzymes separated during the diversification of the “low GC gram-positive” lineage (arrow in Fig. 4). Thus, the POX enzymes probably developed from a PQO protein in an ancestral “low-GC gram-positive” bacterium.
The occurrence of POX proteins instead of PQO enzymes in lactic acid bacteria coincides with the predominately anaerobic metabolism of these bacteria. Lactic acid bacteria obtain energy mainly by substrate-level phosphorylation. The prevailing absence of respiratory capacity and the incomplete citric acid cycle of the lactic acid bacteria preclude that “aerobic” enzymes, such as a PQO protein or the PDHC, would contribute favorably to energy conservation in this bacterial group. In contrast to the PQO protein, the POX enzyme generates the high-energy compound acetyl phosphate, which can be used for ATP production by substrate-level phosphorylation. Furthermore, the POX enzyme is probably more beneficial for the oxidation-reduction balance of the lactobacterial metabolism than PQO or PDHC, since it directly reduces oxygen and therefore avoids the requirement to reoxidize FADH2 or NADH during fermentative growth. It seems likely, therefore, that a POX enzyme would be more advantageous for the growth efficiency of the aerotolerant lactic acid bacteria under aerobic cultivation conditions than a PQO or PDHC enzyme. In accordance with these considerations, a recent functional analysis of the POX enzyme in L. plantarum revealed a predominant role of this protein in the control of acetate production by L. plantarum under aerobic conditions (36). Interestingly, although putative genes coding for components of a PDHC have been identified in the genome sequence of L. plantarum, so far no PDHC activity was detected in cell extracts of this organism (17, 30, 33, 36, 42). These findings suggest that L. plantarum lost a functional PDHC during evolution and thus further substantiate the notion that “aerobic” enzymes, such as the PDHC, would not contribute advantageously to energy conservation in lactic acid bacteria.
Based on our analyses, we propose that POX enzymes have evolved from a PQO protein during the emergence of the lactobacterial lineage within the “low-GC gram-positive” bacteria, possibly in parallel to the adaptation to an anaerobic metabolism in this lineage. As a consequence, POX progenitors lost the typical PQO lipid binding site and the ability to interact with quinones of the respiratory chain and instead evolved a phosphate- and oxygen-dependent reaction mechanism which results in the formation of acetyl phosphate and hydrogen peroxide.
Acknowledgments
We thank Joy Schreiner for critically reading the manuscript.
The support of the EC (grant VALPAN, QLK3-2000-00497), the Fachagentur Nachwachsende Rohstoffe of the BMVEL (grant 04NR004/22000404), and the Grant Agency of the Czech Republic (grant 525/04/0548) is gratefully acknowledged.
REFERENCES
- 1.Abdel-Hamid, A. M., M. M. Attwood, and J. R. Guest. 2001. Pyruvate oxidase contributes to the aerobic growth efficiency of Escherichia coli. Microbiology 147:1483-1498. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Birnboim, H. C. 1983. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 100:243-255. [DOI] [PubMed] [Google Scholar]
- 4.Blake, R. I. I., T. A. O'Brien, R. B. Gennis, and L. P. Hager. 1982. Role of the divalent metal cation in the pyruvate oxidase reaction. J. Biol. Chem. 257:9605-9611. [PubMed] [Google Scholar]
- 5.Blake, R., and L. P. Hager. 1978. Activation of pyruvate oxidase by monomeric and micellar amphiphiles. J. Biol. Chem. 253:1963-1971. [PubMed] [Google Scholar]
- 6.Bowen, T. L., J. Union, D. L. Tumbula, and W. B. Whitman. 1997. Cloning and phylogenetic analysis of the genes encoding acetohydroxyacid synthase from the archaeon Methanococcus aeolicus. Gene 188:77-84. [DOI] [PubMed] [Google Scholar]
- 7.Chang, Y. Y., A. Y. Wang, and J. E. Cronan, Jr. 1994. Expression of Escherichia coli pyruvate oxidase (PoxB) depends on the sigma factor encoded by the rpoS(katF) gene. Mol. Microbiol. 11:1019-1028. [DOI] [PubMed] [Google Scholar]
- 8.Chang, Y. Y., and J. E. Cronan, Jr. 1984. An Escherichia coli mutant in pyruvate oxidase due to altered phospholipid activation of the enzyme. Proc. Natl. Acad. Sci. USA. 81:4348-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, Y. Y., and J. E. Cronan, Jr. 1986. Molecular cloning, DNA sequencing, and enzymatic analyses of two Escherichia coli pyruvate oxidase mutants defective in activation by lipids. J. Bacteriol. 167:312-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, Y. Y., and J. E. Cronan, Jr. 1988. Common ancestry of Escherichia coli pyruvate oxidase and acetohydroxy acid synthases of the branched-chain amino acid biosynthetic pathway. J. Bacteriol. 170:3937-3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang, Y. Y., and J. E. Cronan, Jr. 1995. Detection by site-specific disulfide cross-linking of a conformational change in binding of Escherichia coli pyruvate oxidase to lipid bilayers. J. Biol. Chem. 270:7896-7901. [DOI] [PubMed] [Google Scholar]
- 12.Chang, Y. Y., and J. E. Cronan, Jr. 1997. Sulfhydryl chemistry detects three conformations of the lipid binding region of Escherichia coli pyruvate oxidase. Biochemistry 36:11564-11573. [DOI] [PubMed] [Google Scholar]
- 13.Chang, Y. Y., and J. E. Cronan, Jr. 2000. Conversion of Escherichia coli pyruvate oxidase to an ‘alpha-ketobutyrate oxidase’. Biochem. J. 352:717-724. [PMC free article] [PubMed] [Google Scholar]
- 14.Cocaign-Bousquet, M., and N. D. Lindley. 1995. Pyruvate overflow and carbon flux within the central metabolic pathways of Corynebacterium glutamicum during growth on lactate. Enzyme Microb. Technol. 17:260-267. [Google Scholar]
- 15.Cunningham, C. C., and L. P. Hager. 1971. Crystalline pyruvate oxidase from Escherichia coli. II. Activation by phospholipids. J. Biol. Chem. 246:1575-1582. [PubMed] [Google Scholar]
- 16.Cunningham, C. C., and L. P. Hager. 1971. Crystalline pyruvate oxidase from Escherichia coli. 3. Phospholipid as an allosteric effector for the enzyme. J. Biol. Chem. 246:1583-1589. [PubMed] [Google Scholar]
- 17.Dirar, S., and E. B. Collins. 1973. Aerobic utilization of low concentration of galactose by Lactobacillus plantarum. J. Bacteriol. 78:211-215. [DOI] [PubMed] [Google Scholar]
- 18.Dower, W. J., J. F. Miller, and C. W. Ragsdale. 1988. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 16:6127-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eikmanns, B. J. 2005. Central metabolic pathways of Corynebacterium glutamicum: tricarboxylic cycle and anaplerotic reactions, p. 241-276. In L. Eggeling and M. Bott (ed.), Handbook of Corynebacterium glutamicum. CRC Press, Boca Raton, Fla.
- 20.Eikmanns, B. J., M. Metzger, D. Reinscheid, M. Kircher, and H. Sahm. 1991. Amplification of three threonine biosynthesis genes in Corynebacterium glutamicum and its influence on carbon flux in different strains. Appl. Microbiol. Biotechnol. 34:617-622. [DOI] [PubMed] [Google Scholar]
- 21.Eikmanns, B. J., N. Thum-Schmitz, L. Eggeling, K. U. Ludtke, and H. Sahm. 1994. Nucleotide sequence, expression and transcriptional analysis of the Corynebacterium glutamicum gltA gene encoding citrate synthase. Microbiology 140:1817-1828. [DOI] [PubMed] [Google Scholar]
- 22.Gennis, R. B., and L. P. Hager. 1976. Pyruvate oxidase, p. 493-504. In A. Martonosi (ed.), The enzymes of biological membranes, vol. 2. Plenum, New York, N.Y. [Google Scholar]
- 23.Götz, F., B. Sedewitz, and E. F. Elstner. 1980. Oxygen utilization by Lactobacillus plantarum. I. Oxygen consuming reactions. Arch. Microbiol. 125:209-214. [DOI] [PubMed] [Google Scholar]
- 24.Götz, F., E. F. Elstner, B. Sedewitz, and E. Lengfelder. 1980. Oxygen utilization by Lactobacillus plantarum. II. Superoxide and superoxide dismutation. Arch. Microbiol. 125:215-220. [DOI] [PubMed] [Google Scholar]
- 25.Grabau, C., and J. E. Cronan, Jr. 1984. Molecular cloning of the gene (poxB) encoding the pyruvate oxidase of Escherichia coli, a lipid-activated enzyme. J. Bacteriol. 160:1088-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guest, J. R., and I. T. Creaghan. 1974. Further studies with lipoamide dehydrogenase mutants of Escherichia coli K12. J. Gen. Microbiol. 81:237-245. [DOI] [PubMed] [Google Scholar]
- 27.Hager, L. P., D. M. Geller, and F. Lipmann. 1954. Flavoprotein-catalyzed pyruvate oxidation in Lactobacillus delbrueckii. Fed. Proc. 13:734-738. [PubMed] [Google Scholar]
- 28.Hamilton, S. E., M. Recny, and L. P. Hager. 1986. Identification of the high-affinity lipid binding site in Escherichia coli pyruvate oxidase. Biochemistry 25:8178-8183. [DOI] [PubMed] [Google Scholar]
- 29.Hanahan, D. 1985. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 30.Hickey, M. W., A. J. Hillier, and G. R. Jago. 1983. Metabolism of pyruvate and citrate in lactobacilli. Aust. J. Biol. Sci. 36:487-496. [DOI] [PubMed] [Google Scholar]
- 31.Kalinowski, J., B. Bathe, D. Bartels, N. Bischoff, M. Bott, A. Burkovski, N. Dusch, L. Eggeling, B. J. Eikmanns, L. Gaigalat, A. Goesmann, M. Hartmann, K. Huthmacher, R. Kramer, B. Linke, A. C. McHardy, F. Meyer, B. Mockel, W. Pfefferle, A. Puhler, D. A. Rey, C. Ruckert, O. Rupp, H. Sahm, V. F. Wendisch, I. Wiegrabe, and A. Tauch. 2003. The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins. J. Biotechnol. 104:5-25. [DOI] [PubMed] [Google Scholar]
- 32.Kellmann, J. W., E. Piechersky, and B. Piechulla. 1990. Analysis of the diurnal expression patterns of the tomato chlorophyll a/b binding protein genes. Influence of light and characterization of the gene family. Photochem. Phytobiol. 52:35-41. [DOI] [PubMed] [Google Scholar]
- 33.Kleerebezem, M., J. Boekhorst, R. van Kranenburg, D. Molenaar, O. Kuipers, R. Leer, R. Tarchini, S. A. Peters. H. M. Sandbrink, M. W. E. J. Fiers, W. Stiekema, R. M. K. Lankhorst, P. A. Bron, S. M. Hoffer, M. N. Nierop Groot, R. Kerkhoven, M. de Vries, B. Ursing, W. M. de Vos, and R. J. Siezen. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA. 100:1190-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koffas, M. A., G. Y. Jung, J. C. Aon, and G. Stephanopoulos. 2002. Effect of pyruvate carboxylase overexpression on the physiology of Corynebacterium glutamicum. Appl. Environ. Microbiol. 68:5422-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liebl, W. 1991. The genus Corynebacterium—nonmedical, p. 1157-1171. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes, vol. 2. Springer, New York, N.Y. [Google Scholar]
- 36.Lorquet, F., P. Goffin, L. Muscariello, J. B. Baudry, V. Ladero, M. Sacco, M. Kleerebezem, and P. Hols. 2004. Characterization and functional analysis of the poxB gene, which encodes pyruvate oxidase in Lactobacillus plantarum. J. Bacteriol. 186:3749-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mather, M. W., and R. B. Gennis. 1985. Spectroscopic studies of pyruvate oxidase flavoprotein from Escherichia coli trapped in the lipid-activated form by cross-linking. J. Biol. Chem. 260:10395-10397. [PubMed] [Google Scholar]
- 38.Mather, M., L. M. Schopfer, V. Massey, and R. B. Gennis. 1982. Studies of the flavin adenine dinucleotide binding region in Escherichia coli pyruvate oxidase. J. Biol. Chem. 257:12887-12892. [PubMed] [Google Scholar]
- 39.Moreau, P. L. 2004. Diversion of the metabolic flux from pyruvate dehydrogenase to pyruvate oxidase decreases oxidative stress during glucose metabolism in nongrowing Escherichia coli cells incubated under aerobic phosphate starvation conditions. J. Bacteriol. 186:7364-7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller, Y. A., and G. E. Schulz. 1993. Structure of the thiamine- and flavin-dependent enzyme pyruvate oxidase. Science 259:965-967. [DOI] [PubMed] [Google Scholar]
- 41.Muller, Y. A., G. Schumacher, R. Rudolph, and G. E. Schulz. 1994. The refined structures of a stabilized mutant and of wild-type pyruvate oxidase from Lactobacillus plantarum. J. Mol. Biol. 237:315-335. [DOI] [PubMed] [Google Scholar]
- 42.Murphy, M. G., and S. Condon. 1984. Correlation of oxygen utilisation and hydrogen peroxide accumulation with oxygen induced enzymes in Lactobacillus plantarum cultures. Arch. Microbiol. 138:44-48. [DOI] [PubMed] [Google Scholar]
- 43.Murphy, M. G., L. O′Connor, D. Walsh, and S. Condon. 1985. Oxygen dependent lactate utilization by Lactobacillus plantarum. Arch. Microbiol. 141:75-79. [DOI] [PubMed] [Google Scholar]
- 44.Niebisch, A., and M. Bott. 2001. Molecular analysis of the cytochrome bc1-aa3 branch of the Corynebacterium glutamicum respiratory chain containing an unusual diheme cytochrome c1. Arch. Microbiol. 175:282-294. [DOI] [PubMed] [Google Scholar]
- 45.Pátek, M., B. J. Eikmanns, J. Pátek, and H. Sahm. 1996. Promoters from Corynebacterium glutamicum: cloning, molecular analysis and search for a consensus motif. Microbiology 142:1297-1309. [DOI] [PubMed] [Google Scholar]
- 46.Pátek, M., J. Nesvera, A. Guyonvarch, O. Reyes, and G. Leblon. 2003. Promoters of Corynebacterium glutamicum. J. Biotechnol. 104:311-323. [DOI] [PubMed] [Google Scholar]
- 47.Patton, T. G., K. C. Rice, M. K. Foster, and K. W. Bayles. 2005. The Staphylococcus aureus cidC gene encodes a pyruvate oxidase that affects acetate metabolism and cell death in stationary phase. Mol. Microbiol. 56:1664-1674. [DOI] [PubMed] [Google Scholar]
- 48.Pericone, C. D., S. Park, J. A. Imlay, and J. N. Weiser. 2003. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the Fenton reaction. J. Bacteriol. 185:6815-6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peters-Wendisch, P. G., B. Schiel, V. F. Wendisch, E. Katsoulidis, B. Mockel, H. Sahm, and B. J. Eikmanns. 2001. Pyruvate carboxylase is a major bottleneck for glutamate and lysine production by Corynebacterium glutamicum. J. Mol. Microbiol. Biotechnol. 3:295-300. [PubMed] [Google Scholar]
- 50.Peters-Wendisch, P. G., C. Kreutzer, J. Kalinowski, M. Pátek, H. Sahm, and B. J. Eikmanns. 1998. Pyruvate carboxylase from Corynebacterium glutamicum: characterization, expression and inactivation of the pyc gene. Microbiology 144:915-927. [DOI] [PubMed] [Google Scholar]
- 51.Peters-Wendisch, P. G., V. F. Wendisch, S. Paul, B. J. Eikmanns, and H. Sahm. 1997. Pyruvate carboxylase as an anaplerotic enzyme in Corynebacterium glutamicum. Microbiology 143:1095-1103. [DOI] [PubMed] [Google Scholar]
- 52.Reinscheid, D. J., S. Schnicke, D. Rittmann, U. Zahnow, H. Sahm, and B. J. Eikmanns. 1999. Cloning, sequence analysis, expression and inactivation of the Corynebacterium glutamicum pta-ack operon encoding phosphotransacetylase and acetate kinase. Microbiology 145:503-513. [DOI] [PubMed] [Google Scholar]
- 53.Rice, K. C., B. A. Firek, J. B. Nelson, S. J. Yang, T. G. Patton, and K. W. Bayles. 2003. The Staphylococcus aureus cidAB operon: evaluation of its role in regulation of murein hydrolase activity and penicillin tolerance. J. Bacteriol. 185:2635-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rice, K. C., J. B. Nelson, T. G. Patton, S. J. Yang, and K. W. Bayles. 2005. Acetic acid induces expression of the Staphylococcus aureus cidABC and lrgAB murein hydrolase regulator operons. J. Bacteriol. 187:813-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rice, K. C., T. Patton, S. J. Yang, A. Dumoulin, M. Bischoff, and K. W. Bayles. 2004. Transcription of the Staphylococcus aureus cid and lrg murein hydrolase regulators is affected by sigma factor B. J. Bacteriol. 186:3029-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 57.Sambrook, J., D. W. Russel, N. Irwin, and U. A. Janssen. 2001. Molecular cloning: a laboratory manual, 3rd ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 58.Schäfer, A., A. Tauch, W. Jäger, J. Kalinowski, G. Thierbach, and A. Pühler. 1994. Small mobilizable multi-purpose cloning vectors derived from the E. coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69-73. [DOI] [PubMed] [Google Scholar]
- 59.Schreiner, M. E., and B. J. Eikmanns. 2005. Pyruvate:quinone oxidoreductase from Corynebacterium glutamicum: purification and biochemical characterization. J. Bacteriol. 187:862-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schreiner, M. E., D. Fiur, J. Holátko, M. Pátek, and B. J. Eikmanns. 2005. E1 enzyme of the pyruvate dehydrogenase complex in Corynebacterium glutamicum: molecular analysis of the gene and phylogenetic spects. J. Bacteriol. 187:6005-6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schrumpf, B., A. Schwarzer, J. Kalinowski, A. Pühler, L. Eggeling, and H. Sahm. 1991. A functional split pathway for lysine biosynthesis in Corynebacterium glutamicum. J. Bacteriol. 173:4510-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schrumpf, B., L. Eggeling, and H. Sahm. 1992. Isolation and prominent characteristics of an L-lysine hyperproducing strain of Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 37:566-571. [Google Scholar]
- 63.Sedewitz, B., K. H. Schleifer, and F. Götz. 1984. Physiological role of pyruvate oxidase in the aerobic metabolism of Lactobacillus plantarum. J. Bacteriol. 160:462-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sedewitz, B., K. H. Schleifer, and F. Götz. 1984. Purification and biochemical characterization of pyruvate oxidase from Lactobacillus plantarum. J. Bacteriol. 160:273-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shaw, W. V. 1975. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 43:737-755. [DOI] [PubMed] [Google Scholar]
- 66.Shiio, I., Y. Toride, and S. Sugimoto. 1984. Production of lysine by pyruvate dehydrogenase mutants of Brevibacterium flavum. Agric. Biol. Chem. 48:3091-3098. [Google Scholar]
- 67.Spellerberg, B., D. R. Cundell, J. Sandros, B. J. Pearce, I. Idanpaan-Heikkila, C. Rosenow, and H. R. Masure. 1996. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. 19:803-813. [DOI] [PubMed]
- 68.Tauch, A., I. Homann, S. Mormann, S. Ruberg, A. Billault, B. Bathe, S. Brand, O. Brockmann-Gretza, C. Ruckert, N. Schischka, C. Wrenger, J. Hoheisel, B. Mockel, K. Huthmacher, W. Pfefferle, A. Puhler, and J. Kalinowski. 2002. Strategy to sequence the genome of Corynebacterium glutamicum ATCC 13032: use of a cosmid and a bacterial artificial chromosome library. J. Biotechnol. 95:25-38. [DOI] [PubMed] [Google Scholar]
- 69.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tittmann, K., R. Golbik, S. Ghisla, and G. Hubner. 2000. Mechanism of elementary catalytic steps of pyruvate oxidase from Lactobacillus plantarum. Biochemistry 39:10747-10754. [DOI] [PubMed] [Google Scholar]
- 71.Van der Rest, M. E., C. Lange, and D. Molenaar. 1999. A heat shock following electroporation induces highly efficient transformation of Corynebacterium glutamicum with xenogenic plasmid DNA. Appl. Microbiol. Biotechnol. 52:541-545. [DOI] [PubMed] [Google Scholar]
- 72.Vasicova, P., Z. Abrhamova, J. Nesvera, M. Pátek, H. Sahm, and B. Eikmanns. 1998. Integrative and autonomously replicating vectors for analysis of promoters in Corynebacterium glutamicum. Biotechnol. Tech. 12:743-746. [Google Scholar]
- 73.Venkova-Canova, T., M. Pátek, and J. Nesvera. 2003. Control of rep gene expression in plasmid pGA1 from Corynebacterium glutamicum. J. Bacteriol. 185:2402-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang, A. Y., Y. Y. Chang, and J. E. Cronan, Jr. 1991. Role of the tetrameric structure of Escherichia coli pyruvate oxidase in enzyme activation and lipid binding. J. Biol. Chem. 266:10959-10966. [PubMed] [Google Scholar]
- 75.Williams, F. R., and L. P. Hager. 1966. Crystalline flavin pyruvate oxidase from Escherichia coli. I. Isolation and properties of the flavoprotein. Arch. Biochem. Biophys. 116:168-176. [DOI] [PubMed] [Google Scholar]