Abstract
Papillomaviruses associated with clinical symptoms have been found in many vertebrate species. In this study, we have used an L1 gene consensus PCR test designed to detect a broad spectrum of human skin papillomaviruses to analyze swab samples from healthy skin of 111 animals belonging to 19 vertebrate species. In eight of the species, papillomavirus DNA was found with the following prevalences: chimpanzees, 9 of 11 samples positive; gorillas, 3 of 4; long-tailed macaques, 14 of 16; spider monkeys, 2 of 2; ruffed lemurs, 1 of 2; cows, 6 of 10; European elks, 4 of 4; aurochs, 1 of 1. In total, 53 new putative animal papillomavirus types were found. The results show that skin papillomaviruses can be detected in healthy skin from many different animal species and are sufficiently related genetically to their human counterparts to be identified by a human skin papillomavirus primer set (FAP59 and FAP64).
Papillomaviruses (PVs) are a diverse, epitheliotropic group of viruses which can induce lesions in the stratified squamous epithelia of skin and mucosa. To date, more than 90 human PV (HPV) types have been fully characterized genetically, and, in addition, some 100 putative new types have been identified and partially sequenced. Many PV types from a number of vertebrate species have also been described (27), and some of these animal PV types have been implicated in cancers (5, 26, 28). The finding of PVs in a broad spectrum of distantly related animal species suggests a long coevolutionary history of this group of viruses (3, 8, 22).
Recent reports (1, 2, 4) have shown that HPV is commonly present in the healthy skin of healthy humans as a presumed commensal agent. Here we have investigated skin swab samples from several different vertebrates by using one set of primers (FAP59 and FAP64) (13) previously used for the detection of human skin HPVs.
MATERIALS AND METHODS
Subjects.
Samples were collected in three different zoological gardens from the following primates: 11 chimpanzees (Pan troglodytes), 4 gorillas (Gorilla gorilla), 2 spider monkeys (Ateles geoffroyi), 2 ruffed lemurs (Varecia variegatus variegatus), 7 lemurs (Lemur catta), and 7 animal keepers (Homo sapiens). Furthermore, specimens were taken from four Indian elephants (Elephas maximus), four European elks (Alces alces), one reindeer (Rangifer rangifer), one roe deer (Capreolus capreolus), three European bison (Bison bonasus), one aurochs (Bos taurus primigenus), and one Przewalski's horse (Equus ferus przewalskii). Specimens were also obtained from 16 long-tailed macaques (Macaca fascicularis) from the experimental animal facility of a scientific institute. Finally, samples were collected from the following animals from four different farms: 10 cows (Bos taurus), 8 horses (Equus caballus), 25 pigs (Sus scrofa), 2 dogs (Canis familaris), 5 cats (Felis catus), and 4 hens (Gallus gallus).
One sample was collected from each animal, except from four of the chimpanzees and the four gorillas, from which two consecutive samples were taken 1 day apart. A single sample was also collected from each of seven animal keepers from one of the zoos. From one of them, no. 5, a second set of specimens was taken from the forehead, nose, chin, and from the left and right arms 4 months after the first sampling.
Samples.
Samples were collected with prewetted (0.9% NaCl solution) cotton-tipped swabs (Bio Hospital, Kopparberg, Sweden), which were drawn back and forth five times over the forehead skin or fur within an area of 5 by 10 cm, and then suspended in 1 ml of 0.9% NaCl solution. All samples were kept at 4°C for a maximum of 24 h before being analyzed.
PCR.
All specimens were tested without previous DNA extraction. The final volume of the PCR solution (25 μl) contained 0.75 μM (each) FAP59 and FAP64 primers (13), 0.2% bovine serum albumin (Sigma-Aldrich, Steinheim, Germany), 0.2 mM (each) deoxynucleoside triphosphate, 0.625 U of AmpliTaq Gold DNA polymerase, GeneAmp PCR buffer II, 3.5 mM MgCl2 (Applied Biosystems, Warrington, United Kingdom), and 5 μl of the sample. Forty-five cycles of amplification were performed after denaturation for 10 min at 94°C; each cycle consisted of 94°C for 90 s, 50°C for 90 s, and 72°C for 90 s. H2O without DNA was included as a negative control. HPV20 (a clinical sample) served as positive control. The size of the amplicons was approximately 480 bp.
Cloning and sequence analysis.
PCR amplicons were cloned into the pCR-script SK(+) cloning vector (Stratagene, La Jolla, Calif.). Five clones from each PV-positive sample were sequenced (Big Dye Terminator cycle sequencing kit; Perkin-Elmer) and analyzed with a Perkin-Elmer 373A automated sequencer with both forward and reverse primers. The forward and reverse complementary sequences were aligned with MacMolly computer software (version 3.8).
Two samples (from one ruffed lemur and one animal keeper) could not be cloned, despite several attempts. Therefore, direct DNA sequencing with 4 μl of the PCR products was performed for those two samples.
The DNA sequences obtained were compared with all sequences in GenBank through the BLAST server (National Center for Biotechnology Information; http://www.ncbi.nlm.nih.gov/blast/blast.cgi).
The guidelines from the Papillomavirus Nomenclature Committee 1995 (14th International Papillomavirus Conference, Quebec City, Quebec, Canada) were followed to identify putatively new PV types (9). An isolate is defined as a new PV type if the sequence of its L1 gene displays less than 90% homology with the L1 genes of all types that are already known, it is defined as a subtype if it shows between 90 and 98% homology with a known PV type, and it is defined as a type variant if it displays greater than 98% homology. Since the PCR products obtained in our study represent only part of the L1 gene, the isolated sequences are called putative new PV types instead of PV types. The putative new HPV types described in our studies have the designation FA followed by a number. The putative animal PV types detected have designations consisting of an initial letter combination for the species they were found in, e.g., C for chimpanzee and Mf for Macaca fascicularis, followed by AA and consecutive numbers (e.g., CAA1 and MfAA1).
Phylogenetic analysis.
Phylogenetic analysis was based on multiple alignment with ClustalX (version 1.8) (14, 30), and the alignments were edited with Genedoc (version 2.4.000) (20). Phylip (version 3.5) (11, 12) was used for neighbor-joining and maximum-likelihood analyses. These programs were obtained from the website http://evolution.genetics.washington.edu/phylip/software.html. Furthermore, PAUP (version 4) (30) was used for maximum-parsimony, neighbor-joining, and bootstrap analyses.
The taxonomic system with PV supergroups A to E was used (7, 19). This system was applied to demonstrate relatedness between the PV type candidates and previously established PV types. Supergroup A includes human mucosal PV types. Supergroup C comprises ungulate PVs, supergroup D includes of bovine PV type 4 (BPV4), and supergroup E is a mixture of animal PVs and three human cutaneous PV types. Most of the human cutaneous PV types are found in supergroup B, which is divided into B1 and B2 (formerly referred to as β and γ). B1 is the large skin HPV group, and B2 is a group with only five fully characterized HPV types so far but with a large number of putative HPV types identified (1).
The region of the L1 gene amplified by the primer pair FAP59 and FAP64 was used for the phylogenetic analysis extending from nucleotide 6044 to 6480, relative to the HPV20 sequence. The following PVs were included in the phylogenetic analysis: HPV1, -4, -5, -8, -9, -12, -14, -15, -17, -19 to -25, -36 to -38, -41, -48, -49, -50, -60, -63, -65, -75, -76, and -80, Colobus monkey PV type 2, rhesus monkey PV type 1, chimpanzee PV type 1 (CPV-1), pygmy chimpanzee PV type 1, BPV-1, -2, and -4, ovine PV types 1 and 2, European elk PV (EEPV), deer PV, canine oral PV, cottontail rabbit PV, rabbit oral PV, and Mastomys natalensis (South African mouse) PV. Also included in the analysis were the putative new animal PV types: from chimpanzees, CAA1 to -13; from gorillas, GAA1 to -4; from spider monkeys, SMAA1; from Macaca fascicularis (long-tailed macaque), MfAA1 to -26; from domestic cattle (bovines), BAA1 to -5; from aurochs, AAA1; from European elk, EEAA1 to -3. The DNA sequences from previously characterized PV types were obtained from GenBank (http://www.ncbi.nlm.nih.gov/).
Nucleotide sequence accession numbers.
CAA1 to -13, GAA1 to -4, SMAA1, MfAA1 to -26, BPAA1 to -5, AAA1, EEAA1 to -3, and FA89 to -92 have been submitted to GenBank with the following accession no.: CAA1, AF488684; CAA2, AF488685; CAA3, AF488686; CAA4, AF488687; CAA5, AF488688; CAA6, AF488689; CAA7, AF488690; CAA8, AF489444; CAA9, AF489445; CAA10, AF489446; CAA11, AF489447; CAA12, AF489448; CAA13, AF489449; GAA1, AY081200; GAA2, AY081201; GAA3, AY081202; GAA4, AY081203; SMAA1, AF485374; MfAA1, AF364478; MfAA2, AF364479; MfAA3, AF364480; MfAA4, AF364481; MfAA5, AF364482; MfAA6, AF364483; MfAA7, AF364484; MfAA8, AF364485; MfAA9, AF364486; MfAA10, AF364487; MfAA11, AF364488; MfAA12, AF364489; MfAA13, AF364490; MfAA14, AF364491; MfAA15.1, AF364492; MfAA15.2, AF364493; MfAA16, AF364494; MfAA17, AF364495; MfAA18, AF364496; MfAA19, AF364497; MfAA20, AF364498; MfAA21.1, AF364499; MfAA21.2, AF364500; MfAA22, AF364501; MfAA23, AF364502; MfAA24, AF364503; MfAA25, AF364504; MfAA26, AF364505; BAA1, AF4885375; BAA2, AF4885376; BAA3, AF4885377; BAA4, AF4885378; BAA5, AF4885379; AAA1, AY081956; EEAA1, AY081957; EEAA2, AY081958; EEAA3, AY081959; FA89, AY081196; FA90, AY081197; FA91, AY081198; FA92, AY081199.
RESULTS
PV findings.
Altogether, specimens from 111 animals belonging to 19 vertebrate species were collected, analyzed, and subjected to PV type determination, which resulted in the detection of 53 putative new animal PV types from 7 of the animal species (Table 1).
TABLE 1.
PV DNA prevalence and putative new PV types isolated from 19 different animal species
| Animal | No. tested | No. PV positive | No. of putative new PV types |
|---|---|---|---|
| Chimpanzee | 11 | 9 | 13 |
| Gorilla | 4 | 3 | 4 |
| Long-tailed macaque | 16 | 14 | 26 |
| Ruffed lemur | 2 | 1 | 1a |
| Spider monkey | 2 | 2 | 1 |
| Lemur | 7 | 0 | |
| Elephant, Indian | 4 | 0 | |
| Elk | 4 | 4 | 3 |
| Reindeer | 1 | 0 | |
| Roe deer | 1 | 0 | |
| European bison | 3 | 0 | |
| Aurochs | 1 | 1 | 1 |
| Przewalski's horse | 1 | 0 | |
| Cow | 10 | 6 | 5 |
| Horse | 8 | 0 | |
| Pig | 25 | 0 | |
| Dog | 2 | 0 | |
| Cat | 5 | 0 | |
| Hen | 4 | 0 |
The PCR product of this sample was sequenced directly.
Nine of the 11 chimpanzees (82%) tested were PCR positive with FAP59 and FAP64, and 13 putative new PV types were found. All four chimpanzees from zoo 1 were PV positive, with seven different putative CPV types (CAA) being isolated (Table 2). Each chimpanzee harbored between two and four different putative CPV types, and one, CAA3, was detected in specimens from all four chimpanzees. Five of the seven chimpanzees from zoo 2 were PCR positive, and six putative new CPV types were detected (Table 2). Putative types CAA8 and CAA9 were detected in two different chimpanzees each. Four of the animals were sampled twice with 1 day in between. Three of them were PV positive in one of the two samples, while one was PV DNA positive in both samples, with two putative CPV types (CAA10 and CAA11) being isolated from the first sample and a third putative CPV type (CAA13) being isolated from the second specimen.
TABLE 2.
Putative PV types isolated from chimpanzees and gorillas
| Name of animal | Type(s) isolated on day:
|
|
|---|---|---|
| 1 | 3 | |
| Chimpanzees | ||
| Truntea | CAA1, CAA2, CAA3, CAA4 | —c |
| Trinea | CAA2, CAA3, CAA5 | — |
| Jimmya | CAA3, CAA6 | — |
| Grinnia | CAA3, CAA4, CAA7 | — |
| Marinab | Negative | CAA9, CAA10 |
| Madickenb | Negative | — |
| Malinb | CAA8 | Negative |
| Fridab | CAA8, CAA9 | Negative |
| Marianab | CAA10, CAA11 | CAA13 |
| Fiffib | Negative | — |
| Nillab | CAA12 | — |
| Gorillas | ||
| Nasibub | Negative | Negative |
| Naomib | GAA1 | Negative |
| Irisb | GAA2, GAA3 | GAA3 |
| Efatab | GAA4 | GAA4 |
Sample collected at zoo 1.
Sample collected at zoo 2.
—, no sample collected.
Three of the four gorillas were PCR positive with FAP59 and FAP64, and four putative new gorilla PV types (GAA) were found (Table 2). Each gorilla was sampled twice, with 1 day in between. Two of them were PV positive in both samples, and the same putative gorilla papillomavirus type was found in both samples (GAA3 and GAA4, respectively). One gorilla was PCR negative in both samples, while another was positive in the first sample only.
Eighty-seven percent (14 of 16) of the long-tailed macaques (Macaca fascicularis) were PCR positive, with 26 putative new Macaca fascicularis PV (MfPV; MfAA1 to -26) types identified. These positive macaques harbored between one and three putative MfPV types each. For two of these putatively new MfPV types, two variants each were found: MfAA15.1 and MfAA15.2 (99.54% homology) and MfAA21.1 and MfAA21.2 (98.86% homology). None of the putative MfPV types were detected in more than one animal each. The variants of MfAA15 and MfAA21 were also detected in different monkeys.
Both spider monkeys (Ateles geoffroyi) were PCR positive with the same putative new PV type (SMAA1) in the two animals. One of the two ruffed lemurs was positive, with one DNA sequence being obtained by direct sequencing. This sequence was compared with all sequences in GenBank through the BLAST server, and its closest DNA sequence was HPV50 (68% homology). However, the sequence was neither submitted to GenBank nor included in the phylogenetic analysis, because sequencing was successful only with the forward primer.
All four European elks were PCR positive, and the previously described EEPV type plus three putative new EEPV types (EEAA1 to -3) were identified. All four elks harbored one EEPV type or putative type each, and none of these was detected in more than one animal. The specimen from the aurochs contained one putative new aurochs PV type (AAA1).
Six of the 10 cows tested positive by PCR, and five putative new BPV types (BAA1 to -5) were identified. One or two putative BPV types were found in each of the positive cows. Each of the three putative types, BAA2, BAA3, and BAA4, was detected in two different cows, while BAA1 and BAA5 were each found in one animal only.
All seven animal keepers were PV positive, and in total 18 different PV types or putative types (FA11, FA20, FA33, FA37, FA39, FA41, FA51, FA54, FA66, FA89 to FA92, HPVvs20-4, HPVvs92-1, HPV23, HPV50, and CAA1) were isolated. From one of the animal keepers, no. 5, five different putative PV types were found (FA33, FA66, FA90, and FA91 and CAA1). Interestingly, one of these (CAA1) was also detected in a specimen from a chimpanzee. Four months later, five new specimens (from the forehead, nose, chin, and both arms) were obtained from animal keeper no. 5. The samples from the forehead, nose, and chin were PCR positive and contained the HPV19 and putative HPV types FA33, FA79, FA90, and FA91.
PCR with the primer pair FAP59 and FAP64 could not identify any PV DNA in the samples from the lemurs, elephants, reindeer, roe deer, European bison, Przewalski's horse, horses, hens, cats, dogs, or pigs (Table 1).
The 53 putative new animal types plus the 4 putative new HPV types and their closest PV relatives are presented in Table 3.
TABLE 3.
Fifty-seven putative new PV types and their closest related PV type or putative type
| Putative PV typea | Size (bp) | Closest related PV sequence | Sequence homology (%) |
|---|---|---|---|
| CAA1 | 443 | CAA5 | 68 |
| CAA2 | 443 | HPV50 | 67 |
| CAA3 | 431 | GAA2 | 75 |
| CAA4 | 218 | HPV38 | 79 |
| CAA5 | 443 | CAA1 | 68 |
| CAA6 | 440 | HPV37 | 75 |
| CAA7 | 434 | HPV4 | 70 |
| CAA8 | 440 | HPV37 | 75 |
| CAA9 | 434 | HPV34 | 73 |
| CAA10 | 221 | FA2 | 69 |
| CAA11 | 437 | HPV47 | 74 |
| CAA12 | 437 | FA28 | 79 |
| CAA13 | 437 | FA1 | 80 |
| GAA1 | 425 | HPV4 | 71 |
| GAA2 | 431 | CAA3 | 75 |
| GAA3 | 434 | HPV50 | 76 |
| GAA4 | 443 | HPV5 | 75 |
| MfAA1 | 437 | MfAA15 | 82 |
| MfAA2 | 446 | MfAA4 | 85 |
| MfAA3 | 446 | MfAA23 | 71 |
| MfAA4 | 440 | MfAA11 | 75 |
| MfAA5 | 437 | HPV65 | 68 |
| MfAA6 | 224 | FA61 | 71 |
| MfAA7 | 437 | MfAA11 | 68 |
| MfAA8 | 443 | CAA5 | 67 |
| MfAA9 | 443 | HPV60 | 68 |
| MfAA10 | 443 | HPV65 | 67 |
| MfAA11 | 440 | MfAA2 | 76 |
| MfAA12 | 437 | HPV8 | 69 |
| MfAA13 | 443 | HPV60 | 66 |
| MfAA14 | 437 | FA3 | 70 |
| MfAA15 | 431 | MfAA1 | 82 |
| MfAA16 | 437 | HPV14D | 70 |
| MfAA17 | 431 | CAA13 | 75 |
| MfAA18 | 452 | CAA5 | 69 |
| MfAA19 | 443 | FA3 | 72 |
| MfAA20 | 434 | MfAA1 | 72 |
| MfAA21 | 437 | HPV80 | 68 |
| MfAA22 | 446 | FA43 | 72 |
| MfAA23 | 440 | MfAA3 | 71 |
| MfAA24 | 434 | HPV19 | 66 |
| MfAA25 | 437 | MfAA8 | 80 |
| MfAA26 | 443 | CAA5 | 67 |
| SMAA1 | 428 | HPV18 | 66 |
| BAA1 | 425 | HPV75 | 67 |
| BAA2 | 440 | HPV38 | 65 |
| BAA3 | 221 | FA52 | 72 |
| BAA4 | 437 | CRPV | 61 |
| BAA5 | 428 | HPV17 | 64 |
| AAA1 | 437 | HPV5 | 65 |
| EEAA1 | 224 | FA7 | 68 |
| EEAA2 | 443 | HPV15 | 66 |
| EEAA3 | 443 | HPV12 | 65 |
| FA89 | 440 | FA34 | 85 |
| FA90 | 437 | FA67 | 79 |
| FA91 | 434 | FA81 | 74 |
| FA92 | 431 | FA21 | 77 |
CAA, putative chimpanzee PV type; GAA, putative gorilla PV type; MfAA, putative Macaca fascicularis PV type; SMAA, putative spider monkey PV type; BAA, putative BPV type; AAA, putative aurochs PV type; EEAA, putative European elk PV PV type; FA, putative HPV type; CRPV, cotton-tail rabbit PV.
Phylogenetic analysis.
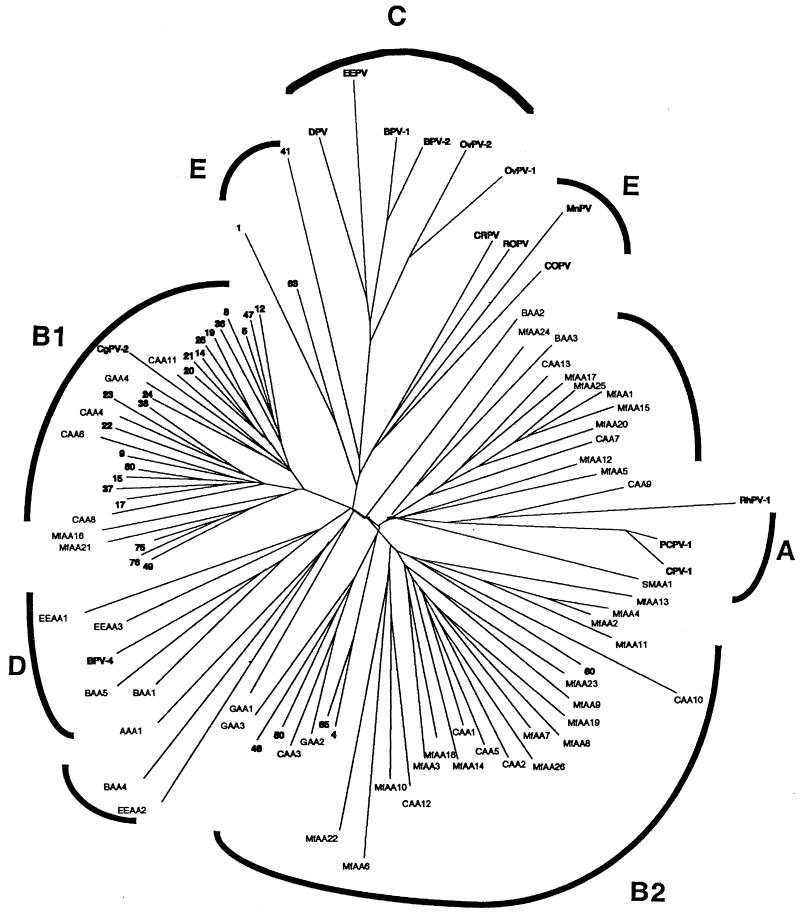
Phylogenetic trees obtained by the neighbor-joining, maximum-parsimony, and maximum-likelihood algorithms were compared. The tree in Fig. 1 shows 30 fully characterized human skin PV types, 15 fully sequenced animal PV types, and the 53 putative new animal PV types found in this study. The tree is divided into the previously described supergroups A to E plus two clusters comprising putative new animal PV types only (new tentative groups) that could not be put into existing supergroups A to E. Supergroup A consists of three previously described animal PV types together with two putative new types. Supergroup B is divided into B1 and B2; B1 comprises 23 previously known skin PV types and 7 putative new animal types, and included in B2 are 5 fully characterized human PV types together with 24 putative new animal PV types. Supergroup D comprises one previously known BPV type (BPV4) and four putative new BPV and elk PV types. No putative new PV types were found in supergroups C and E. Supergroup E was divided into two subgroups, one with animal PV types and the other with HPV types. The two new tentative groups consist of 11 and 4 putative new PV types, respectively. BAA2 does not fit into any of the above-mentioned supergroups, and it may be the first member of another putative supergroup. A phylogenetic analysis based on amino acid alignment was also performed, and the resulting tree was very much the same as that based on nucleic acid alignment.
FIG. 1.
Phylogenetic analysis of the fully characterized human skin papillomavirus types and the previously described animal papillomaviruses (both indicated in boldface), together with the 53 putative animal papillomavirus types found in this study. The tree is divided into the previously described supergroups A, B1, B2, C, D, and E, together with the two new tentative groups. The analysis was based on neighbor-joining evaluation of a segment of the L1 gene. The fully characterized HPV types are indicated by their respective numbers only. OvPV, ovine PV; ROPV, rabbit oral PV; CRPV, cottontail rabbit PV; MnPV, Mastomys natalensis PV; COPV, canine oral PV; PCPV-1, pygmy chimpanzee PV type 1; CgPV-2, colobus monkey PV type 2; DPV, deer PV.
DISCUSSION
The present study shows that not only the healthy skin of humans but also the skin of nonhuman primates and ungulates harbor subclinical PV infections. The prevalences of putative PV infection among chimpanzees (82%), gorillas (75%), and long-tailed macaques (87%) was equal to the one found in a previous study of healthy humans (1). Furthermore, from the relatively small number of samples we were able to detect a large spectrum of putative PV genotypes, all but one of them previously unknown. Thus, the ubiquitousness of skin PVs in nonhuman primates and ungulates seems to be as impressive as what has previously been shown for the human skin PVs.
Of the 19 animal species investigated here, PV DNA could not be detected in 11. However, PV DNA has been isolated previously from some of these animal species, such as elephants (27), reindeer (18), domestic cats (29), and horses (21). For the species where we were unable to detect PV DNA, specimens had been collected from only one or a few animals, except from horses and pigs, where 8 and 25 animals were analyzed, respectively. Although symptoms compatible with PV infection in pigs have been described (27), to our knowledge no swine PV type has been isolated.
No putative new animal PV types were added to supergroups C and E. We therefore aligned the PV sequences of the members of these supergroups to those of the primer pair FAP59 and FAP64. This comparison revealed between 1 and 11 mismatches between the viral sequences and that of FAP59, while 0 to 2 mismatches were seen for FAP64, most of them in the 5′ region. The exceptions were BPV-1 and -2, with one mismatch in FAP59 and two in FAP64, and EEPV, where only one mismatch per primer, both in the 5′ region, was observed. EEPV was actually identified by the FAP59 and FAP64 primers in the present study. Most mismatches were between the sequences of FAP59 and deer PV (11 mismatches). In a previous study (13), the three cutaneous HPVs in group E (HPV1, HPV41, and HPV63) tested negative with the primer pair FAP59 and FAP64. Thus, the difficulty in detecting new or previously described PVs of groups C and E is probably due to bad fitness of the FAP59 primer. Designing a new forward primer for these groups of PVs could possibly reveal new clusters of both human and animal PVs.
Interestingly, 26 putative new PV types were isolated from the laboratory-kept long-tailed macaques, but without any individual putative new MfPV type found in more than one animal. Note that these animals had not been kept in separate cages. The four European elks included in this study lived two by two in separate enclosures and were all PCR positive, each with one unique EEPV type or putative type. The same was also observed among the gorillas, which were all kept in the same enclosure.
The detection of a putative chimpanzee PV type in the skin swab sample from an animal keeper raised the question of whether this was a cross-species infection. New samples were therefore collected from the animal keeper some months after the first one, but the implicated CAA1 DNA sequence could not be detected again, although samples from five different skin sites were analyzed. By the method used here, it is not possible to determine whether the finding represented an established infection or contamination from a chimpanzee. However, to date, no PV type has been shown to have both humans and an animal species as natural hosts (6, 25), although it has been reported that some ungulate PV types can establish infection, under natural or experimental conditions, in hosts other than those they were originally isolated from (17, 23, 24).
Phylogenetic analysis revealed that almost one-half of the new putative PV types belong to group B2, representing a large expansion of this group, which previously had only five fully characterized HPV types. This small group has recently expanded substantially, with 26 putative new human skin PV types being detected with the primer pair FAP59 and FAP64 (1). Furthermore, we observed that putative PV types isolated from primates and ungulates clustered in separate branches on the phylogenetic tree. This pattern was seen in all supergroups, except for the two tentative new supergroups. One of these groups comprised 11 putative new types, 1 isolated from a cow and 10 isolated from nonhuman primates; the other group had 4 members, 3 putative ungulate PV types and 1 from a gorilla.
PVs are assumed to have evolved together with the vertebrates (3, 27), and PVs have been found in a large number of today's species, including humans. Our investigations have shown that healthy human skin harbors multiple genotypes of PV (1) and that establishment of infection with these viruses begins immediately after birth (A. Antonsson, S. Karanfilovska, P. G. Lindqvist, and B. G. Hansson, submitted for publication). Under normal conditions these infections go without clinical symptoms, but in immunosuppressed patients the amount of virus seems to increase and symptoms in the form of warts appear. Furthermore, the possible role of PVs in the development of nonmelanoma skin cancer (10, 15, 16) is far from clarified.
The ubiquity of skin PVs, which has now been demonstrated also to be true for species other than humans, ought to have a better explanation than simply chance, and these findings raise the question as to whether the nature of skin PV infections could be mutualism rather than just commensalism.
Acknowledgments
We thank Carsten Gröndahl and Bengt Holst at Copenhagen Zoo, Torsten Möller at Kolmården Animal Park, Staffan Åkeby at the Skåne Animal Park, Christel Werner, Göran Sjölin, Thomas Jönsson, Stig Janson, and Göran Holgersson for helping us with samples from the animals. Thanks are also due to Axel Janke, Division of Evolutionary Molecular Systematics, Department of Genetics, Lund University, for invaluable help with phylogenetic analysis.
This work was supported by the Cancer Foundation of Malmö University Hospital and the Alfred Österlund Foundation.
REFERENCES
- 1.Antonsson, A., O. Forslund, H. Ekberg, G. Sterner, and B. G. Hansson. 2000. The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. J. Virol. 74:11636-11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astori, G., D. Lavergne, C. Benton, B. Hockmayr, K. Egawa, C. Garbe, and E. M. de Villiers. 1998. Human papillomaviruses are commonly found in normal skin of immunocompetent hosts. J. Investig. Dermatol. 110:752-755. [DOI] [PubMed] [Google Scholar]
- 3.Bernard, H. U. 1994. Coevolution of papillomaviruses with human populations. Trends Microbiol. 2:140-143. [DOI] [PubMed] [Google Scholar]
- 4.Boxman, I. L., R. J. Berkhout, L. H. Mulder, M. C. Wolkers, J. N. B. Bavinck, B. J. Vermeer, and J. ter Schegget. 1997. Detection of human papillomavirus DNA in plucked hairs from renal transplant recipients and healthy volunteers. J. Investig. Dermatol. 108:712-715. [DOI] [PubMed] [Google Scholar]
- 5.Campo, M. S. 1997. Bovine papillomavirus and cancer. Vet. J. 154:175-188. [DOI] [PubMed] [Google Scholar]
- 6.Chan, S. Y., H. U. Bernard, M. Ratterree, T. A. Birkebak, A. J. Faras, and R. S. Ostrow. 1997. Genomic diversity and evolution of papillomaviruses in rhesus monkeys. J. Virol. 71:4938-4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan, S. Y., H. Delius, A. L. Halpern, and H. U. Bernard. 1995. Analysis of genomic sequences of 95 papillomavirus types: uniting typing, phylogeny, and taxonomy. J. Virol. 69:3074-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan, S.-Y., L. Ho, C.-K. Ong, V. Chow, B. Drescher, M. Dürst, J. ter Meulen, L. Villa, J. Luande, H. N. Mgaya, and H.-U. Bernard. 1992. Molecular variants of human papillomavirus type 16 from four continents suggest ancient pandemic spread of the virus and its coevolution with humankind. J. Virol. 66:2057-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Villiers, E. M. 2001. Taxonomic classification of papillomaviruses. Papillomavirus Rep. 12:57-63. [Google Scholar]
- 10.de Villiers, E. M., A. Ruhland, and P. Sekaric. 1999. Human papillomaviruses in non-melanoma skin cancer. Semin. Cancer Biol. 9:413-422. [DOI] [PubMed] [Google Scholar]
- 11.Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783-791. [DOI] [PubMed] [Google Scholar]
- 12.Felsenstein, J. 1982. Numerical methods for inferring evolutionary trees. Q. Rev. Biol. 57:379-404. [Google Scholar]
- 13.Forslund, O., A. Antonsson, P. Nordin, B. Stenquist, and B. G. Hansson. 1999. A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J. Gen. Virol. 80:2437-2443. [DOI] [PubMed] [Google Scholar]
- 14.Jeanmougin, F., J. D. Thompson, M. Gouy, D. G. Higgins, and T. J. Gibson. 1998. Multiple sequence alignment with Clustal X. Trends Biochem. Sci. 23:403-405. [DOI] [PubMed] [Google Scholar]
- 15.Jenson, A. B., S. Geyer, J. P. Sundberg, and S. Ghim. 2001. Human papillomavirus and skin cancer. J. Investig. Dermatol. Symp. Proc. 6:203-206. [DOI] [PubMed] [Google Scholar]
- 16.Kiviat, N. B. 1999. Papillomaviruses in non-melanoma skin cancer: epidemiological aspects. Semin. Cancer Biol. 9:397-403. [DOI] [PubMed] [Google Scholar]
- 17.Martens, A., A. De Moor, J. Demeulemeester, and L. Peelman. 2001. Polymerase chain reaction analysis of the surgical margins of equine sarcoids for bovine papilloma virus DNA. Vet. Surg. 30:460-467. [DOI] [PubMed] [Google Scholar]
- 18.Moreno-Lopez, J., H. Ahola, A. Eriksson, P. Bergman, and U. Pettersson. 1987. Reindeer papillomavirus transforming properties correlate with a highly conserved E5 region. J. Virol. 61:3394-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers, G. 1997. Alignments, p. I-3. In G. Myers, C. Baker, K. Münger, F. Sverdrup, A. McBride, and H. U. Bernard (ed.), Human papillomaviruses 1997. HPV sequence database. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 20.Nicholas, K. B., H. B. Nicholas, Jr., and D. W. Deerfield. 14October2002. Genedoc: analysis and visualization of genetic variation. EMBnet News 4:14. [Online.] http://www.hgmp.mrc.ac.uk/embnet.news/vol4_2/genedoc.html. [Google Scholar]
- 21.O'Banion, M. K., M. E. Reichmann, and J. P. Sundberg. 1986. Cloning and characterization of an equine cutaneous papillomavirus. Virology 152:100-109. [DOI] [PubMed] [Google Scholar]
- 22.Ong, C.-K., S.-Y. Chan, M. S. Campo, K. Fujinaga, P. M. Nazos, V. Labropoulou, H. Pfister, S.-K. Tay, J. ter Meulen, L. L. Villa, and H.-U. Bernard. 1993. Evolution of human papillomavirus type 18: an ancient phylogenetic root in Africa and intratype diversity reflect coevolution with human ethnic groups. J. Virol. 67:6424-6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otten, N., C. von Tscharner, S. Lazary, D. F. Antczak, and H. Gerber. 1993. DNA of bovine papillomavirus type 1 and 2 in equine sarcoids: PCR detection and direct sequencing. Arch. Virol. 132:121-131. [DOI] [PubMed] [Google Scholar]
- 24.Pfister, H., B. Fink, and C. Thomas. 1981. Extrachromosomal bovine papillomavirus type 1 DNA in hamster fibromas and fibrosarcomas. Virology 115:414-418. [DOI] [PubMed] [Google Scholar]
- 25.Shadan, F. F., and L. P. Villarreal. 1993. Coevolution of persistently infecting small DNA viruses and their hosts linked to host-interactive regulatory domains. Proc. Natl. Acad. Sci. USA 90:4117-4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shope, R. E., and E. W. Hurst. 1933. Infectious papillomatosis of rabbits. J. Exp. Med. 68:607-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sundberg, J. P. 1987. Papillomavirus infections in animals, p. 40-103. In K. Syrjänen, L. Gissmann, and L. G. Koss (ed.), Papillomaviruses and human disease. Springer-Verlag, Berlin, Germany.
- 28.Sundberg, J. P., M. K. O'Banion, A. Shima, C. Knupp, and M. E. Reichmann. 1988. Papillomas and carcinomas associated with a papillomavirus in European harvest mice (Micromys minutus). Vet. Pathol. 25:356-361. [DOI] [PubMed] [Google Scholar]
- 29.Sundberg, J. P., M. van Ranst, R. Montali, B. L. Homer, W. H. Miller, P. H. Rowland, D. W. Scott, J. J. England, R. W. Dunstan, I. Mikaelian, and A. B. Jenson. 2000. Feline papillomas and papillomaviruses. Vet. Pathol. 37:1-10. [DOI] [PubMed] [Google Scholar]
- 30.Swofford, D. L. 2002. PAUP∗. Phylogenetic analysis using parsimony (∗and other methods), 4th ed. Sinauer Associates, Sunderland, Mass.
- 31.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. Improved sensitivity of profile searches through the use of sequence weights and gap excision. Comput. Appl. Biol. Sci. 10:19-29. [DOI] [PubMed] [Google Scholar]