Abstract
Herpes simplex virus type 1 (HSV-1) protein ICP27 facilitates the export of viral intronless mRNAs. ICP27 shuttles between the nucleus and cytoplasm, which has been shown to require a leucine-rich nuclear export sequence (NES). ICP27 export was reported to be sensitive to the CRM1 inhibitor leptomycin B (LMB) in HSV-1-infected cells but not in Xenopus oocytes, where ICP27 interacts with the export factor Aly/REF to access the TAP export pathway. Here, we show that ICP27 interacts with Aly/REF in HSV-1-infected mammalian cells and that Aly/REF stimulates export of viral intronless RNAs but does not cross-link to these RNAs. During infection, Aly/REF was no longer associated with splicing factor SC35 but moved into structures that colocalized with ICP27, suggesting that ICP27 recruits Aly/REF from spliceosomes to viral intronless RNAs. Further, ICP27 was found to interact in vivo with TAP but not with CRM1. In vitro export assays showed that ICP27 export was not sensitive to LMB but was blocked by a dominant-negative TAP deletion mutant lacking the nucleoporin interaction domain. These data suggest that ICP27 uses the TAP pathway to export viral RNAs. Interestingly, the leucine-rich N-terminal sequence was required for efficient export, even though ICP27 export was LMB insensitive. Thus, this region is required for efficient ICP27 export but does not function as a CRM1-dependent NES.
Eukaryotic pre-mRNAs are synthesized in the nucleus by RNA polymerase II, after which they are processed by capping at the 5′ end, cleavage and polyadenylation to form the 3′ end, and splicing to remove intervening sequences. After processing, mRNAs must be exported through the nuclear pore complex (NPC) to the cytoplasm for translation. Export of mRNAs requires processing, packaging by RNA-binding proteins into ribonucleoprotein (RNP) complexes, recognition by export factors and translocation through the NPC. Three classes of factors appear to be required for mRNA export: adapter proteins that bind directly to the mRNA, receptor proteins that recognize and bind to adapter proteins, and NPC components termed nucleoporins that mediate export across the nuclear membrane (21, 58).
Two pathways have been uncovered that appear to be responsible for the export of mRNA. The first export receptor to be identified was CRM1, which recognizes nuclear export sequences (NES) that consist of short hydrophobic stretches that are leucine-rich. This sequence was first revealed in the HIV-1 export protein Rev and in the cellular protein PKI (7, 8). Although Rev interacts with CRM1 to export human immunodeficiency virus (HIV) env mRNA, substantial evidence has shown that CRM1 is not a major contributor to mRNA export in metazoans or yeast (6, 35). The human protein TAP and its yeast ortholog Mex67p are the best candidates for mRNA export receptors because they have been shown to shuttle between the nucleus and cytoplasm, cross-link to poly(A)+ RNA, localize at the nuclear pores, and interact directly with nucleoporins (1, 2, 16, 17). TAP was first identified as the cellular factor that interacts with the constitutive transport element (CTE) present in RNAs from type D retroviruses and TAP promotes the export of CTE-containing transcripts (2, 11, 16). In yeast, Mex67p is required for poly(A)+ RNA export and cellular viability (45, 47). Further, overexpression of TAP in Xenopus oocytes or mammalian cells stimulates mRNA export (3, 14) and inactivation of TAP in Caenorhabditis elegans by RNA interference blocked nuclear export of poly(A)+ RNA (56), indicating a direct role in mRNA export.
In metazoans, pre-mRNA splicing is required for rapid and efficient mRNA export (25, 27). Recent studies have begun to unravel the mechanism that couples these two processes. Aly/REF, the metazoan homolog of the yeast export factor Yra1p (53, 59), interacts directly with TAP (55) and is recruited to pre-mRNA sites near exon junctions (23, 24) as part of a multiprotein exon-junction complex (EJC) by a DEAD-box helicase termed UAP56, which functions in spliceosome assembly (28). Aly/REF becomes bound to the spliced mRNP (62) and excess Aly/REF increased the rate and efficiency of mRNA export in vivo (40, 62). In metazoans, intronless mRNAs, which do not associate with spliceosomes and do not acquire EJCs containing Aly/REF are exported less efficiently. Most transcripts encoded by herpes simplex virus 1 (HSV-1) are intronless and therefore require a means to access cellular export pathways efficiently. HSV-1 encodes an essential regulatory protein termed ICP27, which mediates the export of viral intronless mRNAs (41). ICP27 shuttles between the nucleus and cytoplasm (31, 38, 41, 50), and a leucine-rich NES was reported to be required for its export (41). ICP27 cross-links to viral intronless mRNAs in the nucleus and cytoplasm (41), and an RGG motif is required for RNA binding in vivo and in vitro (30, 41). It has been reported that export of ICP27 is sensitive to the CRM1 inhibitor leptomycin B (LMB), suggesting that ICP27 utilizes the CRM1 pathway (51, 52). Further, export of some HSV-1 mRNAs was blocked by LMB, whereas others were exported in the presence of LMB (51), suggesting that another pathway is also utilized by viral mRNAs. In contrast, by using comicroinjection of viral RNA and recombinant ICP27 into Xenopus oocytes, Koffa et al. (20) showed that ICP27 interacts with Aly/REF to recruit TAP and, further, that ICP27 export was insensitive to LMB. Coinjected HSV-1 late mRNAs were exported by the TAP pathway.
To determine which export pathway, CRM1 or TAP, is utilized predominantly by ICP27 in HSV-1-infected mammalian cells, we investigated the interaction of ICP27 with the export factors Aly/REF, TAP, and CRM1. In a yeast two-hybrid screen, we identified Aly/REF as an ICP27-interacting partner. Coimmunoprecipitation and colocalization studies confirmed the interaction in virus-infected cells. Interestingly, the subcellular localization of Aly/REF was altered during infection. Aly/REF colocalizes with splicing factors because of its association with spliceosomes (62). ICP27 inhibits host cell splicing and causes a redistribution of splicing factors (4, 4, 13, 37, 44; K. S. Sciabica, Q. Dai, and R. M. Sandri-Goldin, submitted for publication). Although Aly/REF colocalized with the splicing factor SC35 early in infection, it did not colocalize with redistributed SC35 as infection proceeded. Overexpression of Aly/REF stimulated export of HSV-1 late mRNAs and an ICP27 mutant lacking the Aly/REF binding region was unable to export mRNA. These data suggest that ICP27-mediated inhibition of splicing and its interaction with the spliceosome also results in its recruitment of Aly/REF from pre-mRNAs undergoing splicing to HSV-1 intronless RNAs. Further, by using in vitro export assays to quantify export in the presence or absence of inhibitors, we found that ICP27 export was not sensitive to LMB. However, ICP27 interacted with TAP in vivo and overexpression of a TAP deletion mutant that cannot interact with nucleoporins inhibited ICP27 export. We conclude that TAP is the major pathway used by ICP27 to export HSV-1 late mRNAs. On the other hand, the leucine-rich N-terminal sequence was found to be required for efficient export of the protein, although it does not function as a CRM1-dependent NES.
MATERIALS AND METHODS
Cell lines, viruses, and recombinant plasmids.
Rabbit skin fibroblast (RSF) and 293 cells were grown in minimal essential medium containing 10% fetal calf serum. HSV-1 wild-type strain KOS, ICP27 null mutant 27-LacZ and temperature-sensitive mutant tsLG4 were previously described (49). ICP27 mutant plasmids pCMV-D2ΔS5, pCMV-TAG, pCMV-R1, pΔNLS, pCMV-S5, and pCMV-H17 were described previously (15, 61), as were pΔNES, pICP27GFP, pRevGFP, and pRevNES (41). pRevGFPβ and pICP27GFPβ were constructed by inserting the β-galactosidase coding sequence in-frame between green fluorescent protein (GFP) and the LEF NLS in pRevGFP and pICP27GFP, respectively. In pFlag-Aly/REF, the DNA sequence encoding the Flag epitope was ligated in frame to Aly/REF cDNA from the HeLa library screen. pEGFP-Aly/REF was created by ligating Aly/REF cDNA in frame into pEGFP-C3 (Clontech). pEGFP-TAP was provided by E. Izaurralde (11) and pEGFP-hCRM1 was provided by M. Yoshida (22). Yeast plasmids pDBD-Rsr27, pDBD-R+C27, pDBD-179-512, pDBD-C27, and pDBD-Rsr27ΔC have been described (60). pDBD-NLS/R1/R2 was constructed by inserting a DrdI-SalI fragment of the ICP27 gene in frame into pGBT9 (Clontech). A HeLa cDNA library fused to the Gal4 activation domain (Clontech) was screened as described previously (Sciabica et al., unpublished).
Transfection, infection, and immunoprecipitation procedures.
Cells were transfected with Lipofectamine (Life Technologies) and were infected 24 h later with HSV-1 KOS or 27-LacZ as described previously (61). For experiments in which cells were radiolabeled, [35S]methionine (NEN Dupont) was added beginning 1 h after infection as described previously (61). Nuclear and cytoplasmic extracts were prepared in extraction buffer containing 400 mM NaCl as described previously (41, 60). When extracts were treated with RNase, 2.5 U of RNase A, and 200 U of RNase T1 (Ambion) were added in the presence of protease inhibitors (4 mM Pefabloc and 0.1 μg of leupeptin/ml), and extracts were incubated at 37°C for 1 h before the addition of antibodies. Immunoprecipitations were performed with anti-ICP27 monoclonal antibodies H1119 or H113 (Goodwin Institute) or anti-Flag monoclonal antibody M2 (Sigma) as described previously (43). Extracts were precleared with rabbit anti-mouse immunoglobulin G secondary antibody (Pierce) bound to protein A-Sepharose (Amersham Pharmacia) before primary antibody was added. Proteins were fractionated on sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred to nitrocellulose. Radiolabeled proteins were detected by autoradiography. Blots were subsequently probed with anti-ICP27 or anti-Flag antibodies and detected by enhanced chemiluminescence (ECL).
Immunofluorescent microscopy.
Cells grown on coverslips were transfected and infected with HSV-1 KOS or 27-LacZ as indicated in each figure. Cycloheximide (CH; 100 μg/ml), actinomycin D (ActD; 10 μg/ml), and LMB (25 ng/ml) were added 5 h after infection for 4 h. Cells were fixed in 3.7% formaldehyde, and immunofluorescent staining was performed with anti-ICP27 H1119 or H1113, anti-SC35, or anti-hnRNP A1 4B10 antibodies as described previously (41). Green fluorescent protein-tagged proteins (GFP and enhanced GFP [EGFP]) were visualized directly by fluorescent microscopy. Cells were viewed by fluorescent microscopy at ×100 magnification with a Zeiss Axiovert S100 microscope. Images were pseudocolored and merged by using Adobe Photoshop.
RNA purification, UV irradiation, and RNase protection assays.
Cells were transfected with pFlag-Aly/REF and infected with HSV-1 KOS. When pFlag-Aly/REF was transfected along with pCMV-ICP27, pCMV-R1, or pCMV-L/R, cells were infected with the viral mutant 27-LacZ, which does not express ICP27. At the times indicated, cells were harvested and nuclear and cytoplasmic RNA fractions were prepared and RNase protection analyses were performed as described previously (41, 42). The antisense RNA probes for ICP27, gB, gC, gD, UL49, and thymidine kinase were described previously (15, 41). The 220-nucleotide (nt) antisense Aly/REF probe was transcribed from a T7 transcription plasmid encoding the N-terminal region of Aly/REF. UV irradiation, cell fractionation, and immunoprecipitation of RNA-protein complexes were performed as described previously (41, 42).
In vitro nuclear export assay.
Cells were infected with KOS or transfected with the plasmids indicated, followed by infection with 27-LacZ. Nuclear export assays were carried out 6 h after infection unless otherwise indicated. Cytoplasmic membranes were permeabilized with 40 μl of digitonin (Calbiochem)/ml in transport buffer (20 mM HEPES [pH 7.3], 110 mM potassium acetate, 2 mM magnesium acetate, 1 mM EDTA, 2 mM dithiothreitol). Protease inhibitors, aprotinin, leupeptin, pepstatin A, and trypsin inhibitor (1 μg/ml; Sigma) were added at the time of permeabilization and were present throughout the assay. Cell monolayers were washed three times with cold transport buffer after treatment with digitonin to allow cytoplasmic factors to leak out. After being washed, 50% rabbit reticulocyte lysate (RRL) and an ATP regeneration system (1 mM ATP, 0.2 mM GTP, 5 mM creatine phosphate, 20 U of creatine phosphokinase/ml) was added, and the assay was performed at 30°C in a humidified chamber. Assays were stopped by adding cold transport buffer. Exported proteins were washed away with two ice-cold washes. Proteins retained in the nucleus were harvested by direct lysis of the cells in 2× SDS-PAGE loading buffer (43). Western blot analysis was performed with anti-ICP27 antibodies at a dilution of 1:10,000. The secondary antibody, horseradish peroxidase-conjugated sheep anti-mouse monoclonal antibody was used at a dilution of 1:100,000. Proteins were detected by ECL.
RESULTS
ICP27 interacts with the cellular export factor Aly/REF.
To identify potential interacting partners of ICP27, we screened a HeLa cDNA library by yeast two-hybrid analysis. Of 2.8 × 106 cotransformants screened, 32 colonies grew on His− medium and produced β-galactosidase, thus meeting the criteria for positive interaction. Compared to GenBank entries, one cDNA was identified as encoding a protein homologous to the murine RNA export factor (REF) termed Aly/mREF1-I (55). Further, the predicted protein sequence was nearly identical to the human REF/Aly protein (27, 55). We will refer to this protein as Aly/REF. In a similar two-hybrid screen, Koffa et al. (20) also identified Aly/REF as an ICP27-interacting partner.
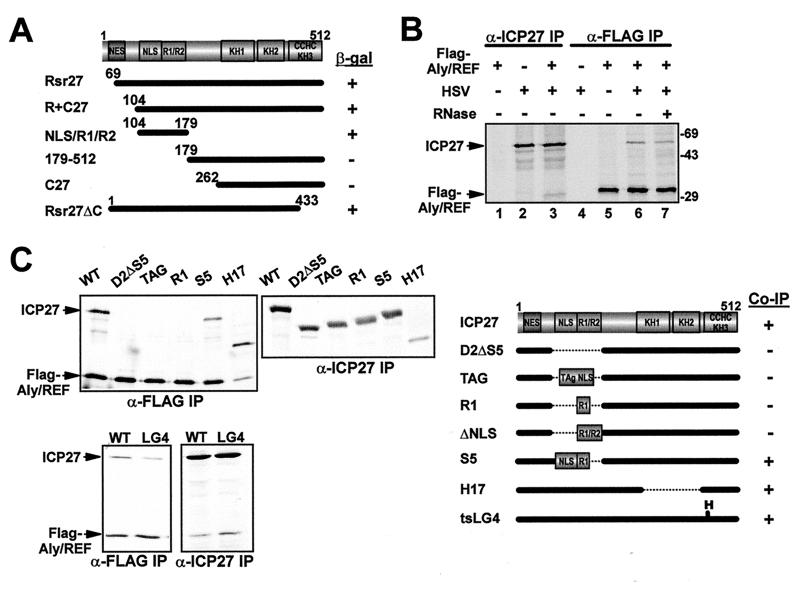
To uncover the region of ICP27 required for the interaction, we cotransformed various deletions of ICP27 with full-length Aly/REF in yeast (Fig. 1A). The region encompassing amino acids 104 to 179, which contains the nuclear localization signal (NLS) and two arginine-rich regions (R1 and R2) was both required and sufficient for interaction with Aly/REF (Fig. 1A). The interaction was confirmed in HSV-1-infected mammalian cells by coimmunoprecipitation experiments (Fig. 1B). Cells were transfected with a plasmid encoding Flag epitope-tagged Aly/REF and subsequently were infected with wild-type HSV-1 KOS (Fig. 1B). ICP27 was seen to coimmunoprecipitate with Flag-Aly/REF in the presence (lane 7) and absence (lane 6) of RNase, and Flag-Aly/REF was seen to coimmunoprecipitate with ICP27 (lane 3). This suggests that the ICP27-Aly/REF interaction does not occur through RNA bridging. To verify the region of ICP27 required for the interaction, we performed immunoprecipitation experiments on extracts from cells that coexpressed Flag-Aly/REF and wild-type or mutant forms of ICP27 (Fig. 1C). Aly/REF was efficiently coprecipitated with mutant H17, which has amino acids 288 to 444 deleted and with mutant LG4, which has a substitution at position 480. This confirms that the interaction with Aly/REF does not require that the C-terminal half of ICP27 be intact. In contrast, Aly/REF did not coimmunoprecipitate with D2ΔS5, which has amino acids 104 to 178 deleted. This corresponds to the region required for interaction with Aly/REF in yeast (Fig. 1A). However, this region includes the NLS of ICP27 (29) and D2ΔS5 is inefficiently localized to the nucleus (15). To exclude this as the reason for the lack of interaction, we tested a mutant termed TAG, which has the NLS from simian virus 40 T antigen inserted into this region and which is efficiently imported (15). TAG did not coimmunoprecipitate with Aly/REF, indicating that all or part of the region between amino acids 104 to 178 was required for interaction (Fig. 1C). This was further delineated with S5, which has a deletion of residues 154 to 178 and which coimmunoprecipitated with Aly/REF. We conclude that amino acids 104 to 153 of ICP27 are necessary for the interaction with Aly/REF in mammalian cells. In vitro binding assays were used by Koffa et al. (20) to map the interaction region to amino acids 10 to 138. By combining our results with theirs, the region of ICP27 required for interaction resides between residues 104 and 138. Interestingly, the NLS of ICP27 lies within residues 110 to 138 (29), suggesting that this region is important both for import and export.
FIG. 1.
ICP27 interacts with Aly/REF. (A) The coding region of ICP27 is shown schematically, including the leucine-rich putative NES, NLS, two arginine-rich regions (R1 or R2), three putative KH domains, and a zinc finger-like motif (CHCC). ICP27 mutations fused to the Gal4 DNA-binding domain were tested for interaction with Aly/REF fused to the Gal4 activation domain as measured by β-galactosidase production. (B) 293 cells were transfected with pFlag-Aly/REF and then mock- or HSV-1 KOS-infected 24 h later. Cells were labeled with [35S]methionine for 5 h beginning 1 h after infection. Extracts were immunoprecipitated with anti-Flag or anti-ICP27 antibodies in the presence or absence of RNase as indicated. (C) 293 cells were cotransfected with pFlag-Aly/REF and plasmids expressing mutants D2ΔS5, ΔNLS, R1, S5, or H17 and later infected with 27-LacZ or the ICP27 viral mutant, tsLG4 at 39.5°C. Labeling with [35S]methionine and immunoprecipitations with anti-ICP27 and anti-Flag antibodies were performed as described above. The positions of the mutants tested for interaction by immunoprecipitation, and the results are shown schematically in the right panel.
Aly/REF colocalizes with ICP27 during infection.
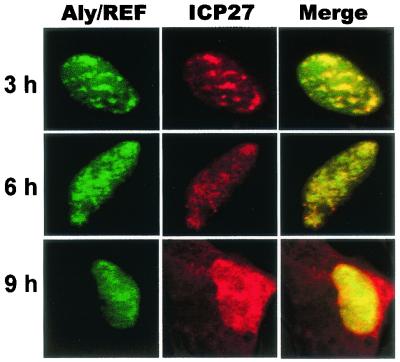
To further analyze the interaction between ICP27 and Aly/REF during viral infection, colocalization studies were performed (Fig. 2). Cells were transfected with a plasmid encoding EGFP-Aly/REF and were subsequently infected with HSV-1 KOS. The merged images show that EGFP-Aly/REF and ICP27 colocalized in the nucleus in coalesced structures over a background of more diffuse staining at 3 and 6 h after infection (Fig. 2). At 9 h when ICP27 is actively shuttling, EGFP-Aly/REF colocalized with ICP27 over regions of the nucleus but was not seen in the cytoplasm. Although Aly/REF has been reported to shuttle to the cytoplasm (27, 55), it was not found associated with exported mRNA and is thought to dissociate soon after export and to be rapidly reimported (23).
FIG. 2.
Aly/REF colocalizes with ICP27. RSF cells were transfected with pEGFP-Aly/REF and were infected with HSV-1 KOS 24 h later. Cells were fixed at the times indicated. Immunofluorescence staining was performed with anti-ICP27 antibody H1119. EGFP expression was detected by direct fluorescence.
Aly/REF does not localize with splicing factor SC35 as HSV-1 infection proceeds.
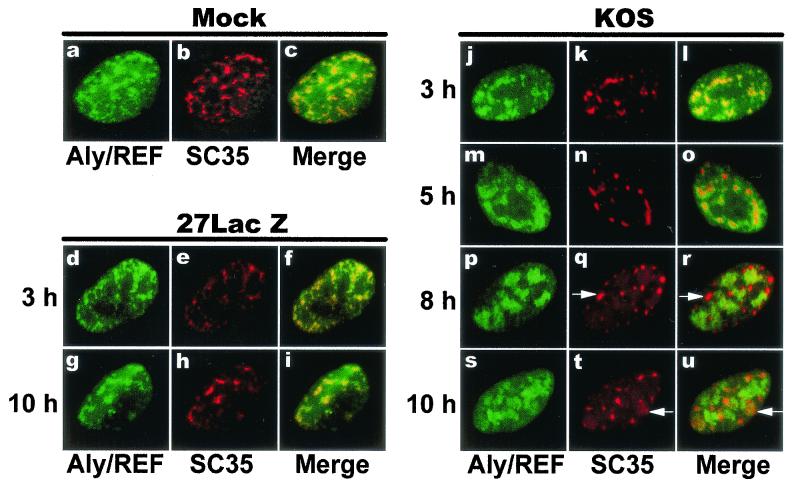
Aly/REF copurifies with the spliceosome (34) and colocalizes with splicing factors (62), and it has been shown to be deposited as part of a protein complex, termed the EJC, on RNA undergoing splicing at a conserved position upstream of exon-exon junctions (24). ICP27 mediates splicing inhibition (4, 12, 13) and causes a redistribution of splicing factors from speckles into rounded, coalesced structures (37, 44; Sciabica et al., unpublished). We looked at the distribution of Aly/REF and the SR splicing protein SC35 in HSV-infected cells. EGFP-Aly/REF colocalized with SC35 speckles in mock-infected cells (Fig. 3c) as reported previously (62). Similar colocalization was seen with the null mutant 27-LacZ (Fig. 3f and i). In contrast, in HSV-1 KOS-infected cells, coalesced ball-like SC35 structures were seen by 6 h after infection and EGFP-Aly/REF was largely excluded from these structures (Fig. 3o, r, and u). Because Aly/REF and ICP27 were seen to colocalize after infection (Fig. 2), these results suggest that ICP27 recruits Aly/REF from spliceosomes to HSV-1 intronless RNAs.
FIG. 3.
Aly/REF colocalization with splicing factor SC35 is altered during HSV infection. RSF cells were transfected with pEGFP-Aly/REF and were later mock infected (panels a to c) or infected with the ICP27 null mutant, 27-LacZ (panels d to i) or wild-type KOS (panels j to u). At the times indicated, cells were fixed and stained with anti-SC35 monoclonal antibody. EGFP fluorescence was detected directly. The merged images show areas of colocalization in yellow. Arrows in panels q, r, t, and u show examples of SC35 structures that do not contain Aly/REF.
Overexpression of Aly/REF stimulates export of HSV-1 late mRNAs.
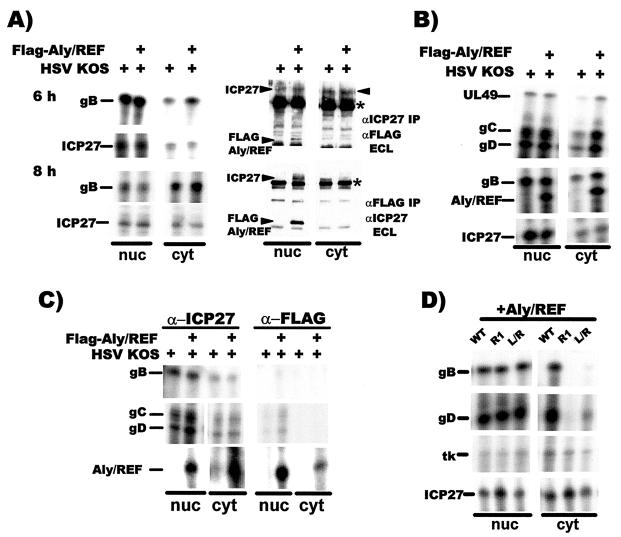
ICP27 facilitates the export of viral intronless mRNAs (20, 41, 50). Further, coinjection of recombinant ICP27 and Aly/REF into Xenopus oocytes stimulated export of two viral mRNAs, but this did not occur with a mutant lacking the Aly/REF interaction region (20). To determine whether Aly/REF plays a role in HSV-1 mRNA export in virus-infected cells, we examined the export of four late intronless mRNAs in cells that were transfected with Flag-Aly/REF and were subsequently infected with HSV-1 KOS. At 6 and 8 h after infection, there was a two- to threefold stimulation in export of gB RNA in cells in which Aly/REF was overexpressed (Fig. 4A, left panels), whereas levels of ICP27 RNA, used as a loading control, were equivalent in the nucleus and cytoplasm in the presence or absence of transfected Aly/REF. The purity of the fractionation was tested by immunoprecipitating portions of the nuclear and cytoplasmic fractions from the 8-h samples with anti-ICP27 or anti-Flag antibodies. Blots were first probed with the antibody used for immunoprecipitation, and proteins were detected by ECL. After the signals were allowed to decay, the blots were subsequently probed with other antibody as indicated (Fig. 4A, right panels). In this manner, both ICP27 and Flag-Aly/REF could be visualized on the same blots where both were present. ICP27 was equivalently expressed in the presence or absence of Flag-Aly/REF and was present in both the nuclear and cytoplasmic fractions because, as shown below, it is actively shuttling at late times after infection (upper right panel). In accord with immunofluorescence results (Fig. 2), Flag-Aly/REF was detected only in the nuclear fraction (lower right panel). Further, these results also showed that ICP27 coimmunopreciptated with Flag-Aly/REF and that Flag-Aly/REF coimmunoprecipitated with ICP27.
FIG. 4.
Overexpression of Aly/REF facilitates export of viral intronless mRNAs. (A) RSF cells were transfected with pFlag-Aly/REF or a control vector plasmid and were infected with HSV-1 KOS. Nuclear and cytoplasmic RNA fractions were prepared and analyzed by RNase protection with probes specific for gB or ICP27 mRNA (left panels). Portions of the 8-h nuclear and cytoplasmic fractions were immunoprecipitated with anti-ICP27 or anti-Flag antibody as indicated (right panels). Blots were subsequently probed first with the antibody used in the immunoprecipitation and detected by ECL. The signals were allowed to decay and the blots were then probed with the other antibody as indicated and again detected by ECL. Asterisks mark heavy-chain immunoglobulin G from the immunoprecipitation that reacted with the secondary antibody used in the immunoblot analysis. (B) Transfected RSF cells expressing Flag-Aly/REF were infected with KOS for 6 h, and nuclear and cytoplasmic RNA fractions were analyzed by RNase protection with probes for UL49, gB, gC, gD, Aly/REF, and ICP27 mRNAs. (C) Transfected RSF cells were infected with KOS, and UV cross-linking was performed 6 h later as described previously (41, 42). RNA-protein complexes were immunoprecipitated with anti-ICP27 or anti-Flag antibodies and RNase protection analysis was performed with probes for gB, gC, gD, and Aly/REF mRNAs as described previously (41). (D) RSF cells were transfected with pFlag-Aly/REF and pCMV-ICP27 or mutants pCMV-R1 or pCMV-L/R and were infected with 27-LacZ virus 24 h after transfection. Nuclear and cytoplasmic RNA fractions prepared 6 h after infection were analyzed by RNase protection.
In a separate experiment, export of gB, UL49, gC, and gD late RNAs was increased two- to threefold 6 h after infection in cells expressing Flag-Aly/REF (Fig. 4B) relative to cells that were not. Further, Aly/REF RNA from the transfected Flag-Aly/REF cDNA was also efficiently exported. Endogenous Aly/REF RNA was not detected in the rabbit fibroblast cells, either because of differences in sequence homology between the probe derived from the human Aly/REF clone or because endogenous Aly/REF was expressed at levels far below those of the transfected cDNA or the viral mRNAs. Again, ICP27 RNA was found in equivalent amounts in the cytoplasmic fractions of cells transfected with Aly/REF and those that were not.
ICP27 has been cross-linked to several viral intronless RNAs (41). Further, Aly/REF binds to cellular transcripts. To determine whether Aly/REF facilitates viral RNA export by its interaction with ICP27 or by binding directly to viral transcripts, UV cross-linking was performed on HSV-1 KOS-infected cells that were or were not transfected with Flag-Aly/REF (Fig. 4C). RNA-protein complexes were purified from nuclear and cytoplasmic fractions by immunoprecipitation with anti-ICP27 or anti-Flag antibody as described previously (41). The viral RNAs for gB, gC, and gD were recovered in both the nuclear and cytoplasmic bound RNA fractions that precipitated with anti-ICP27 antibody, indicating that ICP27 directly bound to these RNAs (Fig. 4C). Aly/REF RNA was also recovered in the ICP27-bound fractions. In contrast, bound gB RNA was not detected in the anti-Flag immunoprecipitated samples, and gC and gD RNAs were not detected in the cytoplasmic fraction and were detected in the nuclear fraction at levels just above background. It is likely that this may be due to ICP27 binding to the RNA and to Aly/REF's interaction with ICP27. However, Flag-Aly/REF bound efficiently to its own transcript (Fig. 4C). These results indicate that Aly/REF does not bind directly to HSV-1 intronless mRNAs to facilitate their export but instead binds to ICP27, which in turn binds to viral mRNAs. This is further supported by the finding that mutant R1, which has a deletion of the Aly/REF binding region from residues 104 to 140 (15) did not export gB and gD RNA when Aly/REF was overexpressed (Fig. 4D), although the ICP27 RGG RNA-binding region was intact and R1 is imported efficiently (15). Thus, Aly/REF is a partner for ICP27-mediated RNA export.
Export of gB and gD RNA was also greatly diminished with mutant L/R, which has four leucines in the putative NES substituted with arginines and is defective in export (41). This suggests that export of viral RNA by ICP27 requires ICP27 binding to the RNA, interaction with Aly/REF, and that the leucine-rich N-terminal sequence must be intact. However, not all HSV-1 transcripts require ICP27 for efficient export. The thymidine kinase transcript encodes cis export elements that interact with hnRNP L (25, 36) and thus is not strictly dependent on ICP27 because there was little difference in its export in the presence of ICP27 compared to mutants R1 and L/R (Fig. 4D). Further, ICP27 RNA was efficiently exported, indicating that ICP27 RNA export is not dependent upon ICP27 protein.
The amount of ICP27 exported from the nucleus increases at later times after infection.
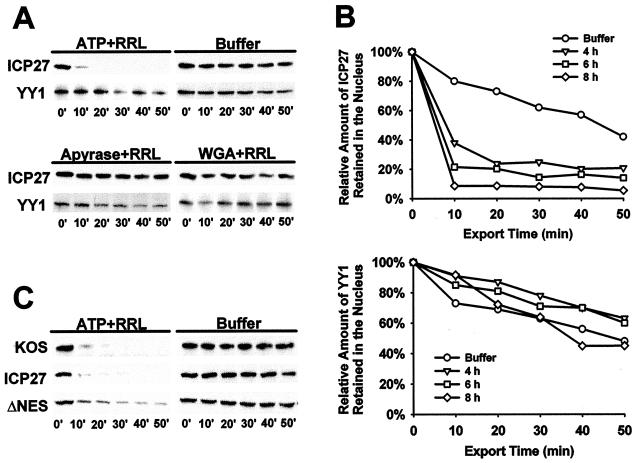
To probe which export receptor is used predominantly by ICP27 in virus-infected mammalian cells, we developed an in vitro nuclear export assay to quantify protein export. In this assay, adapted from reported in vitro export assay protocols (19, 26), HSV-1 KOS-infected cells are permeabilized with digitonin at different times after infection. This disrupts the cytoplasmic membrane so that factors required for translation, protein import, and protein export are washed away after treatment with digitonin. Factors required for protein export are restored by adding RRL and ATP. At different times after permeabilization and addition of RRL and ATP, cells were washed extensively and then suspended in SDS-PAGE sample loading buffer. Western blot analysis was used to detect the amount of ICP27 remaining in the nucleus at each time point. Under these conditions, >90% of ICP27 detected before the addition of digitonin (zero time point) was exported within 20 min, whereas YY1, a 68-kDa nuclear transcription factor that does not shuttle, remained in the nucleus (Fig. 5A). In control experiments, transport buffer alone did not support the export of ICP27. ATP hydrolysis was required because apyrase, an ATPase, inhibited export and, further, ICP27 was retained in the nucleus in the presence of wheat germ agglutinin (Fig. 5A), which blocks the NPC by its high affinity to nucleoporins (46). These results suggest that the assay conditions used can measure nuclear export of ICP27 mediated by an active transport machinery, which requires cytoplasmic factors, ATP hydrolysis and the NPC.
FIG. 5.
An in vitro nuclear export assay to quantify ICP27 export. (A) RSF cells infected with KOS for 6 h were permeabilized by adding digitonin. After depeltion of the cytoplasmic factors, 50% RRL and an ATP regeneration system (ATP) (upper left panel), transport buffer (buffer) alone (upper right panel), apyrase (20 U/ml) and RRL (lower left panel), or wheat germ agglutinin (WGA; 200 μg/ml) and RRL were added. At the times indicated, permeabilized cells were washed in ice-cold transport buffer, and proteins that remained in the nucleus were harvested by adding 2× SDS-PAGE loading buffer. Western blot analysis was performed with anti-ICP27 or anti-YY1 monoclonal antibodies, and the bands were visualized by ECL. (B) Nuclear export assays were performed at 4, 6, and 8 h after viral infection in the presence of 50% RRL and ATP. The results were quantified from scanned X-ray films by using SigmaScan (Jandel Scientific Software). (C) RSF cells were either infected with HSV-1 KOS or were transfected with pCMV-ICP27 (ICP27) or pCMV-DNES, followed by infection with 27-LacZ virus. In vitro export assays were performed 6 h after infection.
In accord with previous immunofluorescence results (50), ICP27 export was more efficient at late times. By 10 min after cells were permeabilized, <10% of ICP27 remained nuclear at 8 h after infection, whereas >25% of the protein remained nuclear 50 min after digitonin addition to cells infected for 4 h (Fig. 5B). In control experiments, endogenous YY1 remained largely nuclear throughout the time course (Fig. 5B). Export of ICP27 was similar in KOS-infected cells, where ICP27 was expressed in cis, and in cells that were transfected with a plasmid encoding ICP27, which were subsequently infected with 27-LacZ, such that ICP27 was expressed in trans (Fig. 5C). Surprisingly, deletion of the leucine-rich NES impaired but did not abolish ΔNES protein export (Fig. 5C). These results suggest that nuclear export of ICP27 is temporally regulated during lytic infection, perhaps because of the requirement for viral RNA transcripts for efficient export (20, 50). HSV-1 mRNA levels increase greatly from early to later times after infection. Further, ICP27 contains another region besides the leucine-rich NES that contributes to its export.
Export of ICP27 is insensitive to treatment with LMB.
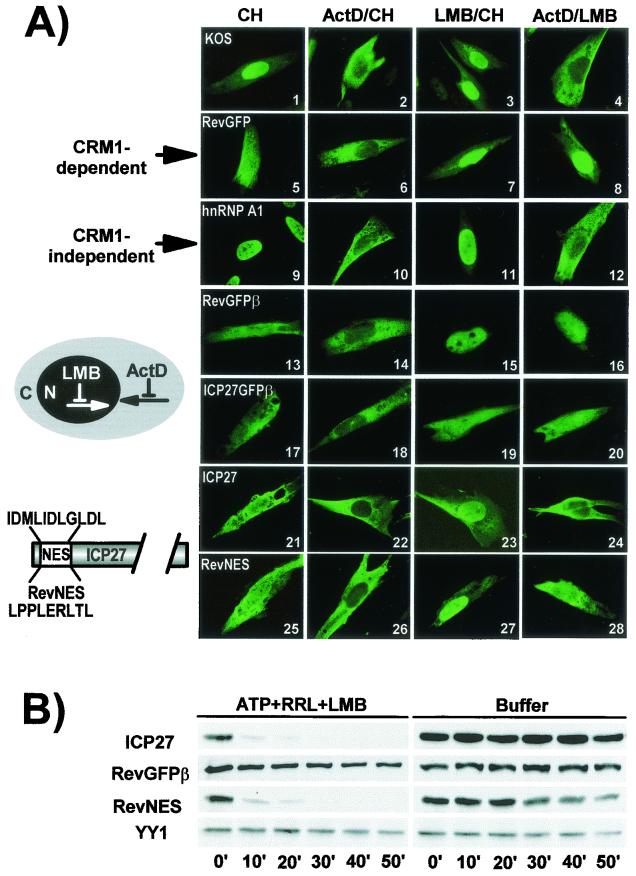
LMB inactivates CRM1 by covalent modification at a cysteine residue in the central conserved region (22). ICP27 encodes a leucine-rich sequence between amino acids 5 and 16 (41), and export was reported to be sensitive to LMB in HSV-infected cells (51, 52). However, in Xenopus oocytes, LMB did not affect export whereas coinjection of excess CTE, which saturates the TAP export receptor, blocked export (20). We examined the effect of LMB on ICP27 export in HSV-infected mammalian cells both by immunofluorescence staining and in vitro export assays. In the immunofluorescence studies, CH was added to inhibit new protein synthesis, and ActD was added to inhibit RNA polymerase II transcription. Import of some shuttling proteins appears to be inhibited by ActD, thus allowing cytoplasmic accumulation of rapidly shuttling proteins (39). In HSV-1 KOS-infected cells, ICP27 was predominantly cytoplasmic in the presence of ActD and CH, and was nearly exclusively cytoplasmic in the presence of LMB and ActD (Fig. 6A, panels 2 and 4). When LMB was added with CH and without ActD, ICP27 was predominantly nuclear, a result similar to the pattern seen with a reporter protein, RevGFP, which has the LMB-sensitive NES from HIV-1 Rev fused to GFP (panel 7). RevGFP also has an NLS from the transcription factor LEF (41). However, unlike ICP27, RevGFP was also predominantly nuclear in the presence of ActD and LMB (panel 8). The cellular protein hnRNP A1, which shuttles through an LMB-insensitive M9 sequence (33), was also confined to the nucleus when treated with LMB and CH (panel 11) but was prominently cytoplasmic when treated with LMB and ActD (panel 12). These results suggest that reimport of ICP27 and hnRNP A1 into the nucleus occurs rapidly in the absence of ActD; thus, these proteins appear to be primarily nuclear with or without LMB treatment. In contrast, the addition of ActD impedes import, thus exported protein accumulated in the cytoplasm. This may be the basis for the differences in the report by Soliman and Silverstein (52) and our results (Fig. 6A) because ActD was not added in the former study, and rapid import of ICP27 could ensue in the presence of LMB.
FIG. 6.
Export of ICP27 is not sensitive to LMB. (A) RSF cells were infected with HSV-1 KOS for 6 h (panels 1 to 4 and 9 to 12) or were transfected with pRevGFP (panels 5 to 8), pRevGFPβ (panels 13 to 16), pICP27GFPβ (panels 17 to 20), pCMV-ICP27 (panels 21 to 24), or pRevNES (panels 25 to 28). Transfected cells were infected with 27-lacZ virus. Cells were treated with CH (100 μg/ml), ActD (10 μg/ml), and LMB (25 ng/ml) as indicated beginning 5 h after infection for 4 h. Cells were fixed in 3.7% formaldehyde. Immunofluorescent staining was performed with anti-ICP27 (panels 1 to 4 and 21 to 28) and anti-hnRNP A1 antibody (panels 9 to 12). GFP fluorescence was visualized directly. (B) RSF cells were transfected with pCMV-ICP27, pRevGFPβ, or pRevNES for 24 h and then were infected with 27-LacZ. LMB (25 ng/ml) was added 5 h after infection and was present throughout the export assays.
Since RevGFP is smaller than the 63-kDa ICP27 protein, we inserted the β-galactosidase coding sequence in frame to GFP, increasing the size of the protein to 160 kDa. Export of RevGFPβ was also sensitive to LMB (panels 15 and 16). The reverse was found when the leucine-rich putative NES of ICP27 was fused to the GFPβ protein (panels 19 and 20). ICP27NES-GFPβ was strongly cytoplasmic in the presence of LMB whether ActD was added or not (panels 19 and 20), which suggests that the LEF NLS in the GFP reporter protein is not as efficient as the ICP27 NLS so that reimport is slower. This further suggests that the ICP27 leucine-rich region, whereas it is, like the Rev NES, is LMB insensitive.
To further analyze the role of the putative NES in ICP27 export, we substituted the Rev NES for the ICP27 leucine-rich region. Cells were transfected with plasmids encoding wild-type ICP27 or Rev-NES-ICP27 and were subsequently infected with 27-LacZ to replicate the conditions of viral infection, including the presence of viral transcripts. Again, wild-type ICP27 did not show sensitivity to LMB (panel 24). Rev NES showed a decreased sensitivity to LMB in the context of the ICP27 protein (compare panels 28 and 16). It is possible that the Rev sequence became partially masked within the ICP27 protein, occluding the interaction with CRM1. Alternatively, the function of the Rev NES might have been blocked by LMB, but another region of ICP27, such as the Aly/REF-binding region, was still able to contribute to export.
To quantify export after treatment with LMB, cells were transfected with plasmids expressing ICP27, RevGFPβ, and RevNES-ICP27 and were later infected with 27-LacZ and then were treated with LMB for 2 h before the addition of digitonin. No other drugs were added. ICP27 was exported efficiently in the presence of LMB (Fig. 6B). In accord with immunofluorescence results, RevGFPβ export was inhibited by LMB (Fig. 6B). In contrast, export of RevNES-ICP27 was similar to that of wild-type ICP27. Endogenous nuclear YY1 was unaffected by this treatment. We conclude that the leucine-rich NES of ICP27, which can serve as an export signal for GFPβ (Fig. 6A, panels 17 to 20) and which contributes to ICP27 export (Fig. 5C), is not LMB sensitive and therefore is unlikely to interact with CRM1. Further, ICP27 appears to encode another export signal because the Rev NES, which has been shown to interact with CRM1 (8) was not blocked by LMB in the context of the ICP27 protein.
A TAP dominant-negative mutant inhibits ICP27 export.
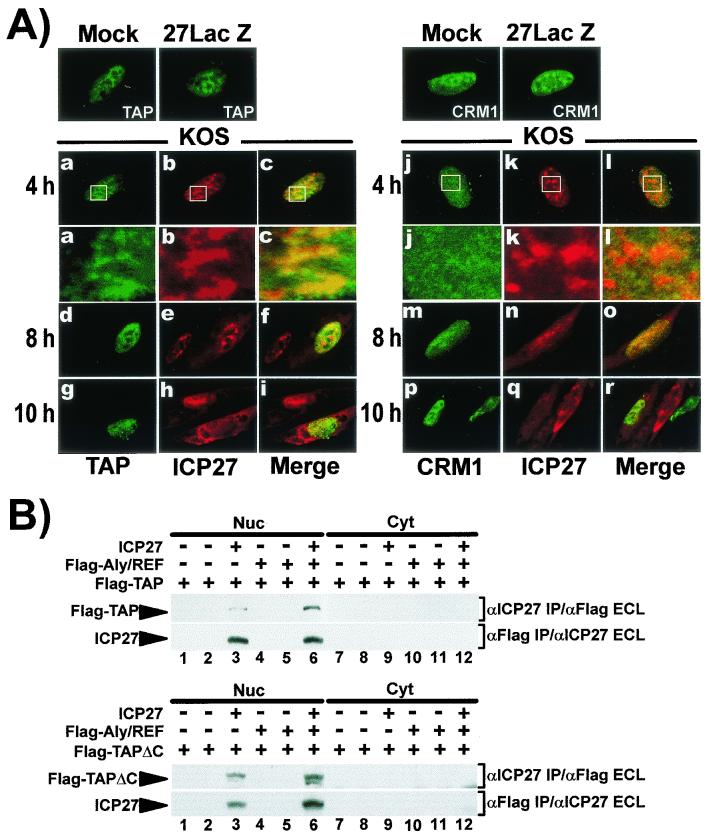
ICP27 interacts with Aly/REF and overexpression of Aly/REF stimulated export of viral mRNA (Fig. 1 and 4). Aly/REF interacts with the export receptor TAP (40, 53, 55), and ICP27 was found to coimmunoprecipitate with TAP (20). Therefore, we investigated the effect of TAP overexpression on ICP27 export. Cells were transfected with EGFP-TAP and were then infected with HSV-1 KOS. ICP27 was seen to colocalize with TAP in the nucleus of cells infected for 4 h (Fig. 7A). This can be visualized more readily in the enlargements of the area marked by rectangles in panels a to c. Further, at 8 and 10 h, ICP27 is more prominently cytoplasmic in the cells that express EGFP-TAP compared to the cells that do not (panels d to i), suggesting that ICP27 export was stimulated by TAP overexpression. In contrast, ICP27 did not appear to colocalize with EGFP-CRM1, but instead was seen in distinct structures, whereas CRM1 was diffusely distributed (panels j to l). Overexpression of EGFP-CRM1 did not appear to stimulate export because an infected cell that did not express EGFP-CRM1 showed more intense cytoplasmic staining of ICP27 than a cell that did express EGF-CRM1 (panel r). Thus, ICP27 did not colocalize with CRM1, nor was its export stimulated. In addition, we were unable to detect interaction between ICP27 and CRM1 in yeast two-hybrid assays or in coimmunoprecipitation experiments in HSV-1 KOS-infected cells (data not shown).
FIG. 7.
ICP27 interacts with TAP but not with CRM1. (A) RSF cells were transfected with pEGFP-TAP or pEGFP-CRM1 for 24 h and then were mock infected, infected with 27-LacZ, or infected with KOS. Cells were fixed at 8 h for mock and 27-LacZ samples and at the times indicated for KOS-infected cells. EGFP was detected by direct fluorescence. Enlargements of the areas marked by rectangles in panels a to c and j to l are shown beneath. (B) RSF cells were transfected with pFlag-TAP (upper panels) or pFlag-TAPΔC, which has a deletion of C-terminal residues 518 to 619 (lower panels). pFlag-Aly/REF was cotransfected where indicated. Transfected cells were mock infected (lanes 1, 4, 7, and 10), infected with 27-LacZ (lanes 2, 5, 8, and 11), or KOS (lanes 3, 6, 9, and 12) for 6 h. Nuclear and cytoplasmic fractions were immunoprecipitated with anti-ICP27 or anti-Flag antibodies as indicated. Immunoblot analysis was performed with the antibodies indicated.
We did find that ICP27 coimmunoprecipitated with Flag-TAP in KOS-infected cells (Fig. 7B, upper panels), and cotransfection with Aly/REF appeared to increase the amount of coprecipitating TAP, suggesting that Aly/REF may bridge the interaction of TAP and ICP27. TAP interacts with nucleoporins through a C-terminal domain, which is critical for its export activity (3). We deleted this domain, from residues 518 to 619, resulting in a protein that retained the regions involved in its interaction with Aly/REF and its heterodimeric partner p15 (3, 57). ICP27 coimmunoprecipitated with Flag-TAPΔC in KOS-infected cells, and Flag-TAPΔC coimmunoprecipitated with ICP27 (Fig. 7B, lower panels).
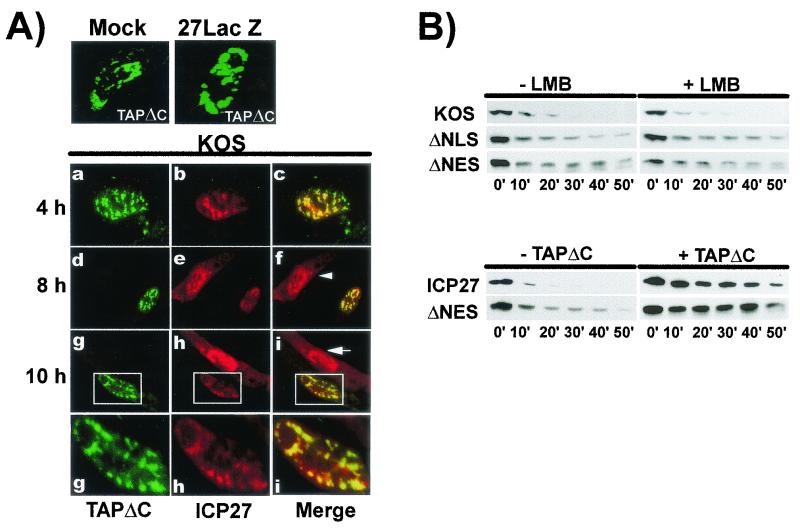
Next, we investigated the effect of overexpression of TAPΔC on ICP27 export because the C-terminal region is critical for the interaction of TAP with the NPC. EGFP-TAPΔC was transfected into cells that were then infected with HSV-1 KOS. ICP27 and EGFP-TAPΔC colocalized in regions of the nucleus at 4, 8, and 10 h after infection (Fig. 8A). Interestingly, in the cells in which TAPΔC was expressed at 8 and 10 h, ICP27 was restricted to the nucleus in structures that colocalized with TAPΔC, whereas in infected cells where TAPΔC was not expressed ICP27 cytoplasmic staining was observed (panels f and i). Thus, overexpression of TAPΔC appeared to inhibit ICP27 export.
FIG. 8.
Dominant-negative TAPΔC blocks export of ICP27. (A) RSF cells were transfected with pEGFP-TAPΔC and then were mock, 27-LacZ, or KOS infected. Cells were fixed at the times indicated. Enlargements of the areas marked by rectangles in panels g to i are shown below. Arrows mark cells where ICP27 is expressed but EGFP-TAPΔC is not. (B) In vitro export assays were performed on cells infected with KOS or transfected with pCMV-ICP27, ΔNLS, or ΔNES, followed by infection with 27-LacZ virus. In the upper panels, LMB or buffer alone (−LMB) was added 5 h after infection for 2 h, at which time the in vitro export assay was performed. In the lower panels, cells were cotransfected with pFlag-TAPΔC or with a control plasmid (−TAPΔC) and ICP27 or ΔNES and then infected with 27-LacZ virus. The export assay was performed 6 h after infection.
This was further supported by in vitro export assays. Cells were infected with KOS or were transfected with ΔNLS, an ICP27 mutant with a deletion of residues 104 to 140, which spans the NLS and the Aly/REF interaction region, or with ΔNES. Transfected cells were subsequently infected with 27-LacZ virus to replicate all conditions of viral infection. LMB had no effect on wild-type ICP27 export, as seen earlier (Fig. 8B). Although the NLS is deleted in ΔNLS, it is efficiently localized to the nucleus because of two arginine-rich sequences adjacent to the NLS (15). Notably, ΔNLS, which lacks the Aly/REF binding site, was inefficiently exported both in the presence or absence of LMB, further suggesting that this region contributes to the export activity of ICP27. ΔNES was similarly inefficiently exported confirming its role in ICP27 export and again, LMB had no effect (Fig. 8B). However, when cells were cotransfected with TAPΔC, export of ICP27 and ΔNES was blocked (lower panels). These data argue that TAP not CRM1 is the export receptor that is utilized by ICP27 in its export function. Further, although it contains a Rev-like leucine-rich sequence that contributes to its export activity, this sequence is not sensitive to LMB. Instead, ICP27 export can be completely inhibited by excess TAPΔC in a dominant-negative fashion.
DISCUSSION
It has been shown that splicing is necessary to form RNP complexes that target mRNA for export (27) and spliceosome-associated proteins have been shown to recruit the factors that imprint mRNA for export (18, 24). Further, Aly/REF has been found to associate with some mRNAs in a splicing-dependent manner (24, 62). Aly/REF and other spliceosome-associated proteins, including SRm160, Y14, DEK, and RNPS1 are deposited at a conserved position 20 to 24 nt upstream of exon-exon junctions to “license” mRNA for export and to facilitate recruitment of TAP to cellular mRNPs (5, 23, 24). Increased concentrations of Aly/REF or TAP can mediate the nuclear export of spliced mRNAs that were engineered such that they do not contain the EJC and would normally be exported inefficiently because the EJC provides a strong binding site for Aly/REF and TAP (23). Further, overexpression of Aly/REF or TAP was required to facilitate transport of inefficiently exported unspliced RNAs by directly binding these intronless mRNAs (40). It has been shown that Aly/REF is recruited to the spliced mRNP by the splicing factor UAP56, a DEAD-box helicase that functions during spliceosome assembly (28). Significantly, the yeast counterpart Sub2p is required for mRNA export through its interaction with the yeast homologue of Aly/REF, Yra1p (54), and the Drosophila homologue, HEL/UAP56, has been reported to be essential for mRNA export in Drosophila (10).
To mediate nuclear export of intronless HSV-1 mRNAs, ICP27 may utilize its interactions with the splicing machinery to access cellular export factors associated with the spliceosome. At early times after infection, ICP27 coimmunoprecipitates with several splicing complex proteins, including U1-70K (43), SR proteins (42), and SAP145 (4), and it has also been shown to colocalize with and redistribute splicing factors into rounded, coalesced structures (37, 44; Sciabica et al., unpublished). ICP27 mediates the inhibition of splicing by its interaction with splicing factors, which contributes to shut off of host protein synthesis (4, 12, 13). Beginning at ca. 6 h after infection, ICP27 begins to shuttle efficiently from the nucleus to the cytoplasm, presumably bound to intronless viral transcripts. We found that the nuclear distribution of Aly/REF changes at this time. At early times, Aly/REF colocalized with SR protein SC35 but later, Aly/REF was found in distinct structures that did not colocalize with SC35 and which resembled viral transcription or replication compartments (Fig. 3). Therefore, we postulate that, although the majority of HSV-1 transcripts are intronless, export and splicing are coupled during infection by the action of ICP27. However, rather than recruiting Aly/REF to the spliceosome, ICP27 recruits Aly/REF away from the spliceosome to sites of HSV-1 transcription. This is further supported by the finding that excess Aly/REF facilitated the export of several intronless viral mRNAs but it did not cross-link to these RNAs, indicating that RNA export was mediated by Aly/REF binding to ICP27. The finding that mutant R1, which lacks the Aly/REF binding region, but retains the RGG RNA-binding domain, failed to export viral RNAs in the presence of excess Aly/REF lends additional credence to this hypothesis.
These studies have also shown that the CRM1-dependent export pathway is not the major route for ICP27 export despite the leucine-rich signal that resembles the Rev NES. Instead, we propose that ICP27 exits the nucleus primarily through the TAP export pathway and is recruited to this pathway through the interaction with Aly/REF. That is, ICP27 binds viral intronless mRNA and recruits the cellular factors Aly/REF and TAP for export of otherwise inefficiently exported mRNAs. Koffa et al. (20) reached a similar conclusion, because they found that export of viral RNA injected into oocytes was stimulated by coinjection of recombinant ICP27 and Aly/REF and was not inhibited by LMB.
The N-terminal leucine-rich region does appear to contribute to ICP27 export because deletion or mutation of the leucines in this region resulted in inefficient export as shown here and in previous studies (41, 42). Further, the leucine-rich sequence was sufficient to confer export activity on the reporter protein GFPβ. This region is distinct from the Aly/REF binding region, which overlaps the NLS, and which also contributes to export because deletion of this region resulted in inefficient export (Fig. 8B). Thus, the putative NES is not sensitive to LMB, and ICP27 was not found to interact with CRM1. Therefore, despite its role in promoting efficient export of ICP27, the N-terminal leucine-rich region is not an NES, which is defined as a sequence that interacts with CRM1 and is sensitive to LMB (6).
Even more surprising was the finding that substitution of the Rev NES in the context of the ICP27 protein resulted in LMB insensitivity, whereas Rev-GFPβ was sensitive to LMB (Fig. 5). Thus, it is possible that the N-terminal leucine-rich region, which appears to be essential for ICP27 function during infection (52) may be important for overall protein conformation, which allows ICP27 to interact with Aly/REF and TAP. Mutation or deletion of this region may decrease interaction with Aly/REF or TAP and thus indirectly contribute to export. This implies that ICP27 encodes a unique leucine-rich region that resembles the Rev and PKI NESs and that contributes to export but not through CRM1. Rather, this region may be important for overall protein conformation. Interestingly, TAP also encodes a leucine-rich region of currently unknown function (3).
There is also the question of the regulated switch in ICP27 function from splicing inhibition to nucleocytoplasmic shuttling. Viral RNA is required for efficient export of ICP27 (20, 50) and expression of late viral mRNA peaks at 6 to 8 h after infection. We found that ICP27 is exported more efficiently at later times (Fig. 5). Thus, the timing of efficient ICP27 shuttling and the expression of viral mRNAs requiring ICP27 for export correlate. It is not clear, however, what dissociates ICP27 from spliceosomes to move to compartments synthesizing viral transcripts. One possibility is that the RGG motif, which is required for ICP27 RNA binding in vivo and in vitro, is methylated during viral infection (30). Arginine methylation has been shown to facilitate the nuclear export of hnRNP proteins (48). Methylation has also been found to direct Sm proteins to the SMN complex for assembly into snRNP core particles (9, 32). Thus, methylation of the RGG box may switch ICP27 to an export protein, perhaps by increasing its binding affinity for RNA or Aly/REF or by altering its conformation such that it dissociates from splicing proteins.
During infection, viral mRNAs compete with cellular mRNAs for access to the RNA export machinery. The model of HSV-1 intronless RNA export emerging is that early in infection, pre-mRNA splicing is inhibited by ICP27 to avoid competition for export between cellular mRNAs and viral mRNAs. Unprocessed cellular RNAs remain bound in stalled spliceosomes. ICP27 also encounters Aly/REF, which associates with spliceosomes, and through an as-yet-undefined switch, begins exporting viral transcripts to the cytoplasm by direct binding to viral RNA and by accessing the TAP pathway via its interaction with Aly/REF.
Acknowledgments
I-H.B.C. and K.S.S. contributed equally to this study.
We thank M. Yoshida for providing LMB and pEGFP-hCRM1, E. Izaurralde for providing pEGFP-TAP, and C. Dehmel for excellent technical assistance.
This work was supported by U.S. Public Health Service grant AI21515 from NIAID. K.S.S. was supported by NIAID T32-AI-07319, and I.-H.B.C. was supported by a University of California Biotechnology training grant.
REFERENCES
- 1.Bachi, A., I. C. Braun, J. P. Rodrigues, N. Pante, K. Ribbeck, C. von Kobbe, U. Kutay, M. Wilm, D. Gorlich, M. Carmo-Fonseca, and E. Izaurralde. 2000. The C-terminal domain of TAP interacts with the nuclear pore complex and promotes export of specific CTE-bearing RNA substrates. RNA 6:136-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bear, J., W. Tan, A. S. Zolotukhin, C. Tabernero, E. A. Hudson, and B. K. Felber. 1999. Identification of novel import and export signals of human TAP, the protein that binds to the Constitutive Transport Element of the type D retrovirus mRNAs. Mol. Cell. Biol. 19:6306-6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun, I. C., A. Herold, M. Rode, E. Conti, and E. Izaurralde. 2001. Overexpression of TAP/p15 heterodimers bypasses nuclear retention and stimulates nuclear mRNA export. J. Biol. Chem. 276:20536-20543. [DOI] [PubMed] [Google Scholar]
- 4.Bryant, H. E., S. Wadd, A. I. Lamond, S. J. Silverstein, and J. B. Clements. 2001. Herpes simplex virus IE63 (ICP27) protein interacts with spliceosome-associated protein 145 and inhibits splicing prior to the first catalytic step. J. Virol. 75:4376-4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullen, B. R. 2000. Connections between the processing and nuclear export of mRNA: evidence for an export license? Proc. Natl. Acad. Sci. USA 97:4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cullen, B. R. 2000. Nuclear RNA export pathways. Mol. Cell. Biol. 20:4181-4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer, U., J. Huber, W. C. Boelens, I. W. Mattaj, and R. Luhrmann. 1995. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell 82:475-483. [DOI] [PubMed] [Google Scholar]
- 8.Fornerod, M., M. Ohno, M. Yoshida, and I. W. Mattaj. 1997. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90:1051-1060. [DOI] [PubMed] [Google Scholar]
- 9.Freisen, W. J., S. Paushkin, A. Wyce, S. Massenet, G. S. Pesiridis, G. Van Duyne, J. Rappsilber, M. Mann, and G. Dreyfuss. 2001. The methylosome, a 20S complex containing JBP1 and pICln, produces dimethylarginine-modified Sm proteins. Mol. Cell. Biol. 21:8289-8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gatfield, D., H. LeHir, C. Schmitt, I. C. Braun, T. Kocher, M. Wilm, and E. Izaurralde. 2001. The DexH/D box protein HEL/UAP56 is essential for mRNA export in Drosophila. Curr. Biol. 11:1716-1721. [DOI] [PubMed] [Google Scholar]
- 11.Gruter, P., C. Tabernero, C. von Kobbe, C. Schmitt, C. Saavedra, A. Bacchi, M. Wilm, B. K. Felber, and E. Izaurralde. 1998. TAP, the human homologue of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol. Cell 1:649-659. [DOI] [PubMed] [Google Scholar]
- 12.Hardwicke, M. A., and R. M. Sandri-Goldin. 1994. The herpes simplex virus regulatory protein ICP27 can cause a decrease in cellular mRNA levels during infection. J. Virol. 68:4797-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy, W. R., and R. M. Sandri-Goldin. 1994. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J. Virol. 68:7790-7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herold, A., M. Suyama, J. P. Rodrigues, I. C. Braun, U. Kutay, M. Carmo-Fonseca, P. Bork, and E. Izaurralde. 2000. TAP (NXF1) belongs to a multigene family of putative RNA export factors with a conserved modular architecture. Mol. Cell. Biol. 20:8996-9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibbard, M. K., and R. M. Sandri-Goldin. 1995. Arginine-rich regions succeeding the nuclear localization region of the HSV-1 regulatory protein ICP27 are required for efficient nuclear localization and late gene expression. J. Virol. 69:4656-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang, Y., and B. R. Cullen. 1999. The human Tap protein is a nuclear mRNA export factor that contains novel RNA-binding and nucleocytoplasmic transport sequences. Genes Dev. 13:1126-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katahira, J., K. Straber, A. Podtelejnikov, M. Mann, J. U. Jung, and E. Hurt. 1999. The Mex-67p-mediated nuclear mRNA export pathway is conserved from yeast to human. EMBO J. 18:2593-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kataoka, N., J. Yong, V. N. Kim, F. Velazquez, R. A. Perkinson, F. Wang, and G. Dreyfuss. 2000. Pre-mRNA splicing imprints mRNA in the nucleus with a novel RNA-binding protein that persists in the cytoplasm. Mol. Cell 6:673-682. [DOI] [PubMed] [Google Scholar]
- 19.Kehlenbach, R. H., A. Dickmanns, and L. Gerace. 1998. Nucleocytoplasmic shuttling factors including Ran and CRM1 mediate nuclear export of NFAT in vitro. J. Cell Biol. 141:863-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koffa, M. D., J. B. Clements, E. Izaurralde, S. Wadd, S. A. Wilson, I. W. Mattaj, and S. Kuersten. 2001. Herpes simplex virus ICP27 protein provides viral mRNAs with access tot he cellular mRNA export pathway. EMBO J. 20:5769-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komeili, A., and E. K. O'Shea. 2001. New perspectives on nuclear transport. Annu. Rev. Genet. 35:341-364. [DOI] [PubMed] [Google Scholar]
- 22.Kudo, N., B. Wolff, T. Sekimoto, E. P. Schreiner, Y. Yoneda, M. Yanagida, S. Horinouchi, and M. Yoshida. 1998. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp. Cell Res. 242:540-547. [DOI] [PubMed] [Google Scholar]
- 23.LeHir, H., D. Gatfield, E. Izaurralde, and M. J. Moore. 2001. The exon-exon junction complex provides a binding platform for factors involved in mRNA export and nonsense-mediated mRNA decay. EMBO J. 20:4987-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeHir, H., E. Izaurralde, L. E. Maquat, and M. J. Moore. 2000. The spliceosome deposits multiple proteins 20-24 nucleotides upstream of mRNA exon-exon junctions. EMBO J. 19:6860-6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, X., and J. E. Mertz. 1995. HnRNP L binds a cis-acting RNA sequence element that enables intron-independent gene expression. Genes Dev. 9:1766-1780. [DOI] [PubMed] [Google Scholar]
- 26.Love, D. C., T. D. Sweitzer, and J. A. Hanover. 1998. Reconstitution of HIV-1 Rev nuclear export: independent requirements for nuclear import and export. Proc. Natl. Acad. Sci. USA 95:10608-10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo, M. J., and R. Reed. 1999. Splicing is required for rapid and efficient mRNA export in metazoans. Proc. Natl. Acad. Sci. USA 96:14937-14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo, M. J., Z. Zhou, K. Magni, C. Christoforides, J. Rappsilber, M. Mann, and R. Reed. 2001. Pre-mRNA splicing and mRNA export linked by direct interactions between UAP56 and Aly. Nature 413:644-647. [DOI] [PubMed] [Google Scholar]
- 29.Mears, W. E., V. Lam, and S. A. Rice. 1995. Identification of nuclear and nucleolar localization signals in the herpes simplex virus regulatory protein ICP27. J. Virol. 69:935-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mears, W. E., and S. A. Rice. 1996. The RGG box motif of the herpes simplex virus ICP27 protein mediates an RNA-binding activity and determines in vivo methylation. J. Virol. 70:7445-7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mears, W. E., and S. A. Rice. 1998. The herpes simplex virus immediate-early protein ICP27 shuttles between the nucleus and cytoplasm. Virology 242:128-137. [DOI] [PubMed] [Google Scholar]
- 32.Meister, G., C. Eggert, D. Buhler, H. Brahms, C. Kambach, and U. Fischer. 2001. Methylation of Sm proteins by a complex containing PRMT5 and the putative U snRNP assembly factor pICln. Curr. Biol. 11:1990-1994. [DOI] [PubMed] [Google Scholar]
- 33.Michael, W. M., M. Choi, and G. Dreyfuss. 1995. A nuclear export signal in hnRNP A1: a signal-mediated, temperature-dependent nuclear protein export pathway. Cell 83:415-422. [DOI] [PubMed] [Google Scholar]
- 34.Neubauer, G., A. King, J. Rappsilber, C. Calvio, M. Watson, P. Ajuh, J. Sleeman, A. Lamond, and M. Mann. 1998. Mass spectrometry and EST-database searching allows characterization of the multi-protein spliceosome complex. Nat. Genet. 20:46-50. [DOI] [PubMed] [Google Scholar]
- 35.Neville, M., and M. Rosbash. 1999. The NES-Crm1 export pathway is not a major mRNA export route in Saccharomyces cerevisiae. EMBO J. 18:3746-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otero, G. C., and T. J. Hope. 1998. Splicing-independent expression of the herpes simplex virus type 1 thymidine kinase gene is mediated by three cis-acting RNA subelements. J. Virol. 72:9889-9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phelan, A., M. Carmo-Fonseca, J. McLauchlan, A. I. Lamond, and J. B. Clements. 1993. A herpes simplex virus type 1 immediate-early gene product, IE63, regulates small nuclear ribonucleoprotein distribution. Proc. Natl. Acad. Sci. USA 90:9056-9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phelan, A., and J. B. Clements. 1997. Herpes simplex virus type 1 immediate early protein IE63 shuttles between nuclear compartments and the cytoplasm. J. Gen. Virol. 78:3327-3331. [DOI] [PubMed] [Google Scholar]
- 39.Pinol-Roma, S., and G. Dreyfuss. 1992. Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature 355:730-732. [DOI] [PubMed] [Google Scholar]
- 40.Rodrigues, J. P., M. Rode, D. Gatfield, B. J. Blencowe, M. Carmo-Fonseca, and E. Izaurralde. 2001. REF proteins mediate the export of spliced and unspliced mRNAs from the nucleus. Proc. Natl. Acad. Sci. USA 98:1030-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sandri-Goldin, R. M. 1998. ICP27 mediates herpes simplex virus RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 12:868-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandri-Goldin, R. M. 1998. Interactions between an HSV regulatory protein and cellular mRNA processing pathways. Methods 16:95-104. [DOI] [PubMed] [Google Scholar]
- 43.Sandri-Goldin, R. M., and M. K. Hibbard. 1996. The herpes simplex virus type 1 regulatory protein ICP27 coimmunoprecipitates with anti-Sm antiserum and an the C terminus appears to be required for this interaction. J. Virol. 70:108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandri-Goldin, R. M., M. K. Hibbard, and M. A. Hardwicke. 1995. The C-terminal repressor region of HSV-1 ICP27 is required for the redistribution of small nuclear ribonucleoprotein particles and splicing factor SC35; however, these alterations are not sufficient to inhibit host cell splicing. J. Virol. 69:6063-6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos-Rosa, H., H. Moreno, G. Simos, A. Segref, B. Fahrenkrog, N. Pante, and E. Hurt. 1998. Nuclear mRNA export requires complex formation between Mex67p and Mtr2p at the nuclear pores. Mol. Cell. Biol. 18:6826-6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmitt, I., and L. Gerace. 2001. In vitro analysis of nuclear transport mediated by the C-terminal shuttle domain of Tap. J. Biol. Chem. 276:42355-42363. [DOI] [PubMed] [Google Scholar]
- 47.Segref, A., K. Sharma, V. Doye, A. Hellwig, J. Huber, R. Luhrmann, and E. Hurt. 1997. Mex67p, a novel factor for nuclear mRNA export, binds to both poly(A)+ RNA and nuclear pores. EMBO J. 16:3256-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen, E. C., M. F. Henry, V. H. Weiss, S. R. Valentini, P. A. Silver, and M. S. Lee. 1998. Arginine methylation facilitates the nuclear export of hnRNP proteins. Genes Dev. 12:679-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith, I. L., M. A. Hardwicke, and R. M. Sandri-Goldin. 1992. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology 186:74-86. [DOI] [PubMed] [Google Scholar]
- 50.Soliman, T. M., R. M. Sandri-Goldin, and S. Silverstein. 1997. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J. Virol. 71:9188-9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soliman, T. M., and S. J. Silverstein. 2000. Herpesvirus mRNAs are sorted for export via CRM1-dependent and -independent pathways. J. Virol. 74:2814-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soliman, T. M., and S. J. Silverstein. 2000. Identification of an export control sequence and a requirement for the KH domains in ICP27 from herpes simplex virus type 1. J. Virol. 74:7600-7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Straber, K., and E. Hurt. 2000. Yra1p, a conserved nuclear RNA-binding protein, interacts directly with Mex67p and is required for mRNA export. EMBO J. 19:410-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Straber, K., and E. Hurt. 2001. Splicing factor Sub2p is required for nuclear mRNA export through its interaction with Yra1p. Nature 413:648-652. [DOI] [PubMed] [Google Scholar]
- 55.Stutz, F., A. Bachi, T. Doerks, I. C. Braun, B. Seraphin, M. Wilm, P. Bork, and E. Izaurralde. 2000. REF, an evolutionary conserved family of hnRNP-like proteins, interacts with TAP/Mex67p and participates in mRNA nuclear export. RNA 6:638-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan, W., A. S. Zolotukhin, J. Bear, D. J. Patenaude, and B. Felber. 2000. The mRNA export of Caenorhabditis elegans is mediated by Ce-NFX-1, an ortholog of human TAP/NFX and Saccharomyces cerevisiae Mex67p. RNA 6:1762-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weigand, H. L., G. A. Coburn, Y. Zeng, Y. Kang, H. P. Bogerd, and B. R. Cullen. 2002. Formation of Tap/NXT1 heterodimers activates Tap-dependent nuclear mRNA export by enhancing recruitment to nuclear pore complexes. Mol. Cell. Biol. 22:245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zenklusen, D., and F. Stutz. 2001. Nuclear export of mRNA. FEBS Lett. 498:150-156. [DOI] [PubMed] [Google Scholar]
- 59.Zenklusen, D., P. Vinciguerra, Y. Strahm, and F. Stutz. 2001. The yeast hnRNP-like proteins Yra1p and Tra2p participate in mRNA export through interaction with Mex67p. Mol. Cell. Biol. 21:4219-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhi, Y., and R. M. Sandri-Goldin. 1999. Analysis of the phosphorylation sites of the herpes simplex virus type 1 regulatory protein ICP27. J. Virol. 73:3246-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhi, Y., K. S. Sciabica, and R. M. Sandri-Goldin. 1999. Self interaction of the herpes simplex virus type 1 regulatory protein ICP27. Virology 257:341-351. [DOI] [PubMed] [Google Scholar]
- 62.Zhou, Z., M. J. Luo, K. Straesser, J. Katahira, E. Hurt, and R. Reed. 2001. The protein Aly links pre-messenger-RNA splicing to nuclear export in metazoans. Nature 407:401-405. [DOI] [PubMed] [Google Scholar]