Abstract
C57BL/6 (B6) mice infected with LP-BM5 retroviruses develop disease, including an immunodeficiency similar to AIDS. This disease, murine AIDS (MAIDS), is inhibited by in vivo anti-CD154 monoclonal antibody treatment. The similar levels of insusceptibility of CD40−/− and CD154−/− B6 mice indicate that CD154/CD40 molecular interactions are required for MAIDS. CD4+ T and B cells, respectively, provide the CD154 and CD40 expression needed for MAIDS induction. Here, the required CD154/CD40 interaction is shown to be independent of CD80 and CD86 expression: CD80/CD86−/− B6 mice develop MAIDS after LP-BM5 infection.
After infection with the LP-BM5 murine leukemia retrovirus isolate, certain inbred strains of mice, such as the highly susceptible C57BL/6 (B6) strain, develop a complex disease syndrome which includes immunodeficiency. This murine leukemia retrovirus mixture contains ecotropic, recombinant mink cell cytopathic-focus-inducing, and replication-negative/defective viruses, with the defective genome serving as the proximal agent causing the syndrome (2, 9, 17, 18, 31). Since many features of LP-BM5-induced disease are similar to those seen in human immunodeficiency virus-infected individuals, this syndrome has been designated murine AIDS (MAIDS). Disease similarities include activation-related parameters such as hypergammaglobulinemia (hyper-Ig), splenomegaly, and lymphadenopathy; immunodeficiency-related responses involving severely dampened T- and B-cell responses; increased susceptibility to infection, disease progression, and death when exposed to environmental pathogens that normally cause limited infections; and the development of terminal B-cell lymphomas (5, 7, 22, 23, 25, 29, 30, 32, 40).
According to previous studies, B6 mice infected by LP-BM5 after in vivo depletion of either CD4+ T cells or B cells do not develop MAIDS (7, 39, 40). These studies, however, did not address the possibility that the involvement of other cellular subsets is required for MAIDS pathogenesis and did not experimentally address the possible interaction of these subsets nor the molecular bases for interaction. Given that CD154/CD40 ligation has been shown to be critical in many immune responses involving the interaction of CD4+ T cells with professional antigen-presenting cells (APC) (1, 19, 20, 33, 35), we recently examined the involvement of CD154/CD40 interactions in the LP-BM5-induced initiation and progression of MAIDS. In one approach, Green et al. treated LP-BM5-infected mice in vivo with anti-CD154 monoclonal antibody (MAb) (13, 15). By interrupting CD154/CD40 signaling by anti-CD154 treatment during the first week postinfection (13) or starting at 3 to 4 weeks after LP-BM5 infection with a chronic MAb treatment schedule (15), MAIDS-associated splenomegaly, hyper-Ig, and immunodeficiency were efficiently inhibited. In addition, Green et al. and other researchers have shown that CD154 (14) and CD40 (14, 21) knockout (ko) B6 mice are resistant to LP-BM5-induced MAIDS. Thus, CD154/CD40 interactions are necessary for both the initiation and the progression of MAIDS. Most recently, we have defined the cellular subsets required to provide CD154 or CD40 expression for MAIDS pathogenesis (14). Using a series of adoptive transfers into B6 nude mice, we demonstrated that CD4+, but not CD8+, T cells must express CD154 for induction of MAIDS. Analogous experiments involved successful reconstitution of disease susceptibility by transfer of donor CD40+ B cells, but not CD40+ macrophages or dendritic cells, into normally MAIDS-resistant, LP-BM5-infected CD40 ko mice.
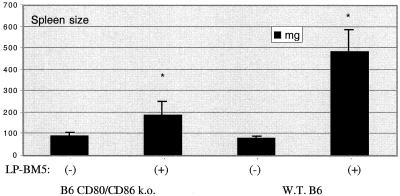
Although ligation of CD40 on professional APC induces a number of overlapping signal transduction pathways, a central paradigm is that many of the ultimate functional consequences are determined by the known upregulation of the costimulatory ligands for CD28 and CTLA-4, namely, B7-1 (CD80) and B7-2 (CD86) (6, 20, 34-36, 38). To determine whether the requirement for CD40 ligation by CD154 for MAIDS pathogenesis depends on this classic upregulation of CD80 and/or CD86, we infected B6.CD80/CD86 double-ko mice, which were originally derived as previously described (3), with LP-BM5 virus. Infected mice were assessed 6 to 11 weeks postinoculation via the standard panel of readouts by which Green et al. and other researchers have evaluated MAIDS pathogenesis (2, 5, 7, 9, 13-15, 18, 20, 31). The spleen weight for infected CD80/CD86 ko mice more than doubled relative to that of uninfected ko mice, whereas the spleen weight for infected B6 mice was about six times that for uninfected B6 mice (Fig. 1). The finding of the relative increase in spleen weight in LP-BM5-infected B6 wild-type (WT) versus B6 CD80/CD86 ko mice was repeated in a second initial experiment.
FIG. 1.
Spleen size (by weight) at 8.5 weeks postinfection of LP-BM5-infected B6 CD80/CD86 ko mice and B6 WT mice. Mean spleen sizes and standard deviations represent four mice per group. *, P < 0.05 by the Student t test.
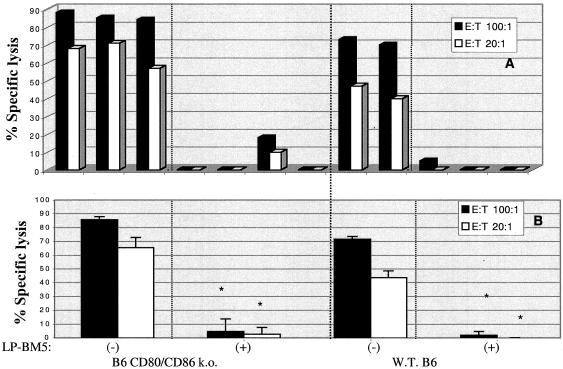
All other disease parameters, including both those indicative of the activational events and those indicative of the immunosuppressive effects, clearly indicated that B6 CD80/CD86 double-ko mice are susceptible to LP-BM5-induced MAIDS. For example, the ability to generate allogeneic CTL responses was severely impaired, as one index of T-cell immunodeficiency (Fig. 2). Spleen cells from either uninfected or infected CD80/CD86 ko or WT (H-2b) B6 mice were cultured in vitro with irradiated LB27.4 (H-2bxd) cells, and 6 days later allogeneic (anti-H-2d) CTL activity was assessed by standard 51Cr release assays. As evidenced by both analysis of individual mice (Fig. 2A) and analysis of the mean activity levels (± standard deviations) of mouse groups (Fig. 2B), similar levels of dramatic loss in the ability to generate allogeneic CTL responses were seen for the CD80/CD86 ko and WT LP-BM5-infected mice. Similar results were observed in the second initial experiment.
FIG. 2.
Allogeneic CTL response (at 8.5 weeks postinfection) of spleen cells from LP-BM5-infected B6 CD80/CD86 ko mice and B6 WT mice. At the terminal time point, mice were sacrificed and their spleen cells (H-2b) were mixed with irradiated LB27.4 tumor cells (H-2 b/d); 6 days later, the resulting effector cells were used in a 51Cr release assay against P815 target cells (anti-H-2d). Values for individual mice (A) and mean ± standard deviation (B) percent specific lysis values are shown. *, P < 0.05 by the Student t test; E:T, effector-to-target cell ratio.
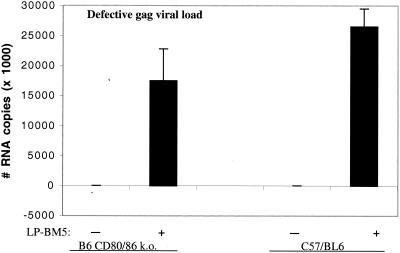
Thus, LP-BM5-infected CD80/CD86 ko mice are clearly susceptible to MAIDS pathogenesis. However, it was unclear whether their susceptibility was as complete as that seen in infected B6 mice. That is, using splenomegaly as representative of the activational events of MAIDS, less disease was seen, whereas levels of allogeneic CTL generation, along with those of lipopolysaccharide (LPS) and concanavalin A (ConA) responsiveness (data not shown), which represent the immunodeficiency parameters, suggested nearly equivalent levels of disease in these two mouse strains. To eliminate the possibility that the precise virus dose usually employed was responsible for this potential differential disease penetrance as revealed by activational versus immunosuppressive parameter values, a virus titration experiment was also conducted. The results were clear and argued against a critical role for virus dose (data not shown). Although somewhat higher or lower levels of disease were obtained by varying the inoculating virus dose, the relationship of the different disease parameters between B6 and B6.CD80/CD86 ko mice was maintained. Similarly, to address the expression and spread of the causative defective virus, the viral loads of infected B6 and B6.CD80/CD86 ko mice were assessed. RNA was isolated from splenic tissue, and reverse-transcribed cDNA was subjected to real-time quantitative PCR amplification by using previously described primers (12) with specificity for the MAIDS-defective gag gene. We found roughly similar amounts of defective gag RNA copies in LP-BM5-infected CD80/CD86 ko and B6 mice (Fig. 3), and in a repeat experiment (data not shown) there was almost no difference in the amounts of defective gag RNA copies for infected CD80/CD86 ko versus B6 mice.
FIG. 3.
Viral load in uninfected (−) and infected (+ [9 weeks postinfection]) CD80/CD86 ko and WT mouse spleen tissue. Total RNA was isolated and DNase I treated. One microgram of DNA-free RNA was reverse transcribed to cDNA by using random hexamer priming. Real-time PCR was performed for BM5-defective gag RNA by amplifying 0.5 μl (∼300 pg) of cDNA in the presence of SYBR Green stain on an iCycler iQ instrument (Bio-Rad). Amplification conditions were 95°C for 8 min followed by 40 cycles of 94°C for 15 s, 63°C for 45 s, and 72°C for 15 s. The number of PCR cycles required for SYBR green fluorescence to cross a threshold at which there was a significant increase in change in fluorescence Ct-threshold cycle) was measured by using iCycler iQ software. Total cDNA input was normalized to β-actin expression, which was measured by using primers (forward, 5′-agagggaaatcgtgcgtgac; reverse, 5′-caatagtgatgacctggccgt) in parallel PCRs. Copy numbers of BM5-defective gag RNA were determined using a standard curve that was generated by using the cloned 246-bp defective gag amplicon. In a repeat experiment, the numbers of BM5-defective gag RNA copies found in spleens of CD80/CD86 ko and B6 mice were nearly identical.
To further evaluate MAIDS disease susceptibility and its degree of severity in LP-BM5-infected B6 CD80/CD86 ko mice, it was important to more carefully consider comparisons to the disease phenotypes of infected B6.CD154 and B6.CD40 ko mice. In particular, Green et al. (14) and the laboratory of Kikutani et al. (21) have reported that while CD40−/− mice are essentially MAIDS resistant upon infection with LP-BM5, they show some limited signs of MAIDS compared to fully resistant strains such as nude and CD154−/− mice. Although all standard disease parameters were assessed, we emphasize here those not already presented in Fig. 1 and 2.
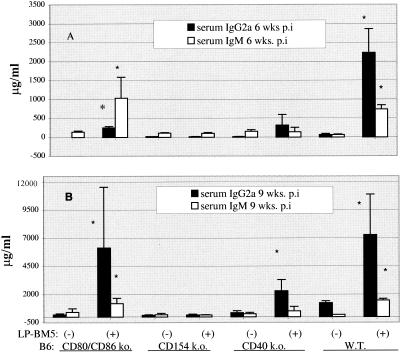
In experiments which included CD154 ko and CD40 ko mice, we again found that spleen weights for infected CD80/CD86 ko mice were about twofold higher than those of uninfected CD80/CD86 ko mice (data not shown). Along with splenomegaly as an indicator of the activational aspects of MAIDS, we measured levels of serum immunoglobulin G2a (IgG2a) and IgM as a part of the generalized hyper-Ig of MAIDS. At an early time point (6 weeks postinfection), IgM levels were about eightfold higher for infected than for uninfected CD80/CD86 ko mice (Fig. 4A), with similar results observed in a replicate experiment comparing these four mouse strains. For prototype susceptible B6 mice, the increse to a similar absolute value for 6-week IgM levels was somewhat greater at 13-fold upon infection, but this was merely due to different baseline IgM levels. The increases for CD80/CD86 ko and B6 mice were strongly indicative of the presence of MAIDS, especially in comparison to the IgM values for infected CD154 and CD40 ko mice, which were essentially the same as those for the uninfected controls. Absolute serum IgG2a levels at 6 weeks postinfection were higher for infected B6 mice than those for infected CD80/CD86 ko mice, which were similar to those for CD40 ko mice. CD40 ko mice have previously been reported to demonstrate partially elevated Ig levels upon LP-BM5 infection (14, 21). However, at 9 weeks postinfection, IgG2a and IgM levels for CD80/CD86 ko and B6 mice were similarly elevated and clearly distinguishable from those of the other ko strains, consistent with equivalent disease only in these two strains (Fig. 4B).
FIG. 4.
Serum IgM and IgG2a response (at 6 weeks [A] and 9 weeks [B] postinfection) in LP-BM5-infected B6.CD80/CD86 ko, CD154 ko, CD40 ko, and WT mice. Enzyme-linked immunosorbent assays were conducted by using goat anti-mouse IgM and IgG2a capture antibodies followed by standard reagents, as previously described (12-15). *, P < 0.05 by the Student t test.
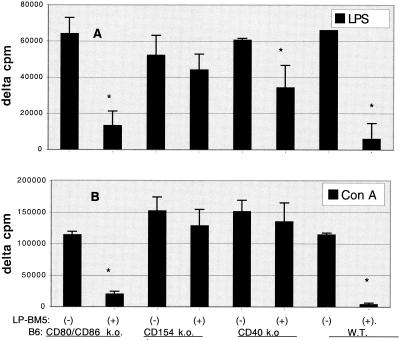
The responses to mitogens for T versus B cells were also evaluated as indices of immunodeficiency. Like those from B6 mice, spleen cells from infected CD80/CD86 ko mice had greatly diminished ConA and LPS responses, i.e., only approximately 20% of those for uninfected CD80/CD86 ko mice (Fig. 5). In contrast, ConA responses for both infected CD154 and CD40 ko mice were consistently nearly identical to those for their uninfected counterparts. Similarly, LPS responses for infected CD154 ko mice were largely unaffected (85% of those for uninfected CD154 ko mice). The LPS response for infected CD40 ko mice was about 60% of that for uninfected CD40 ko mice, as discussed above (14, 21). In summary, for the mitogen studies, infected CD80/CD86 ko mice were clearly immunodeficient compared to the MAIDS-resistant CD154−/− and CD40−/− controls. As with allogeneic CTL generation as an index of immunodeficiency, the mitogen responses of CD80/CD86 ko mice were nearly as severely diminished as those of B6 mice, the prototype susceptible strain.
FIG. 5.
Spleen cell response to T-cell mitogen (ConA) and B-cell mitogen (LPS). Mice were left untreated (−) or were subjected to LP-BM5 virus infection for 9 (A) or 10 (B) weeks (+). After the mice were sacrificed, spleen cell suspensions were cultured for 3 days with 2 μg of ConA/ml and, in the case of the CD80/CD86 ko mouse responders, irradiated WT B6 spleen cells (responder/spleen cell ratio, 10:1) to provide CD80/CD86-expressing APC, which are required for T-cell proliferation as previously reported (37), or with 10 μg of LPS/ml or with medium only. Cellular proliferation was assessed by a terminal 6-h incorporation of [3H]thymidine. Mean values for delta counts per minute ± standard deviations represent four mice per group. *, P < 0.05 by the Student t test.
In conclusion, the data clearly demonstrate that the CD80/CD86−/− strain is MAIDS susceptible. For this double-ko strain, all MAIDS readouts, including those measuring activational parameters, readily distinguished uninfected mice from those undergoing disease progression in response to LP-BM5 infection (Fig. 1 and 4). Furthermore, CD80/CD86 ko spleen cells from infected mice displayed similarly dramatic levels of immunodeficiency (Fig. 2 and 5), and cell subpopulation changes, based on Thy 1.2, CD4, and CD8 expression at 11 weeks postinfection with LP-BM5, compared to prototype susceptible B6 mice (data not shown). Consistent with these data, there was no evidence for differences in the viral loads for the causative defective genome in the B6 and B6.CD80/CD86 ko strains (Fig. 3).
It is worth discussing further the degree of MAIDS susceptibility in LP-BM5-infected CD80/CD86 ko mice, particularly respecting the readouts indicating activational events. The spleen size of infected CD80/CD86 ko mice was consistently about twofold larger than that of the uninfected ko mice, while the spleen size of infected WT B6 mice was approximately sixfold larger than that of uninfected B6 mice (Fig. 1). This relative difference in splenomegaly may be the result of an intrinsic defect of (uninfected) CD80/CD86 ko spleen cells in response to certain stimuli that induce proliferation, for example, to ConA stimulation. As has been reported previously (37), we consistently found that after stimulation with ConA, spleen cells from uninfected CD80/CD86 ko mice proliferated approximately 10-fold less than uninfected WT B6 mouse spleen cells whereas LPS mitogenesis was normal (data not shown). By the addition of irradiated WT (CD80/CD86+) B6 spleen cells as APC, we were able to restore the level of response of CD80/CD86 ko mouse responder T cells to that of WT B6 T cells. But the percent inhibition of ConA responsiveness caused by LP-BM5 infection of CD80/CD86 ko mice was virtually the same with or without WT APC. This lack of full proliferative potential of CD80/CD86 ko T cells in response to stimuli mediated by cell-to-cell interaction is in keeping with various functional deficits that have been reported for this double ko strain (8, 28, 37). This reduction of levels of cell proliferation and/or splenomegaly in response to LP-BM5 infection was not cell subpopulation specific, however, based on our assessment by flow cytometry of phenotypic markers defining these subsets (data not shown).
We also observed somewhat less disease penetrance in CD80/CD86 ko mice with respect to certain aspects of the activational parameter, hyper-Ig (Fig. 4). Consistent with these findings, the lab of Swain has also reported data which suggest that the activational versus immunodeficiency aspects of MAIDS can be separated in certain settings (16). However, in our studies, the less than fully increased serum Ig levels for infected CD80/CD86−/− mice were isotype and time point dependent. Certainly, by the terminal time point, the hyper-Ig readouts were as fully developed for infected CD80/CD86−/− as for B6 mice (Fig. 4).
Our results indicating the clear independence of MAIDS susceptibility and CD80/CD86 expression are seemingly at odds with recent reports that soluble CTLA4Ig treatment can inhibit MAIDS. In one study, very partial inhibition was observed with delayed kinetics of MAIDS pathogenesis after in vivo chronic treatment with soluble CTLA4Ig (10). Alternatively, the transgenic overexpression of CTLA4Ig (11) led to more substantial, though still transient and incomplete, inhibition of MAIDS. The reasons for this apparent discrepancy are unclear. First, however, a different MAIDS virus isolate (Du5H) was employed versus our use of LP-BM5. Second, even though a greater resistance to MAIDS was observed as a result of the continuous transgenic expression of CTLA4Ig (11) compared to that observed as a result of the periodic injection of soluble CTLA4Ig (10), observable disease still occurred, especially at later time points, and there was no decrease in defective viral load (11). Third, it has been suggested that some of the observed functional effects of CTLA4Ig treatment may not be due simply to blockade of CD80/CD86 but are rather a consequence of the engagement of the Ig tail with Fc receptors or the complement system (24). Finally, although we are unaware of any supporting physiological data, it is theoretically possible that additional novel ligands for CD28/CTLA-4 exist whose function is blocked by CTLA4Ig, perhaps with induction of subsequent regulatory events (4). In addition, our results do not preclude the involvement of other previously described receptor-ligand pairs that may also be considered broadly as costimulatory molecules. For example, chronic in vivo MAb treatment with anti-LFA-1 and/or anti-ICAM-1 partially reduced MAIDS severity to a variable extent (26).
Because CD80/CD86 interactions are not necessary for the LP-BM5-induced cascade of cellular and molecular responses characteristic of MAIDS pathogenesis, further investigation of upstream activational signaling events initiated by CD154/CD40 ligation will obviously be needed to shed further light on the molecular basis for this requirement for disease.
Acknowledgments
The authors thank Bob Rich, On Ho, Arti Gaur, and Meghan Brennan for many helpful discussions concerning this study.
This work was supported by U.S. Public Health Service grant CA50157. The DMS irradiation facilities and the flow cytometers were the generous gift of the Fannie Rippel Foundation and are partially supported by National Institutes of Health core grant CA23108 for the Norris Cotton Cancer Center.
REFERENCES
- 1.Allen, R. C., R. J. Armitage, M. E. Conley, H. Rosenblatt, N. A. Jenkins, N. G. Copeland, M. A. Bedell, S. Edelhoff, J. Disteche, and D. K. Simoneaux. 1993. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science 259:990-995. [DOI] [PubMed] [Google Scholar]
- 2.Aziz, D. C., Z. Hanna, and P. Jolicoeur. 1989. Severe immunodeficiency disease induced by a defective murine leukemia virus. Nature (London) 338:505-508. [DOI] [PubMed] [Google Scholar]
- 3.Borriello, F., M. P. Sethna, S. D. Boyd, A. N. Schweitzer, E. A. Tivol, D. Jacoby, T. B. Strom, E. M. Simpson, G. J. Freeman, and A. H. Sharpe. 1997. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity 6:303-313. [DOI] [PubMed] [Google Scholar]
- 4.Brodie, D., A. V. Collins, A. Laboni, J. A. Fennelly, L. M. Sparks, X. N. Xu, P. A. van der Merwe, and S. J. Davis. 2000. Licos, a primordial costimulatory ligand? Curr. Biol. 10:333-336. [DOI] [PubMed] [Google Scholar]
- 5.Buller, R. M. L., R. A. Yetter, T. N. Fredrickson, and H. C. Morse III. 1987. Abrogation of resistance to severe mousepox in C57BL/6 mice infected with LP-BM5 murine leukemia viruses. J. Virol. 61:383-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carreno, B. M., and M. Collins. 2002. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu. Rev. Immunol. 20:29-53. [DOI] [PubMed] [Google Scholar]
- 7.Cerny, A. A., W. Hugin, R. R. Hardy, K. Hayakawa, R. M. Zinkernagel, M. Makino, and H. C. Morse III. 1990. B cells are required for induction of T cell abnormalities in a murine retrovirus-induced immunodeficiency syndrome. J. Exp. Med. 171:315-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, T. T., C. Jabs, R. A. Sobel, V. K. Kuchroo, and A. H. Sharpe. 1999. Studies in B7-deficient mice reveal a critical role for B7 costimulation in both induction and effector phases of experimental autoimmune encephalomyelitis. J. Exp. Med. 190:733-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chattopadhyay, S. K., H. C. Morse III, M. Makino, S. K. Ruscetti, and J. W. Hartley. 1989. A defective virus is associated with induction of a murine retrovirus-induced immunodeficiency syndrome, MAIDS. Proc. Natl. Acad. Sci. USA 86:3862-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Leval, L., S. Colombi, S. Debrus, M. A. Demoitie, R. Greimers, P. Linsley, M. Moutschen, and J. Boniver. 1998. CD28-B7 costimulatory blockade by CTLA4Ig delays the development of retrovirus-induced murine AIDS. J. Virol. 72:5285-5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Leval, L., S. Debrus, P. Plane, J. Boniver, M. Mountschen. 1999. Mice transgenic for a soluble form of murine cytotoxic T lymphocyte antigen 4 are refractory to murine acquired immune deficiency syndrome development. Immunology 98:630-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giese, N. A., T. Giese, and H. C. Morse III. 1994. Murine AIDS is an antigen-driven disease: requirements for major histocompatibility complex class II expression and CD4+ T cells. J. Virol. 68:5819-5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green, K. A., K. M. Crassi, J. D. Laman, A. Schoneveld, R. R. Strawbridge, T. M. Foy, R. J. Noelle, and W. R. Green. 1996. Antibody to the ligand for CD40 (gp39) inhibits murine AIDS-associated splenomegaly, hypergammaglobulinemia, and immunodeficiency in disease-susceptible C57BL/6 mice. J. Virol. 70:2569-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green, K. A., R. J. Noelle, B. G. Durell, and W. R. Green. 2001. Characterization of the CD154-positive and CD40-positive cellular subsets required for pathogenesis in retrovirus-induced murine immunodeficiency. J. Virol. 75:3581-3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green, K. A., R. J. Noelle, and W. R. Green. 1998. Evidence for a continued requirement for CD40/CD40 ligand (CD154) interactions in the progression of LP-BM5 retrovirus-induced murine AIDS. Virology 241:260-268. [DOI] [PubMed] [Google Scholar]
- 16.Harris, D. P., S. Koch, L. M. Mullen, and S. L. Swain. 2001. B cell immunodeficiency fails to develop in CD4-deficient mice infected with BM5: murine AIDS as a multistep disease. J. Immunol. 166:6041-6049. [DOI] [PubMed] [Google Scholar]
- 17.Hartley, J. W., T. N Fredrickson, R. A. Yetter, M. Makino, and H. C. Morse III. 1989. Retrovirus-induced murine acquired immunodeficiency syndrome: natural history of infection and differing susceptibility of inbred mouse strains. J. Virol. 63:1223-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang, M., C. Simard, and P. Jolicoeur. 1989. Immunodeficiency and clonal growth of target cells induced by helper-free defective retrovirus. Science 246:1614-1617. [DOI] [PubMed] [Google Scholar]
- 19.Hugo, P., J. W. Kappler, and P. C. Marrack. 1993. Positive selection of TcR alpha beta thymocytes: is cortical epithelium an obligatory participant in the presentation of major histocompatibility complex protein? Immunol. Rev. 135:134-155. [DOI] [PubMed] [Google Scholar]
- 20.Jones, K. W., and C. J. Hackett. 1996. Activated T hybridomas induce upregulation of B7-1 on bystander B lymphoma cells by a contact-dependent interaction utilizing CD40 ligand. Cell. Immunol. 174:42-53. [DOI] [PubMed] [Google Scholar]
- 21.Kawabe, T., T. Naka, K. Yoshida, T. Tanaka, H. Fujiwara, S. Suematsu, N. Yoshida, T. Kishimoto, and H. Kikutani. 1994. The immune responses in CD40 deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity 3:167-178. [DOI] [PubMed] [Google Scholar]
- 22.Klinken, S. P., T. N. Fredrickson, J. W. Hartley, R. A. Yetter, and H. C. Morse III. 1988. Evolution of B cell lineage lymphomas in mice with a retrovirus-induced immunodeficiency syndrome, MAIDS. J. Immunol. 140:1123-1131. [PubMed] [Google Scholar]
- 23.Klinman, D. M., and H. C. Morse III. 1989. Characteristics of B cell proliferation and activation in murine AIDS. J. Immunol. 142:1144-1149. [PubMed] [Google Scholar]
- 24.Lane, P., C. Burdet, S. Hubele, D. Scheidegger, U. Muller, F. McConnell, and M. Kosco-Vilbois. 1994. B cell function in mice transgenic for MCTLA4-Hγ1: lack of germinal centers correlated with poor affinity maturation and class switching despite normal priming of CD4+ T cells. J. Exp. Med. 179:819-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Legrand, E., R. Daculsi, and J. F. Duplan. 1981. Characteristics of the cell populations involved in extrathymic lymphosarcoma induced in C57BL/6 mice by RadLV-RS. Leuk. Res. 5:223-233. [DOI] [PubMed] [Google Scholar]
- 26.Makino, M. Y. Kazuhiko, M. Azuma, Y. Okada, Y. Hitoshi, H. Yagita, K. Takatsu, and K. Komuro. 1995. Rapid development of murine AIDS is dependent on signals provided by CD54 and CD11a. J. Immunol. 155:974-981. [PubMed] [Google Scholar]
- 27.McAdam, A. J., B. E. Gewurz, E. A. Farkash, and A. H. Sharpe. 2000. Either B7 costimulation or IL-2 can elicit generation of primary alloreactive CTL. J. Immunol. 165:3088-3093. [DOI] [PubMed] [Google Scholar]
- 28.McAdam, A. J., E. A. Farkash, B. E. Gewurz, and A. H. Sharpe. 2000. B7 costimulation is critical for antibody class switching and CD8+ cytotoxic T-lymphocyte generation in the host response to vesicular stomatitis virus. J. Virol. 74:203-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morse, H. C., III, R. A. Yetter, C. S. Via, R. R. Hardy, A. Cerny, K. Hayakawa, A. W. Hugin, M. W. Miller, K. L. Homes, and G. M. Shearer. 1989. Functional and phenotypic alterations in T cell subsets during the course of MAIDS, a murine retrovirus-induced immunodeficiency syndrome. J. Immunol. 143:844-850. [PubMed] [Google Scholar]
- 30.Mosier, D. E., R. A. Yetter, and H. C. Morse III. 1987. Functional T lymphocytes are required for a murine retrovirus-induced immunodeficiency disease (MAIDS). J. Exp. Med. 165:1732-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosier, D. E., R. A. Yetter, and H. C. Morse III. 1985. Retroviral induction of acute lymphoproliferative disease and profound immunosuppression in adult C57BL/6 mice. J. Exp. Med. 161:766-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pattengale, P. K., C. R. Taylor, P. Twomey, S. Hill, J., Jonasson, T. Beardsley, and M. Haas. 1982. Immunopathology of B cell lymphomas induced in C57BL/6 mice by dualtropic murine leukemia virus (MuLV). Am. J. Pathol. 107:362-377. [PMC free article] [PubMed] [Google Scholar]
- 33.Pulendran, B., J. Lingappa, M. K. Kennedy, J. Smith, M. Teepe, A. Rudensky, C. R. Maliszewski, and E. Maraskovsky. 1997. Developmental pathways of dendritic cells in vivo. J. Immunol. 159:2222-2231. [PubMed] [Google Scholar]
- 34.Ranheim, E. A., and T. J. Kipps. 1995. Tumor necrosis factor-a facilitates induction of CD80 (B7-1) and CD54 on human B cells by activated T cells: complex regulation by IL-4, IL-10 and CD40L. Cell. Immunol. 161:226-235. [DOI] [PubMed] [Google Scholar]
- 35.Ranheim, E. A., and T. J. Kipps. 1993. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J. Exp. Med. 177:925-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy, M., A. Aruffo, J. Ledbetter, P. Linsley, M. Kehry, and R. Noelle. 1995. Studies on the interdependence of gp39 and B7 expression and function during antigen-specific immune responses. Eur. J. Immunol. 25:596-603. [DOI] [PubMed] [Google Scholar]
- 37.Schweitzer, A. N., and A. H. Sharpe. 1998. Studies using antigen-presenting cells lacking expression of both B7-1 (CD80) and B7-2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J. Immunol. 161:2762-2771. [PubMed] [Google Scholar]
- 38.Sharpe, A. H., and G. J. Freeman. 2002. The B7-CD28 superfamily. Nat. Rev. Immunol. 2:116-126. [DOI] [PubMed] [Google Scholar]
- 39.Simard, C., S. J Klein, T. Mak, and P. Jolicoeur. 1997. Studies of the susceptibility of nude, CD4 knockout, and SCID mutant mice to the disease induced by the murine AIDS defective virus. J. Virol. 71:3013-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yetter, R. A., R. M. L. Buller, J. S. Lee, K. L. Elkins, D. E. Mosier, T. N. Fredrickson, and H. C. Morse III. 1988. CD4+T cells are required for development of a murine retrovirus-induced immunodeficiency syndrome (MAIDS). J. Exp. Med. 168:623-635. [DOI] [PMC free article] [PubMed] [Google Scholar]