Abstract
Hepatitis B virus (HBV) transgenic mice expressing rat hepatocyte nuclear factor 3β (HNF3β) were generated by breeding HBV transgenic mice with transgenic mice that constitutively overexpress the rat HNF3β polypeptide in the liver. HBV 3.5-, 2.4- and 2.1-kb transcripts were reduced 2- to 4-fold in these mice relative to the HBV transgenic mouse controls. In contrast, the abundance of viral replication intermediates was profoundly reduced in HBV transgenic mice by overexpression of HNF3β. This results, in part, from the preferential reduction in the level of the pregenomic 3.5-kb RNA relative to the precore 3.5-kb RNA. Therefore, it is apparent that increased expression of HNF3β modestly reduces viral transcription and dramatically inhibits replication in vivo in the HBV transgenic mouse. This suggests that altering the activity of this transcription factor in vivo in chronic HBV carriers might be therapeutically beneficial.
The hepatitis B virus (HBV) is a 42-nm enveloped particle possessing an internal 28-nm nucleocapsid (5, 14). The 3.2-kb partially double-stranded circular viral genome and the HBV polymerase are present within the nucleocapsid. When HBV infects the liver, the nucleocapsid is released from the viral envelope, and the HBV DNA is transported into the nucleus of the hepatocyte. In the nucleus, the viral genome is converted into a 3.2-kbp covalently closed circular (CCC) DNA molecule that serves as the template for transcription by the host RNA polymerase (5, 20). The viral CCC DNA is transcribed, producing the 3.5-, 2.4-, 2.1-, and 0.7-kb viral transcripts that encode the HBV core and polymerase polypeptides, the large surface antigen polypeptide, the middle and major surface antigen polypeptides, and the X-gene polypeptide, respectively (14, 19). The pregenomic 3.5-kb transcript is also reverse transcribed by the viral polymerase to produce the HBV genome within the nucleocapsid (23). The synthesis of viral DNA from pregenomic RNA converts the immature nucleocapsids into mature nucleocapsids that bind the envelope antigen polypeptides in the endoplasmic reticulum membrane and subsequently bud into the lumen of the endoplasmic reticulum. The virus particles are then transported through the Golgi apparatus and released from the hepatocytes into the bloodstream, where they can infect additional hepatocytes (5, 14).
Because HBV replication occurs by reverse transcription of the pregenomic transcript, it is apparent that the regulation of the level of synthesis of viral RNAs will also modulate HBV replication. Viral transcription is regulated by ubiquitous and liver-enriched transcription factors (10, 19, 20, 24). The role of liver-enriched transcription factors in controlling viral RNA synthesis suggested that transcription restricts viral biosynthesis to hepatocytes. This contention was supported by the observation that the liver-enriched nuclear hormone receptors, HNF4 and PPARα plus RXRα, are essential for HBV pregenomic RNA synthesis and viral replication in nonhepatoma cell lines (21). This study also demonstrated that nuclear hormone receptor-mediated viral replication was negatively regulated by HNF3. HNF3 appears to inhibit the synthesis of the HBeAg-encoding precore 3.5-kb transcript to a lesser extent than the pregenomic 3.5-kb transcript. This may promote the HBeAg-mediated inhibition of viral replication and result in a larger decrease in viral replication than 3.5-kb RNA synthesis (21, 22).
In this study, the roles of HNF3β in regulating viral transcription and replication in vivo were examined by using the HBV transgenic mouse model of chronic HBV infection (8). The HBV transgenic mice were bred with transgenic mice that overexpress the HNF3β polypeptide in the liver (16). The rat HNF3β-expressing HBV transgenic mice were examined for viral transcription and replication in the liver. These mice demonstrate that elevated levels of HNF3β expression inhibit viral replication in the liver in a manner similar to that observed in cell culture. Viral replication is reduced by HNF3β overexpression to a significantly greater extent than the HBV 3.5-kb RNA. This suggests that physiological or therapeutic stimuli that modulate HBV 3.5-kb RNA synthesis to a modest extent may have significant antiviral potential.
MATERIALS AND METHODS
Transgenic mice.
The production and characterization of the HBV transgenic mouse lineage 1.3.32 have been described previously (8). These HBV transgenic mice contain a single copy of the terminally redundant, 1.3-genome-length copy of the HBVayw genome integrated into the mouse chromosomal DNA. High levels of HBV replication occur in the livers of these mice. The mice used in the breeding experiments were homozygous for the HBV transgene and were maintained on the C57BL/6 genetic background.
The production and characterization of the TTR-HNF3β (T-77) transgenic mice have been described previously (16). These mice express the rat HNF3β polypeptide from a transgene construct by using the mouse transthyretin promoter to direct the transcription of the rat HNF3β cDNA in the liver and brain (16, 25). TTR-HNF3β (T-77) transgenic mice display growth retardation, depletion of hepatic glycogen storage, elevated levels of bile acids in the serum, and severe ataxia (11, 16, 25). The mice used in the breeding experiments were hemizygous for the TTR-HNF3β (T-77) transgene and were maintained on the CD1 genetic background (16).
TTR-HNF3β (T-77) HBV transgenic mice were generated by mating the HBV transgenic mice with the TTR-HNF3β (T-77) transgenic mice. The resulting TTR-HNF3β (T-77) HBV transgenic F1 mice were screened for the HBV transgene and TTR-HNF3β (T-77) transgene by PCR analysis of tail DNA. Tail DNA was prepared by incubating 1 cm of tail in 500 μl of a mixture containing 100 mM Tris hydrochloride (pH 8.0), 200 mM NaCl, 5 mM EDTA, and 0.2% (wt/vol) sodium dodecyl sulfate (SDS) containing 100 μg of proteinase K per ml for 16 to 20 h at 55°C. Samples were centrifuged at 14,000 rpm in an Eppendorf 5417C microcentrifuge for 5 min, and the supernatant was precipitated with 500 μl of isopropanol. DNA was pelleted by centrifugation at 14,000 rpm in an Eppendorf 5417C microcentrifuge for 5 min and subsequently dissolved in 100 μl of 5 mM Tris hydrochloride (pH 8.0)-1 mM EDTA. The HBV transgene was identified by PCR analysis with the oligonucleotides TCGATACCTGAACCTTTACCCCGTTGCCCG (oligo XpHNF4-1; HBV coordinates 1133 to 1159) and TCGAATTGCTGAGAGTCCAAGAGTCCTCTT (oligo CpHNF4-2; HBV coordinates 1683 to 1658) and 1 μl of tail DNA. The TTR-HNF3β (T-77) transgene was identified by PCR analysis with the oligonucleotides AAAGTCCTGGATGCTGTCCGAG and CAGACATGATAAGATACATTGATG and 1 μl of tail DNA. The samples were subjected to 42 amplification cycles involving denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension from the primers at 72°C for 2 min. A PCR product of 551 bp indicated the presence of the HBV transgene. A PCR product of approximately 300 bp indicated the presence of the TTR-HNF3β (T-77) transgene. The 20-μl reaction conditions used were as described by the manufacturer (PGC Scientifics) and contained 1.5 U of Taq DNA polymerase.
HBV DNA and RNA analysis.
Total DNA and RNA were isolated from livers of 4-week-old HBV transgenic mice as described previously (2, 18). DNA (Southern) filter hybridization analyses were performed with 20 μg of HindIII-digested DNA (18). Filters were probed with 32P-labeled HBVayw genomic DNA (4) to detect HBV sequences. RNA (Northern) filter hybridization analyses were performed with 10 μg of total cellular RNA as described previously (18). Filters were probed with 32P-labeled HBVayw genomic DNA to detect HBV sequences and the mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH) cDNA to detect the GAPDH transcript used as an internal control (17).
RNase protection assays were performed with the Pharmingen Riboquant kit, and riboprobes were synthesized by using the Ambion Maxiscript kit as described by the manufacturers. The mouse HNF3α, HNF3β, and HNF3γ transcripts and the rat HNF3β transcript were detected by RNase protection analysis with 20 μg of total cellular RNA as previously described (16). Transcription initiation sites for the 3.5-kb HBV transcripts were examined with 20 μg of total cellular RNA and a 333-nucleotide-long (HBV coordinates 1990 to 1658) 32P-labeled HBV riboprobe. The transcription initiation site for the 2.4-kb HBV transcript was examined with 20 μg of total cellular RNA and a 327-nucleotide-long (HBV coordinates 2708 to 3034) 32P-labeled HBV riboprobe. As an internal control for the RNase protection analysis, a 32P-labeled mouse ribosomal protein L32 gene riboprobe spanning 101 nucleotides of exon 3 was utilized (3). All riboprobes contained additional flanking vector sequences that are not protected by HBV transgenic mouse RNA.
Filter hybridization and RNase protection analysis were quantitated by phosphorimaging with a Packard Cyclone Storage Phosphor System.
HBV antigen analysis.
HBeAg analysis was performed with 20 μl of mouse serum and the HBe radioimmunoassay as described by the manufacturer (DiaSorin). The level of antigen was determined in the linear range of the assay. Immunohistochemical detection of HBcAg in paraffin-embedded mouse liver sections was performed as previously described (8).
RESULTS
Effect of rat HNF3β expression on mouse HNF3 isoforms in the liver of HBV transgenic mice.
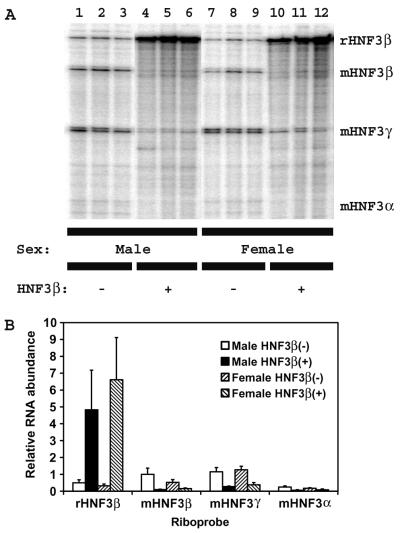
Homozygous HBV transgenic mice were bred with hemizygous TTR-HNF3β (T77) transgenic mice, and control rat HNF3β(−) HBV transgenic mice and rat HNF3β expressing hemizygous HNF3β(+) HBV transgenic mice were identified in the F1 generation. For these studies, 4-week old HBV transgenic mice that lacked the rat HNF3β transgene were used as controls and compared with their rat HNF3β-expressing HBV transgenic littermates. However, similar results were also obtained with 8-week-old HBV transgenic mice (K. Banks and A. McLachlan, unpublished data). Initially, the effect of the expression of the rat HNF3β polypeptide on the levels of expression of the mouse HNF3 isoforms in the liver of the HBV transgenic mice was examined by RNase protection analysis (Fig. 1). The rat HNF3β riboprobe detected both the rat and mouse HNF3β transcripts (Fig. 1A). Using this riboprobe, it is estimated that the rat HNF3β transcript is expressed at an approximately 10-fold-higher level in the rat HNF3β-expressing HBV transgenic mice than the mouse HNF3β transcript is expressed in the control rat HNF3β(−) HBV transgenic mice (Fig. 1B). The mouse HNF3β transcript is decreased up to 10-fold in the rat HNF3β-expressing HBV transgenic mice (Fig. 1B). These observations are consistent with the previous finding that the HNF3β polypeptide is expressed at an approximately fivefold-higher level in the rat HNF3β-expressing transgenic mice than in control transgenic mice (16). The mouse HNF3α and HNF3γ transcripts are also decreased between two- and fivefold in the rat HNF3β-expressing HBV transgenic mice compared with control rat HNF3β(−) HBV transgenic mice (Fig. 1B). These changes in the levels of HNF3 isoform expression in the livers of the HBV transgenic mice are similar to those originally described for the rat HNF3β-expressing transgenic mice (16).
FIG. 1.
RNase protection analysis of the rat HNF3β, mouse HNF3α, mouse HNF3β, and mouse HNF3γ transcripts from the livers of HBV transgenic mice. (A) Groups of three mice of each sex and genotype are shown. The rat HNF3β, mouse HNF3α, mouse HNF3β, and mouse HNF3γ riboprobes used were described previously (16). The rat HNF3β, mouse HNF3β, mouse HNF3γ, and mouse HNF3α RNAs protect fragments of 480 (rHNF3β), 332 (mHNF3β), 231 (mHNF3γ), and 150 (mHNF3α) nucleotides, respectively. −, HBV transgenic mice lacking the rat HNF3β transgene; +, HBV transgenic mice hemizygous for the rat HNF3β transgene. (B) Quantitative analysis of the rat HNF3β, mouse HNF3β, mouse HNF3γ, and mouse HNF3α RNA levels in HBV transgenic mice. The levels of the HNF3 RNAs are reported relative to the mouse HNF3β RNA present in the liver of control male rat HNF3β(−) HBV transgenic mice, which is designated as having a relative activity of 1.0. The mean HNF3 RNA levels plus standard deviations derived from six male rat HNF3β(−) HBV transgenic mice, six male rat HNF3β(+) HBV transgenic mice, seven female rat HNF3β(−) HBV transgenic mice, and four female rat HNF3β(+) HBV transgenic mice are shown. The lower levels of the mouse HNF3 RNAs in the rat HNF3β-expressing HBV transgenic mice are statistically significantly different from their levels in the control rat HNF3β(−) HBV transgenic mice by Student's t test (P < 0.05).
Effect of rat HNF3β expression on HBeAg synthesis in HBV transgenic mice.
HBV transgenic mice of each genotype were assayed for the level of HBeAg in their sera. HBeAg is translated from the 3.5-kb precore RNA and therefore indirectly indicates nucleocapsid promoter activity (7, 8). HBeAg in the sera of male and female rat HNF3β-expressing HBV transgenic mice were approximately 30 and 60%, respectively, of the levels observed in the control rat HNF3β(−) HBV transgenic mice of the same sex. This observation suggests the level of the 3.5-kb precore RNA was slightly reduced in the livers of the rat HNF3β-expressing HBV transgenic mice.
Effect of rat HNF3β expression on viral 3.5- and 2.1-kb transcript synthesis in HBV transgenic mice.
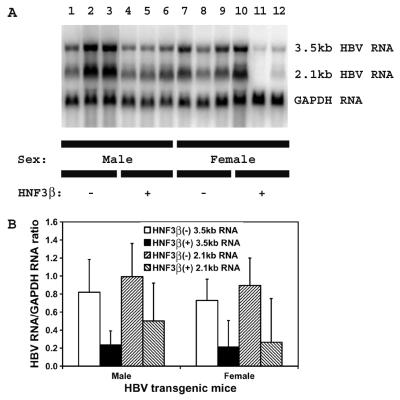
The rat HNF3β-expressing HBV transgenic mice were examined for their steady-state levels of HBV transcripts by analysis of the total liver RNA (Fig. 2). The levels of the HBV 3.5- and 2.1-kb transcripts in the livers of the rat HNF3β-expressing HBV transgenic mice were two- to threefold lower than the levels of these viral transcripts in control rat HNF3β(−) HBV transgenic mice (Fig. 2). This result is consistent with the lower level of serum HBeAg in rat HNF3β-expressing HBV transgenic mice and the observation that HNF3β modestly inhibits HBV transcription in cell culture (21, 22).
FIG. 2.
RNA (Northern) filter hybridization analysis of HBV transcripts in the livers of HBV transgenic mice. (A) Groups of three mice of each sex and genotype are shown. The GAPDH transcript was used as an internal control for the quantitation of the HBV 3.5- and 2.1-kb RNAs. The probes used were HBVayw genomic DNA plus GAPDH cDNA. −, HBV transgenic mice lacking the rat HNF3β transgene; +, HBV transgenic mice hemizygous for the rat HNF3β transgene. (B) Quantitative analysis of the HBV 3.5- and 2.1-kb RNA levels in HBV transgenic mice. The mean HBV 3.5- and 2.1-kb RNA levels plus standard deviations derived from six male rat HNF3β(−) HBV transgenic mice, six male rat HNF3β(+) HBV transgenic mice, seven female rat HNF3β(−) HBV transgenic mice, and four female rat HNF3β(+) HBV transgenic mice are shown. The lower levels of the HBV 3.5- and 2.1-kb RNA in the rat HNF3β-expressing HBV transgenic mice are statistically significantly different from their levels in the control rat HNF3β(−) HBV transgenic mice by a Student's t test (P < 0.05).
Effect of rat HNF3β expression on viral replication in HBV transgenic mice.
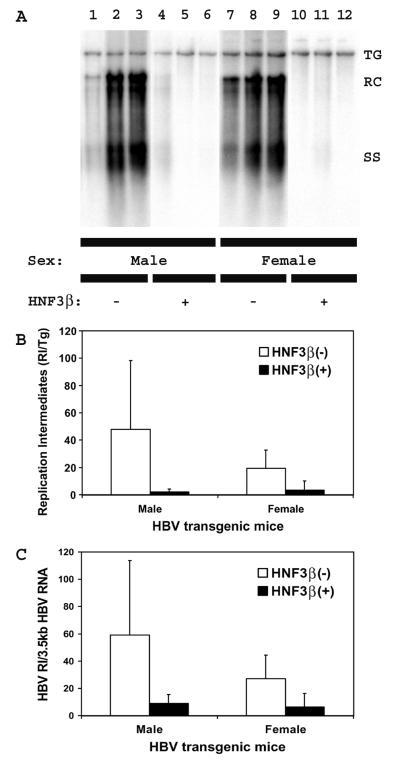
The levels of replication intermediates in the livers of the rat HNF3β-expressing HBV transgenic mice were approximately 23- and 6-fold lower in male and female mice, respectively, than those observed in the control rat HNF3β(−) HBV transgenic mice (Fig. 3). From this observation, it is apparent that the reductions in the levels of viral replication intermediates are considerably greater than the reductions in the level of the viral 3.5-kb transcripts in the rat HNF3β-expressing HBV transgenic mice (Fig. 2). The ratio of viral replication intermediates to HBV 3.5-kb transcripts in individual mice indicates that in rat HNF3β-expressing HBV transgenic mice, four- to sevenfold less viral replication occurs per 3.5-kb transcript compared with the level in control HBV transgenic mice (Fig. 3C). This finding suggests that slightly reduced levels of HBV 3.5-kb RNA can be associated with dramatically reduced levels of viral replication.
FIG. 3.
DNA (Southern) filter hybridization analysis of HBV DNA replication intermediates in the livers of HBV transgenic mice. (A) Groups of three mice of each sex and genotype are shown. The HBV transgene (TG) was used as an internal control for the quantitation of the HBV replication intermediates. The probe used was HBVayw genomic DNA. TG, HBV transgene; RC, HBV relaxed circular replication intermediates; SS, HBV single-stranded replication intermediates; −, HBV transgenic mice lacking the rat HNF3β transgene; +, HBV transgenic mice hemizygous for the rat HNF3β transgene. (B) Quantitative analysis of the HBV DNA replication intermediate (RI) levels in HBV transgenic mice. The mean HBV DNA replication intermediate levels plus standard deviations derived from six male rat HNF3β(−) HBV transgenic mice, six male rat HNF3β(+) HBV transgenic mice, seven female rat HNF3β(−) HBV transgenic mice, and four female rat HNF3β(+) HBV transgenic mice are shown. The lower levels of the HBV DNA replication intermediates in the rat HNF3β-expressing HBV transgenic mice are statistically significantly different from their levels in the control rat HNF3β(−) HBV transgenic mice by Student's t test (P < 0.05). (C) Quantitative analysis of the ratio of HBV DNA replication intermediates synthesized per unit of HBV 3.5-kb RNA present in HBV transgenic mice. The mean HBV DNA to 3.5-kb RNA ratio plus standard deviations derived from six male rat HNF3β(−) HBV transgenic mice, six male rat HNF3β(+) HBV transgenic mice, seven female rat HNF3β(−) HBV transgenic mice, and four female rat HNF3β(+) HBV transgenic mice are shown. The lower ratio of HBV DNA to 3.5-kb RNA in the rat HNF3β-expressing HBV transgenic mice are statistically significantly different from their ratios in the control rat HNF3β(−) HBV transgenic mice by Student's t test (P < 0.05).
Effect of rat HNF3β expression on viral precore and pregenomic RNA synthesis in HBV transgenic mice.
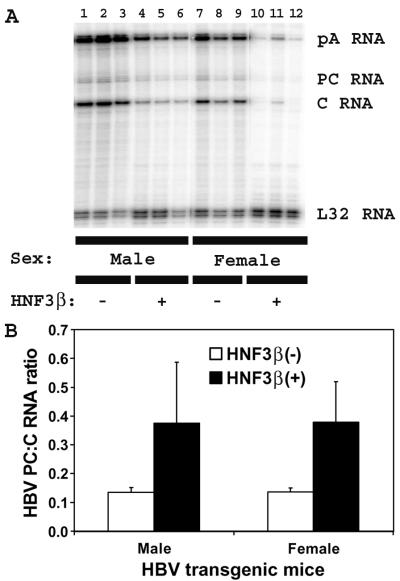
The possibility that rat HNF3β expression might differentially reduce the levels of the HBV precore and pregenomic 3.5-kb RNAs was examined by RNase protection analysis (Fig. 4). The ratio of precore to pregenomic RNA is approximately threefold higher in HNF3β-expressing HBV transgenic mice than in control rat HNF3β(−) HBV transgenic mice (Fig. 4B). This is similar to the effect of HNF3β expression on precore and pregenomic RNA synthesis from HBV genomic DNA in nonhepatoma cells (21, 22). This suggests that modulation of the relative level of expression of precore to pregenomic RNA is a general property of HNF3β-mediated regulation of HBV transcription, and it probably contributes to the greater reduction in viral replication compared with 3.5-kb RNA synthesis in the rat HNF3β-expressing HBV transgenic mice (Fig. 3C).
FIG. 4.
RNase protection analysis mapping the transcription initiation sites of the precore (PC) and pregenomic (C) transcripts from the livers of HBV transgenic mice. (A) Groups of three mice of each sex and genotype are shown. The 3′ ends of the all the HBV transcripts corresponding to the polyadenylation site (pA) of these RNAs also generated a protected fragment in this analysis. The riboprobes used included the HBVayw sequence spanning nucleotide coordinates 1990 to 1658 and the mouse ribosomal protein L32 gene riboprobe spanning 101 nucleotides of exon 3. The 3.5-kb HBV RNAs protect fragments of 283 (pA), 206 (PC), and 175 (C) nucleotides, respectively. The mouse ribosomal protein L32 RNA protects a fragment of 101 nucleotides, designated L32, when probed with the L32 probe. −, HBV transgenic mice lacking the rat HNF3β transgene; +, HBV transgenic mice hemizygous for the rat HNF3β transgene. (B) Quantitative analysis of the HBV precore/pregenomic RNA ratios in HBV transgenic mice. The mean HBV precore/pregenomic RNA ratios plus standard deviations derived from six male rat HNF3β(−) HBV transgenic mice, six male rat HNF3β(+) HBV transgenic mice, seven female rat HNF3β(−) HBV transgenic mice, and four female rat HNF3β(+) HBV transgenic mice are shown. The higher HBV precore/pregenomic RNA ratios in the rat HNF3β-expressing HBV transgenic mice are statistically significantly different from their ratios in the control rat HNF3β(−) HBV transgenic mice by Student's t test (P < 0.05).
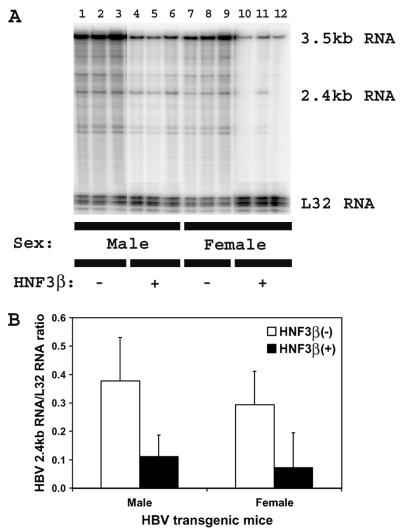
Effect of rat HNF3β expression on viral 2.4-kb RNA synthesis in HBV transgenic mice.
RNase protection analysis of the HBV 2.4-kb RNA initiation site demonstrated that the levels of the HBV 2.4-kb transcripts in the livers of the rat HNF3β-expressing HBV transgenic mice were approximately fourfold lower than the levels of these viral transcripts in control rat HNF3β(−) HBV transgenic mice (Fig. 5). This suggests that synthesis of both HBV 3.5- and 2.4-kb RNA is inhibited by HNF3β expression in the context of viral replication. This might be mediated by inhibiting RNA elongation rather than transcription initiation, as noted previously for the synthesis of HBV 3.5-kb RNA from HBV genomic DNA in transient transfection analysis (22). Therefore, from the analysis of rat HNF3β-expressing HBV transgenic mice, it is apparent that HNF3β coordinately downregulates the expression of each of the viral RNAs in vivo from HBV genomic DNA in a manner similar to that previously observed in cell culture (21, 22).
FIG. 5.
RNase protection analysis mapping the transcription initiation site of the large surface antigen transcript from the livers of HBV transgenic mice. (A) Groups of three mice of each sex and genotype are shown. The riboprobes used included the HBVayw sequence spanning nucleotide coordinates 3054 to 2708 and the mouse ribosomal protein L32 gene riboprobe spanning 101 nucleotides of exon 3. The 3.5-kb HBV RNA protects a fragment of 327 nucleotides and the 2.4-kb HBV RNA protects a fragment of 226 nucleotides. The mouse ribosomal protein L32 RNA protects a fragment of 101 nucleotides, designated L32, when probed with the L32 probe. −, HBV transgenic mice lacking the rat HNF3β transgene; +, HBV transgenic mice hemizygous for the rat HNF3β transgene. (B) Quantitative analysis of the HBV 2.4-kb RNA levels in HBV transgenic mice. The mean HBV 2.4-kb RNA levels plus standard deviations derived from six male rat HNF3β(−) HBV transgenic mice, six male rat HNF3β(+) HBV transgenic mice, seven female rat HNF3β(−) HBV transgenic mice, and four female rat HNF3β(+) HBV transgenic mice are shown. The lower levels of the HBV 2.4-kb RNAs in the rat HNF3β-expressing HBV transgenic mice are statistically significantly different from their levels in the control rat HNF3β(−) HBV transgenic mice by Student's t test (P < 0.05).
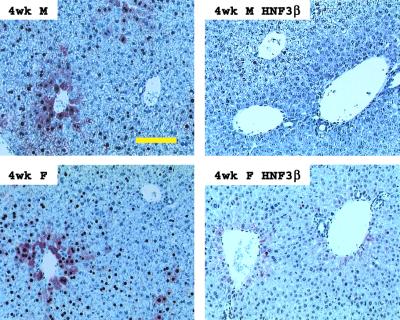
Effect of HNF3β expression on viral HBcAg distribution within the livers of HBV transgenic mice.
Immunohistochemical analysis of the livers of HBV transgenic mice demonstrated that HNF3β influences the distribution of HBcAg within the liver lobule (Fig. 6). In both male and female control rat HNF3β(−) HBV transgenic mice, the HBcAg staining is both nuclear and cytoplasmic in the hepatocytes located around the central vein. HBcAg staining is limited to the nuclei of hepatocytes located further from the central vein and is considerably reduced within hepatocytes surrounding the portal vein. In contrast, rat HNF3β-expressing HBV transgenic mice display very limited HBcAg staining that is limited to the nuclei of a few hepatocytes adjacent to the central vein. These staining patterns correlate with the reduction in pregenomic RNA synthesis and viral replication observed in the HNF3β-expressing HBV transgenic mice (Fig. 3 and 4).
FIG. 6.
Immunohistochemical staining of HBcAg in the livers of HBV transgenic mice. Nuclear staining of HBcAg is observed throughout the liver, whereas cytoplasmic staining is located primarily in the centrolobular hepatocytes in the livers of control rat HNF3β(−) HBV transgenic mice (left panels). Rat HNF3β(+) HBV transgenic mice expressing the rat HNF3β transgene display minimal HBcAg staining in their hepatocytes (right panels). Results for 4-week-old male (M) and female (F) mice are shown. The yellow size bar represents 500 μm.
DISCUSSION
The transcription factors that regulated HBV RNA synthesis have been extensively examined (10, 12, 13, 20, 24). The roles of specific liver-enriched transcription factors in determining the level of HBV pregenomic RNA expression and viral replication have also been characterized in cell culture (21). These studies have demonstrated that the nuclear hormone receptors, HNF4 and RXRα plus PPARα, are a major determinant restricting HBV viral replication to cells of hepatic origin (21). These analyses also demonstrated that the liver-enriched transcription factor HNF3 negatively regulated nuclear hormone receptor-mediated HBV pregenomic RNA synthesis and viral replication in cell culture (21, 22). In this study, the possibility that HNF3β might also negatively regulate viral transcription and replication in vivo in a manner similar to that observed in cell culture was investigated by using an HBV transgenic mouse model (8). This was achieved by characterizing the viral transcripts and replication intermediates in HBV transgenic mice expressing the rat HNF3β polypeptide in their liver (8, 16).
HBV transgenic mice were bred with transgenic mice expressing the rat HNF3β polypeptide in their liver utilizing the transthyretin promoter to produce rat HNF3β-expressing HBV transgenic mice (8, 16). The rat HNF3β-expressing HBV transgenic mice were shown to express approximately 10-fold-higher levels of the rat HNF3β transcript than the endogenous mouse HNF3β transcript (Fig. 1) (16). The expression of the rat HNF3β polypeptide also results in the reduction of the level of expression of the endogenous mouse HNF3α and HNF3γ transcripts in rat HNF3β-expressing HBV transgenic mice (Fig. 1) (16). The levels of expression of additional liver-enriched transcription factors that can modulate HBV transcription, including C/EBPα, C/EBPβ, HNF1α, and HNF4α, are not greatly affected by the alteration in the levels of HNF3 isoform expression in rat HNF3β-expressing transgenic mice (16). In addition, the physiological alteration in the livers of the rat HNF3β-expressing HBV transgenic mice at 4 weeks of age did not result in measurable liver damage or detectable expression of the cytokines tumor necrosis factor alpha (TNF-α), alpha/beta interferon (IFN-α/β), and IFN-γ (K. E. Banks and A. McLachlan, unpublished data). Consequently, the effect of the expression of the rat HNF3β polypeptide on viral transcription and replication in the rat HNF3β-expressing HBV transgenic mice is not influenced by cytokine-mediated processes that can downregulate HBV viral replication (1, 6).
The levels of the HBV transcripts in the rat HNF3β-expressing HBV transgenic mice were compared with those of the control rat HNF3β(−) HBV transgenic mice (Fig. 2, 4, and 5). As observed in cell culture (21, 22), elevated levels of HNF3β were associated with a modest decrease in the level of expression of the two major HBV transcripts. This observation demonstrates that the negative effect of a specific transcription factor on HBV transcription and replication observed in cell culture can be recapitulated in vivo in the HBV transgenic mouse model system. The mechanism of HNF3β-mediated inhibition of viral transcription in vivo probably does not involve decreasing HBV promoter activity, because HNF3 has been shown to activate the level of transcription from the nucleocapsid and large surface antigen promoters in reporter gene analysis (9, 15). In addition, elevated levels of HNF3β in transgenic mice are associated with increased levels of expression of HNF3-responsive genes, such as the insulin-like growth factor binding protein 1 gene (16). Additional cell culture analysis with viral replication-competent genomes suggests HNF3β may reduce viral transcription by interfering with the transcriptional elongation step rather than affecting promoter activity (22).
RNase protection analysis demonstrated that the increased expression of HNF3β affects the relative level of expression of the precore and pregenomic 3.5-kb RNA transcripts in HBV transgenic mice (Fig. 4). The ratio of the precore to pregenomic transcripts increases approximately threefold due to the expression of HNF3β. This observation is similar to the effect of HNF3β expression on precore and pregenomic RNA synthesis in cell culture (21, 22). Therefore, HNF3β preferentially inhibits pregenomic RNA synthesis compared with precore RNA in vivo, as well as in cell culture. HNF3β also inhibits the level of the HBV 2.4-kb transcript approximately fourfold in vivo (Fig. 5). This indicates that all of the HBV transcripts are susceptible to HNF3β-mediated inhibition of RNA synthesis. Therefore, increased expression of HNF3β appears to coordinately downregulate HBV transcription. The coordinate downregulation of the HBV transcription by HNF3β would be expected to translate into a corresponding reduction in viral replication.
The level of viral replication intermediates and associated cytoplasmic core protein in the liver of the rat HNF3β-expressing HBV transgenic mice was dramatically reduced compared with the control rat HNF3β(−) HBV transgenic mice (Fig. 3 and 6). The reduction in viral replication intermediates due to the elevated level of HNF3β expression is greater than the reduction in HBV 3.5-kb RNA (Fig. 3C). This observation is due in part to the increase in HNF3β expression decreasing pregenomic RNA synthesis to a greater extent than precore RNA synthesis (Fig. 4B), which appears to contribute to the disproportionate decrease in viral replication compared with HBV RNA synthesis. Regardless of the mechanisms of HNF3β-mediated downregulation of HBV replication, it appears that any physiological stimulus that modulates HNF3 isoform expression may significantly affect virus synthesis. In addition, increasing the level of the HNF3β isoform in the liver may represent an appropriate target for HBV antiviral therapy.
Acknowledgments
We thank Luca G. Guidotti and Francis V. Chisari (The Scripps Research Institute, La Jolla, Calif.) for providing the HBV transgenic mice. We are grateful to Susan L. Uprichard (The Scripps Research Institute) for the plasmid pmGAPDH containing the mouse GAPDH cDNA. We are grateful to Margie Chadwell for preparation and staining of tissue sections. We thank Francis V. Chisari for many helpful discussions and critical reading of the manuscript.
This work was supported by a postdoctoral fellowship from the West China University of Medical Sciences of the People's Republic of China to H.T. and by Public Health Service grant AI30070 from the National Institutes of Health.
Footnotes
Publication no. 14998-CB from The Scripps Research Institute.
REFERENCES
- 1.Chisari, F. V. 2000. Viruses, immunity, and cancer: lessons from hepatitis B. Am. J. Pathol. 156:1118-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 3.Dudov, K. P., and R. P. Perry. 1984. The gene family encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell 37:457-468. [DOI] [PubMed] [Google Scholar]
- 4.Galibert, F., E. Mandart, F. Fitoussi, P. Tiollais, and P. Charnay. 1979. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature 281:646-650. [DOI] [PubMed] [Google Scholar]
- 5.Ganem, D., and H. E. Varmus. 1987. The molecular biology of the hepatitis B viruses. Annu. Rev. Biochem. 56:651-693. [DOI] [PubMed] [Google Scholar]
- 6.Guidotti, L. G., and F. V. Chisari. 2000. Cytokine-mediated control of viral infections. Virology 273:221-227. [DOI] [PubMed] [Google Scholar]
- 7.Guidotti, L. G., C. M. Eggers, A. K. Raney, S. Y. Chi, J. M. Peters, F. J. Gonzalez, and A. McLachlan. 1999. In vivo regulation of hepatitis B virus replication by peroxisome proliferators. J. Virol. 73:10377-10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guidotti, L. G., B. Matzke, H. Schaller, and F. V. Chisari. 1995. High-level hepatitis B virus replication in transgenic mice. J. Virol. 69:6158-6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson, J. L., A. K. Raney, and A. McLachlan. 1995. Characterization of a functional hepatocyte nuclear factor 3 binding site in the hepatitis B virus nucleocapsid promoter. Virology 208:147-158. [DOI] [PubMed] [Google Scholar]
- 10.Kosovsky, M. J., I. Qadri, and A. Siddiqui. 1998. The regulation of hepatitis B virus gene expression: an overview of the cis- and trans-acting components, p. 21-50. In R. Koshy and W. H. Caselmann (ed.), Hepatitis B virus: molecular mechanisms in disease and novel strategies for therapy. Imperial College Press, London, United Kingdom.
- 11.Lee, Y.-H., B. Sauer, and F. J. Gonzalez. 1998. Laron dwarfism and non-insulin-dependent diabetes mellitus in the Hnf-1α knockout mouse. Mol. Cell. Biol. 18:3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raney, A. K., A. J. Easton, and A. McLachlan. 1994. Characterization of the minimal elements of the hepatitis B virus large surface antigen promoter. J. Gen. Virol. 75:2671-2679. [DOI] [PubMed] [Google Scholar]
- 13.Raney, A. K., A. J. Easton, D. R. Milich, and A. McLachlan. 1991. Promoter-specific transactivation of hepatitis B virus transcription by a glutamine- and proline-rich domain of hepatocyte nuclear factor 1. J. Virol. 65:5774-5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raney, A. K., and A. McLachlan. 1991. The biology of hepatitis B virus, p. 1-37. In A. McLachlan (ed.), Molecular biology of the hepatitis B virus. CRC Press, Boca Raton, Fla.
- 15.Raney, A. K., P. Zhang, and A. McLachlan. 1995. Regulation of transcription from the hepatitits B virus large surface antigen promoter by hepatocyte nuclear factor 3. J. Virol. 69:3265-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rausa, F. M., Y. Tan, H. Zhou, K. W. Yoo, D. B. Stolz, S. C. Watkins, R. R. Franks, T. G. Unterman, and R. H. Costa. 2000. Elevated levels of hepatocyte nuclear factor 3β in mouse hepatocytes influence expression of genes involved in bile acid and glucose homeostasis. Mol. Cell. Biol. 20:8264-8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabath, D. E., H. E. Broome, and M. B. Prystowsky. 1990. Glyceraldehyde-3-phosphate dehydrogenase mRNA is a major interleukin 2-induced transcript in a cloned T-helper lymphocyte. Gene 91:185-191. [DOI] [PubMed] [Google Scholar]
- 18.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 19.Schaller, H., and M. Fischer. 1991. Transcriptional control of hepadnavirus gene expression. Curr. Top. Microbiol. Immunol. 168:21-39. [DOI] [PubMed] [Google Scholar]
- 20.Tang, H., K. E. Banks, A. L. Anderson, and A. McLachlan. 2001. Hepatitis B virus transcription and replication. Drug News Perspect. 14:325-334. [PubMed] [Google Scholar]
- 21.Tang, H., and A. McLachlan. 2001. Transcriptional regulation of hepatitis B virus by nuclear hormone receptors is a critical determinant of viral tropism. Proc. Natl. Acad. Sci. USA 98:1841-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang, H., and A. McLachlan. 2002. Mechanisms of inhibition of nuclear hormone receptor-dependent hepatitis B virus replication by hepatocyte nuclear factor 3β. J. Virol. 76:8572-8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Will, H., W. Reiser, T. Weimer, E. Pfaff, M. Buscher, R. Sprengle, R. Cattaneo, and H. Schaller. 1987. Replication strategy of human hepatitis B virus. J. Virol. 61:904-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yen, T. S. B. 1993. Regulation of hepatitis B virus gene expression. Semin. Virol. 4:33-42. [Google Scholar]
- 25.Zhou, H., D. E. Hughes, M. L. Major, K. Yoo, C. Pesold, and R. H. Costa. 2001. Atypical mouse cerebellar development is caused by ectopic expression of the forkhead box transcription factor HNF-3beta. Gene Expr. 9:217-236. [DOI] [PMC free article] [PubMed] [Google Scholar]