Abstract
Cells require optimal substrate stiffness for normal function and differentiation. The mechanisms for sensing matrix rigidity and durotaxis, however, are not clear. Here we showed that control, Shp2−/−, integrin β1−/−, and talin1−/− cell lines all spread to a threefold greater area on fibronectin (FN)-coated rigid polyacrylamide surfaces than soft. In contrast, RPTPα−/− cells spread to the same area irrespective of rigidity on FN surfaces but spread 3× greater on rigid collagen IV-coated surfaces than soft. RPTPα and αvβ3 integrins were shown previously to be colocalized at leading edges and antibodies to αvβ3 blocked FN rigidity sensing. When FN beads were held with a rigid laser trap at the leading edge, stronger bonds to the cytoskeleton formed than when held with a soft trap; whereas back from the leading edge and in RPTPα−/− cells, weaker bonds were formed with both rigid and soft laser traps. From the rigidity of the trap, we calculate that a force of 10 pN generated in 1 s is sufficient to activate the rigidity response. We suggest that RPTPα and αvβ3 at the leading edge are critical elements for sensing FN matrix rigidity possibly through SFK activation at the edge and downstream signaling.
INTRODUCTION
The stiffness of extracellular matrix (ECM) dramatically affects many cellular processes, such as motility (1,2), phagocytosis (3), and cell differentiation (4). However, different cell types prefer different matrix rigidities (5). Normal fibroblasts do not spread fully, form extensive focal contacts, or grow on soft substrates (2,6). Endothelial cells from human umbilical vein are more spread and have larger lumens and less branching on stiffer collagen gels (7). Myocytes only formed myotubes with striations on gels of intermediate stiffness, and changes in myoblast behavior were related to differences in stiffness of healthy and diseased muscle tissue (5,8). On the other hand, hepatocytes maintain a differentiated phenotype only on soft materials, and changes in mechanical properties during liver disease may be partially responsible for the deterioration of hepatocyte networks (5,9). Oncogenically transformed cells can grow on gelatin-coated materials softer than 100 Pa, whereas nontransformed cells cannot survive (10,11). Primary neuron cultures from mouse spinal cords branched more frequently on soft substrates (12). Thus, the rigidity response can contribute to differentiation of cells within a particular tissue (5) and is a major factor in cancers and other disease states as well (13,14).
In fibroblast cells, matrix-integrin interactions on the active lamellipodia cause indirect attachment of integrins to actin filaments (15). As the filaments are moved rearward by myosin motors, they generate force on the matrix when it resists movement. Force increases rapidly with small movements when the matrix is rigid or more slowly with larger movements when the matrix is soft (16). Fibroblasts sense substrate rigidity and move toward rigid areas both in three dimensions (4,17) and in two dimensions, a phenomenon defined as durotaxis (1), by an unknown process(es). Generation of periodic contractions in extending lamellipodia appears to be linked to the mechanical probing of the ECM rigidity by the cell (18). However, to date, no specific molecule has been shown to be the sensor of the fibronectin (FN) matrix rigidity although periodic rows of β3 integrin clusters were observed in spreading cells (18).
αvβ3 integrin has important roles in the migration and invasion of melanoma cells (19,20), vascular endothelial cells (21), and primary tumor growth and metastasis in vivo (20,21). It forms a complex with RPTPα, a receptor-like protein tyrosine phosphatase, at the leading edge early in spreading (22). RPTPα was identified previously as a component involved in the cellular response to force (22). Gene inactivation of RPTPα delays spreading on FN, impairs activation of Src family kinases (SFK) (23,24), and compromises correct positioning of pyramidal neurons during development of mouse hippocampus (25). Those studies identified RPTPα as a key component for proper radial neuronal migration (25). In this study, we investigated the function of αvβ3 integrin and RPTPα in FN matrix rigidity sensing by measuring the cell spread area on different stiffness polyacrylamide gel surfaces and the strength of cytoskeleton bonding to FN-coated beads with laser traps of different stiffness. Our data indicate that rigidity sensing involves position-dependent changes in force on the RPTPα/αvβ3 complex at the leading edge.
METHODS AND MATERIALS
Cell culture and materials
Mouse fibroblast cells (FAK+/+, FAK−/−, RPTPα+/+, RPTPα−/−, Shp2+/+, Shp2−/−, β1M−/−, Talin1−/−) were maintained at 37°C and 5% CO2 in Dulbecco's Modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Invitrogen), 2 mM L-glutamine, 50 μg/ml streptomycin, and 50 units/ml penicillin. Anti-αvβ3 (clone LM609, Chemicon, Temecula, CA) antibody was included at optimal concentration in the integrin binding inhibition experiments.
Cell spreading on polyacrylamide substrates and microscopy
The polyacrylamide substrates were prepared and coated with FN and collagen IV as described previously (2). The flexibility of the substrate was manipulated by maintaining the total acrylamide concentration at 8% while varying the bis-acrylamide components between 0.4% (rigid surface) and 0.03% (soft surface). The Young's modulus of the polyacrylamide substrates was measured and calculated as described by Pelham et al. (2). The uniformity of FN coating on the substrate surface was examined by coating the gels with Alexa 568 labeled FN and observed by immunofluorescence microscopy. Experiments were performed 15 h after the cells were plated on the polyacrylamide gel at a low density. Phase contrast images were recorded with a cooled charge-coupled device camera attached to an Olympus IX81 equipped with a 10× objective. The spread area of individual cells was quantified with Image J software. At least 50 cells were counted for each cell line under each condition.
Breaking events assay
Cells were plated on acid-washed, silane-treated coverslips coated with laminin (40 μg/ml). With an optical-gradient laser trap as described previously (26), FN-coated beads were held at the leading edge or 2 μm away from the leading edge of the lamellipodia for 3 s, then released by turning off the laser power. If the bead bound to the cell membrane, the laser was turned on again. The movement of the bead restrained by the laser trap was recorded until the bead was finally pulled out of the trap. Breaking events were defined by the rapid movement of beads to the center of the laser trap after the cell started to move the bead rearward.
RESULTS AND DISCUSSION
Rigidity-dependent spreading requires RPTPα
To determine which protein(s) may be involved in the rigidity-sensing process, we screened a number of knockout cell lines for their ability to spread on rigid versus soft polyacrylamide substrates coated with FN (see Fig. 1 A). As shown in Fig. 1 B, the spread areas of wild-type cells (RPTPα+/+, FAK+/+, Shp2+/+) were two- to threefold greater on rigid than on soft surfaces. Similarly, Shp2−/−, talin1−/−, and integrin β1−/− cells spread more on rigid than on soft surfaces.
FIGURE 1.
Screening of FN rigidity sensing response of different cell lines. (A) Control cells were spread on rigid versus soft FN-coated polyacrylamide substrates. The spread area of individual cells was measured with Image J. Scale bar, 40 μm. (B) Spread area of different cell lines on rigid and soft FN substrates. Results shown are the mean ± SD of at least 50 cells. Asterisks indicate significant statistical difference between the rigid and soft surface, p < 0.01.
In contrast, FAK−/− cells had only a very small contact area on rigid surfaces but spread to the same area as controls on soft polyacrylamide surfaces. Since myosin or Rho-kinase (ROCK) inhibition caused FAK−/− cell contact areas to increase to control cell areas on glass (27), we believed that hypercontraction produced the small contact area. In our assay of FAK−/− cell spreading on rigid FN polyacrylamide gels, ROCK inhibition caused an increase in the contact area (data not shown). Consequently, we believed that rigidity was sensed in the FAK−/− cells and the normal increase in contraction on rigid surfaces was accentuated in the absence of FAK.
We found one mutant cell line, RPTPα−/− cells, that spread to the same area on soft and rigid surfaces (p < 0.21, t-test) (Figs. 1 B and 3 B). When cells were cultured on FN-coated surfaces with varying stiffness (Fig. 2 B) by changing the concentration of bis-acrylamide (0.03%, 0.08%, 0.2%, and 0.4%), RPTPα+/+cells spread to larger areas as the matrix rigidity increased, whereas RPTPα−/− cells did not show the stiffness-dependent spreading behavior (Fig. 2 C). When the level of RPTPα in wild-type cells was reduced by SiRNA (Fig. 2 A), cells spread to an even smaller area than RPTPα−/− cells and were unable to sense the matrix rigidity changes (Fig. 2 C). Thus, we suggest that RPTPα is critical for the matrix rigidity sensing process.
FIGURE 3.
Rigidity sensing of FN matrix is dependent on αvβ3 integrins and RPTPα, whereas rigidity sensing on collagen IV matrix is RPTPα independent. (A) Spread area of RPTPα+/+ and RPTPα−/− cells plated on FN-coated polyacrylamide substrate with or without 10 μg/ml LM609. (B) The morphologies of RPTPα−/− cells on FN and Collagen IV (Col)-coated substrates. (C) Spread area of RPTPα+/+ and RPTPα−/− cells plated on collagen IV-coated polyacrylamide substrates. Results shown are the mean ± SD of at least 50 cells. Asterisks indicate a significant statistical difference between the rigid and soft surface, p < 0.01. Scale bar, 40 μm.
FIGURE 2.
Rigidity sensing of FN matrix is RPTPα dependent. (A) Western blot showing the expression of RPTPα in control (CT) and knockdown (A1 and A2) cells. (B) Mechanical properties of polyacrylamide substrate. The Young's modulus (N/m2) of polyacrylamide gels with a range of bis-acrylamide (0.03%, 0.08%, 0.2%, and 0.4%) to acrylamide (8%) was measured as described by Pelham et al. (2). (C) The spread areas of RPTPα+/+, RPTPα−/−, and RPTPα knockdown (A2) cells on polyacrylamide substrates of different rigidities. Results shown are the mean ± SD of at least 50 cells.
αvβ3 integrin involvement in the rigidity sensing process
Because RPTPα formed a complex with αvβ3 at the leading edge (22), we incubated control fibroblasts with anti-αvβ3 monoclonal antibody LM609 at 10 μg/ml (28,29) during cell spreading. As shown in Fig. 3 A, RPTPα+/+ cells treated with LM609 showed no difference in spread area on rigid versus soft FN polyacrylamide surfaces (p < 0.1, t-test), which indicated that the cells lost their ability to sense FN rigidity when the binding of αvβ3 to FN was blocked. Treatment of RPTPα−/− cells with LM609 had no effects on the spread area (p < 0.45, t-test). Thus, αvβ3 integrin was an important component for FN rigidity sensing at the leading edge during early spreading, possibly through an interaction with RPTPα.
To determine if RPTPα−/− cells could sense matrix rigidity through other integrins that did not bind to RPTPα (22), we plated cells on rigid versus soft polyacrylamide substrate coated with collagen IV, which binds to the α1β1 integrin (30). Both control and RPTPα−/− cells spread to threefold greater area on the rigid collagen IV surfaces (Fig. 3 C). Thus, it was clear that α1β1 integrins can participate in rigidity sensing through a mechanism that does not involve RPTPα.
Matrix rigidity sensing at the leading edge
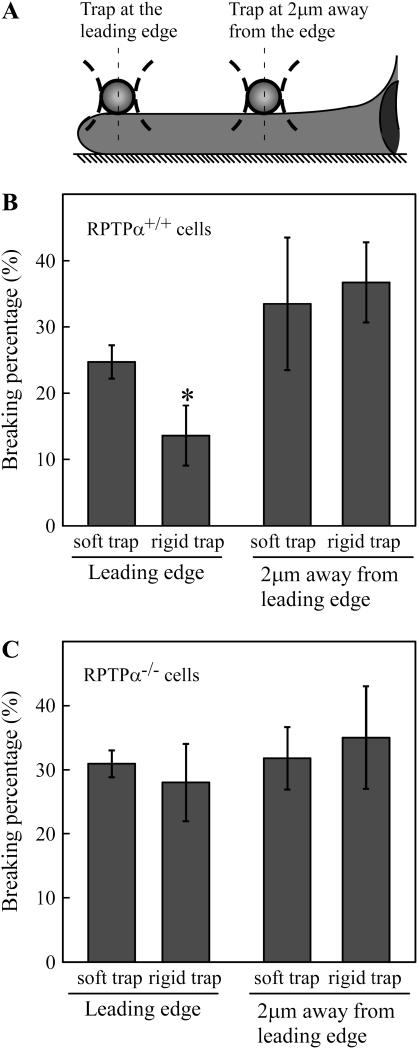
Because the two membrane proteins (αvβ3 and RPTPα) implicated in rigidity sensing were at the leading edge (22), we compared rigidity sensing at the edge and two microns back from the edge using the laser trap microscope (Fig. 4 A). The rigidity of beads held in the laser trap was directly proportional to the laser power. There were two cellular responses to rigid surfaces: 1), activation of leading edge extension (18), and 2), increased strengthening of bonds between integrins and the cytoskeleton with increased recruitment of focal complex proteins (22). Increased extension was manifested as an increase in spread area (as above); however, increased strengthening of bonds could be measured by increased resistance of beads to movement by the laser trap. To quantify bond strength, we measured the frequency of breaking of bonds between FN beads and the actin cytoskeleton as the cell moved the beads toward the nucleus at the rate of ∼60 nm/s. With soft (0.02 pN/nm) laser trap, there was nearly twice the number of breaking events than with rigid (0.18 pN/nm) trap (Fig. 4 B), which indicated that the rigid trap caused stronger bonds to the cytoskeleton to form at the leading edge. When beads were placed 2 microns back from the edge, there was the same number of breaking events for both rigid and soft laser trap, matching the weaker bonding with the soft trap at the edge. Thus, the rigid trap was only sensed at the leading edge, where it increased the strengthening of FN-cytoskeleton linkages.
FIGURE 4.
Rigidity sensing is related to the strength of FN bead linkages to the cytoskeleton and is RPTPα dependent. (A) Schematic graph showing the position of rigid or soft trap on the lamellipodia of a spreading cell. (B) Breaking percentage of FN beads placed on the lamellipodia of RPTPα+/+ cells. (C) Breaking percentage of FN beads placed on the lamellipodia of RPTPα−/− cells. Results shown are the mean ± SD of three independent experiments. Asterisks indicate significant statistical difference between the two groups, p < 0.01.
An alternative explanation for the increased bonding to the cytoskeleton with the increased laser trap rigidity was that the increased light intensity caused photo-induced cross-linking. To control for possible photodamage, we made the soft trap appear rigid by rapidly shifting the stage 500 nm to the point of highest force in the trap, causing a rapid increase in force. When the 0.02 pN/nm laser trap was moved 500 nm from the cell center to generate higher force instantaneously, we found fewer bond-breaking events (data not shown). This indicated that the soft trap could mimic rigid trap by a rapid increase in the force on the bead, which caused stronger bonds to form between the beads and the cytoskeleton without laser photodamage.
If RPTPα was involved in the bead response to laser trap rigidity, then RPTPα−/− cells should not show rigidity dependence in the frequency of breaking events even at the edge. Indeed, the frequency of breaking events was the same for soft and rigid laser traps at the edge (Fig. 4 C), and the high fraction of breaking events was similar to the frequency when beads were placed 2 microns back from the edge. Thus, we found that the edge response to laser traps rigidity was dependent upon RPTPα.
Rigidity sensing downstream of αvβ3 and RPTPα
In this study we demonstrate that the membrane proteins RPTPα and αvβ3 are involved in the sensing of FN matrix rigidity at the leading edge during early spreading. Further steps in the rigidity-sensing pathway are in question. Previous experiments have demonstrated that αvβ3 integrins and RPTPα are involved in the activation of SFKs during early spreading (22). Either the absence of RPTPα or inhibition of FN binding to αvβ3 (with an antibody or an inhibitory peptide, Gpen) significantly reduces SFK activation (22), which parallels the effects on rigidity sensing. In contrast, talin1−/− cells, which are defective in force-dependent reinforcement of FN bead-cytoskeleton bonds but have normal SFK activation (31), display normal rigidity sensing behavior (Fig. 1 B). Thus, we suggest that SFK activation is critical in the FN rigidity sensing process.
Previous studies indicated that the sensing of rigid substrates involves either the stimulation of a tyrosine kinase or inhibition of a tyrosine phosphatase (2). With more rigid substrates, there is greater tyrosine phosphorylation in NIH3T3 fibroblasts; and cells on soft substrates spread to the area of cells on rigid substrates after treatment with PAO, a phosphatase inhibitor (2). Our data suggest more specifically that the RPTPα-dependent activation of SFK is essential for the rigidity sensing process on FN. In studies of the cellular response to substrate stretch, cytoskeleton stretch activates SFK-dependent phosphorylation of p130Cas (32) and p130Cas−/− cells are defective in matrix rigidity sensing (A. Kostic and M. P. Sheetz, unpublished results). P130Cas is also localized to the leading edge (33). Thus, rigidity sensing appears to involve SFK phosphorylation of substrates at the leading edge.
Models of rigidity sensing
Mechanically, there are two basic mechanisms that could account for the cell's ability to sense matrix rigidity: 1), a time-dependent change in force or 2), a position-dependent change in force. Our data show that rigidity sensing is position dependent and from the characteristics of the laser traps, we can estimate the level of force and the distances involved. With the rapid displacement of the 0.02 pN/nm laser trap by 500 nm, a force of ∼10 pN is produced that is sufficient to cause increased attachment to the cytoskeleton. Therefore, we suggest that a rapid application of 10 pN of force can elicit the rigidity response. In the case of the rigid trap (0.18 pN/nm), a force of 10–20 pN is reached after 50–100 nm of displacement. Another way of considering the rigidity response is to consider the velocity of actin filament movement in lamellipodia, 60 nm/s (19). At that velocity, the force of 10–20 pN will be reached within 1–2 s. Thus, the rigidity response can be caused either by the cell pulling on a rigid surface or by an active matrix pulling on the cell. In both cases, a rise in force on the cytoskeleton should occur within 50–100 nm of the initial binding site to elicit a rigidity response (Fig. 5).
FIGURE 5.
Model of FN matrix rigidity sensing. On a rigid surface, the quick rise in force to a threshold level X will expose a site in the linker protein complex within the leading edge, where SFK is localized and can cause the reinforcement of this site by phosphorylating the linker protein complex. The reinforced site can then stabilize the edge and cause further cell extension. On soft surfaces, however, the force will only rise to X farther away from the edge, and SFKs are not available to phosphorylate the substrate to reinforce the site.
At a biochemical level, the increase in phosphorylation can be explained by several different mechanisms. One possibility that fits with components known to be involved is a position and force-dependent phosphorylation (Fig. 5). If a phosphorylation site in the FN-cytoskeleton linkage is exposed by force and the kinase that reacts with that site is restricted to the leading edge, then only in the rigid case will the kinase be close enough to the site to phosphorylate it. Force-dependent activation of p130Cas for SFK phosphorylation has been observed upon cytoskeleton stretch (Y. Sawada, M. Tamada, O. Cherniavskaya, and M. P. Sheetz, unpublished results), and SFKs are able to bind to matrix binding sites. Thus, we believe that FN rigidity sensing occurs at the leading edge of active lamellipodia and produces signals that cause cell migration toward more rigid FN matrices or durotaxis. Further studies are required to define the critical proteins and the actual mechanism of tyrosine phosphorylation in response to substrate rigidity.
Acknowledgments
We thank G. Giannone, O. Rossier, and H. Xenias for microscope support, and A. Kostic, M. Tamada, A. S. Meshel, H. G. Doebereiner, Y. Sawada, and J. Liu for helpful discussion.
This work was supported by a grant from NIH to M.P.S.
References
- 1.Lo, C. M., H. B. Wang, M. Dembo, and Y. L. Wang. 2000. Cell movement is guided by the rigidity of the substrate. Biophys. J. 79:144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pelham, R. J. Jr., and Y. Wang. 1997. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA. 94:13661–13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beningo, K. A., C. M. Lo, and Y. L. Wang. 2002. Flexible polyacrylamide substrata for the analysis of mechanical interactions at cell-substratum adhesions. Methods Cell Biol. 69:325–339. [DOI] [PubMed] [Google Scholar]
- 4.Cukierman, E., R. Pankov, D. R. Stevens, and K. M. Yamada. 2001. Taking cell-matrix adhesions to the third dimension. Science. 294:1708–1712. [DOI] [PubMed] [Google Scholar]
- 5.Georges, P. C., and P. A. Janmey. 2005. Cell type-specific response to growth on soft materials. J. Appl. Physiol. 98:1547–1553. [DOI] [PubMed] [Google Scholar]
- 6.Yeung, T., P. C. Georges, L. A. Flanagan, B. Marg, M. Ortiz, M. Funaki, N. Zahir, W. Ming, V. Weaver, and P. A. Janmey. 2005. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton. 60:24–34. [DOI] [PubMed] [Google Scholar]
- 7.Sieminski, A. L., R. P. Hebbel, and K. J. Gooch. 2004. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp. Cell Res. 297:574–584. [DOI] [PubMed] [Google Scholar]
- 8.Engler, A. J., M. A. Griffin, S. Sen, C. G. Bonnemann, H. L. Sweeney, and D. E. Discher. 2004. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 166:877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semler, E. J., C. S. Ranucci, and P. V. Moghe. 2000. Mechanochemical manipulation of hepatocyte aggregation can selectively induce or repress liver-specific function. Biotechnol. Bioeng. 69:359–369. [DOI] [PubMed] [Google Scholar]
- 10.Wang, H. B., M. Dembo, and Y. L. Wang. 2000. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am. J. Physiol. Cell Physiol. 279:C1345–C1350. [DOI] [PubMed] [Google Scholar]
- 11.Putman, D. L., D. K. Park, J. S. Rhim, A. F. Steuer, and R. C. Ting. 1977. Correlation of cellular aggregation of transformed cells with their growth in soft agar and tumorigenic potential. Proc. Soc. Exp. Biol. Med. 155:487–494. [DOI] [PubMed] [Google Scholar]
- 12.Flanagan, L. A., Y. E. Ju, B. Marg, M. Osterfield, and P. A. Janmey. 2002. Neurite branching on deformable substrates. Neuroreport. 13:2411–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kusaka, K., Y. Harihara, G. Torzilli, K. Kubota, T. Takayama, M. Makuuchi, M. Mori, and S. Omata. 2000. Objective evaluation of liver consistency to estimate hepatic fibrosis and functional reserve for hepatectomy. J. Am. Coll. Surg. 191:47–53. [DOI] [PubMed] [Google Scholar]
- 14.Wong, J. Y., A. Velasco, P. Rajagopala, and Q. Pham. 2003. Directed movement of vascular smooth muscle cells on gradient-compliant hydrogels. Langmuir. 19:1908–1913. [Google Scholar]
- 15.Felsenfeld, D. P., D. Choquet, and M. P. Sheetz. 1996. Ligand binding regulates the directed movement of beta1 integrins on fibroblasts. Nature. 383:438–440. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz, U. S., N. Q. Balaban, D. Riveline, A. Bershadsky, B. Geiger, and S. A. Safran. 2002. Calculation of forces at focal adhesions from elastic substrate data: the effect of localized force and the need for regularization. Biophys. J. 83:1380–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandeville, J. T., and F. R. Maxfield. 1997. Effects of buffering intracellular free calcium on neutrophil migration through three-dimensional matrices. J. Cell. Physiol. 171:168–178. [DOI] [PubMed] [Google Scholar]
- 18.Giannone, G., B. J. Dubin-Thaler, H. G. Dobereiner, N. Kieffer, A. R. Bresnick, and M. P. Sheetz. 2004. Periodic lamellipodial contractions correlate with rearward actin waves. Cell. 116:431–443. [DOI] [PubMed] [Google Scholar]
- 19.Aznavoorian, S., M. L. Stracke, J. Parsons, J. McClanahan, and L. A. Liotta. 1996. Integrin alphavbeta3 mediates chemotactic and haptotactic motility in human melanoma cells through different signaling pathways. J. Biol. Chem. 271:3247–3254. [DOI] [PubMed] [Google Scholar]
- 20.Li, X., B. Chen, S. D. Blystone, K. P. McHugh, F. P. Ross, and D. M. Ramos. 1998. Differential expression of alphav integrins in K1735 melanoma cells. Invasion Metastasis. 18:1–14. [DOI] [PubMed] [Google Scholar]
- 21.Kiosses, W. B., S. J. Shattil, N. Pampori, and M. A. Schwartz. 2001. Rac recruits high-affinity integrin alphavbeta3 to lamellipodia in endothelial cell migration. Nat. Cell Biol. 3:316–320. [DOI] [PubMed] [Google Scholar]
- 22.von Wichert, G., G. Jiang, A. Kostic, K. De Vos, J. Sap, and M. P. Sheetz. 2003. RPTP-alpha acts as a transducer of mechanical force on alphav/beta3-integrin-cytoskeleton linkages. J. Cell Biol. 161:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponniah, S., D. Z. Wang, K. L. Lim, and C. J. Pallen. 1999. Targeted disruption of the tyrosine phosphatase PTPalpha leads to constitutive downregulation of the kinases Src and Fyn. Curr. Biol. 9:535–538. [DOI] [PubMed] [Google Scholar]
- 24.Su, J., M. Muranjan, and J. Sap. 1999. Receptor protein tyrosine phosphatase alpha activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr. Biol. 9:505–511. [DOI] [PubMed] [Google Scholar]
- 25.Petrone, A., F. Battaglia, C. Wang, A. Dusa, J. Su, D. Zagzag, R. Bianchi, P. Casaccia-Bonnefil, O. Arancio, and J. Sap. 2003. Receptor protein tyrosine phosphatase alpha is essential for hippocampal neuronal migration and long-term potentiation. EMBO J. 22:4121–4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang, G., G. Giannone, D. R. Critchley, E. Fukumoto, and M. P. Sheetz. 2003. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature. 424:334–337. [DOI] [PubMed] [Google Scholar]
- 27.Chen, B. H., J. T. Tzen, A. R. Bresnick, and H. C. Chen. 2002. Roles of Rho-associated kinase and myosin light chain kinase in morphological and migratory defects of focal adhesion kinase-null cells. J. Biol. Chem. 277:33857–33863. [DOI] [PubMed] [Google Scholar]
- 28.Avizienyte, E., A. W. Wyke, R. J. Jones, G. W. McLean, M. A. Westhoff, V. G. Brunton, and M. C. Frame. 2002. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nat. Cell Biol. 4:632–638. [DOI] [PubMed] [Google Scholar]
- 29.Levinson, H., J. E. Hopper, and H. P. Ehrlich. 2002. Overexpression of integrin alphav promotes human osteosarcoma cell populated collagen lattice contraction and cell migration. J. Cell. Physiol. 193:219–224. [DOI] [PubMed] [Google Scholar]
- 30.Kaido, T., M. Yebra, V. Cirulli, and A. M. Montgomery. 2004. Regulation of human beta-cell adhesion, motility, and insulin secretion by collagen IV and its receptor alpha1beta1. J. Biol. Chem. 279:53762–53769. [DOI] [PubMed] [Google Scholar]
- 31.Giannone, G., G. Jiang, D. H. Sutton, D. R. Critchley, and M. P. Sheetz. 2003. Talin1 is critical for force-dependent reinforcement of initial integrin-cytoskeleton bonds but not tyrosine kinase activation. J. Cell Biol. 163:409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamada, M., M. P. Sheetz, and Y. Sawada. 2004. Activation of a signaling cascade by cytoskeleton stretch. Dev. Cell. 7:709–718. [DOI] [PubMed] [Google Scholar]
- 33.Abassi, Y. A., M. Rehn, N. Ekman, K. Alitalo, and K. Vuori. 2003. p130Cas couples the tyrosine kinase Bmx/Etk with regulation of the actin cytoskeleton and cell migration. J. Biol. Chem. 278:35636–35643. [DOI] [PubMed] [Google Scholar]