Abstract
The Epstein-Barr virus (EBV) immediate-early protein BZLF1 mediates the switch between the latent and lytic forms of EBV infection and has been previously shown to induce a G1/S block in cell cycle progression in some cell types. To examine the effect of BZLF1 on cellular gene expression, we performed microarray analysis on telomerase-immortalized human keratinocytes that were mock infected or infected with a control adenovirus vector (AdLacZ) or a vector expressing the EBV BZLF1 protein (AdBZLF1). Cellular genes activated by BZLF1 expression included E2F-1, cyclin E, Cdc25A, and a number of other genes involved in cell cycle progression. Immunoblot analysis confirmed that BZLF1 induced expression of E2F-1, cyclin E, Cdc25A, and stem loop binding protein (a protein known to be primarily expressed during S phase) in telomerase-immortalized keratinocytes. Similarly, BZLF1 increased expression of E2F-1, cyclin E, and stem loop binding protein (SLBP) in primary tonsil keratinocytes. In contrast, BZLF1 did not induce E2F-1 expression in normal human fibroblasts. Cell cycle analysis revealed that while BZLF1 dramatically blocked G1/S progression in normal human fibroblasts, it did not significantly affect cell cycle progression in primary human tonsil keratinocytes. Furthermore, in EBV-infected gastric carcinoma cells, the BZLF1-positive cells had an increased number of cells in S phase compared to the BZLF1-negative cells. Thus, in certain cell types (but not others), BZLF1 enhances expression of cellular proteins associated with cell cycle progression, which suggests that an S-phase-like environment may be advantageous for efficient lytic EBV replication in some cell types.
Epstein-Barr virus (EBV) is a human gammaherpesvirus and is known to be the causative agent of infectious mononucleosis (37, 57). EBV infection has also been associated with a growing number of malignancies (48, 57). EBV can infect cells in either the latent or lytic form. EBV infection in B cells is predominantly latent, and only a small subset of the viral genes is expressed (57). Expression of one of the EBV immediate-early (IE) proteins, BZLF1 or BRLF1, induces the switch from latent to lytic infection, allowing the virus to form new particles and be transferred to new host cells (15, 16, 55, 60, 73, 81). Although the role of epithelial cell infection in normal EBV pathogenesis remains controversial (24, 65, 66), it is clear that EBV infection is important in the development of certain epithelial tumors (in particular, nasopharyngeal carcinoma) (48). In addition, in immunocompromised patients, EBV causes lesions involving epithelial cells along the lateral border of the tongue known as oral hairy leukoplakia (OHL). OHL is primarily, if not exclusively, due to the lytic form of EBV infection (76).
BZLF1 is a transcriptional activator which binds to AP-1-like sequences (17, 25, 26, 30, 36, 39, 74), and binding sites for BZLF1 are present in most early EBV promoters, as well as the BRLF1 IE promoter (2, 13, 22, 25, 49, 54). BZLF1 initially activates expression of BRLF1. BZLF1 and BRLF1 together are then sufficient to activate expression of the lytic viral replication proteins, followed by the subsequent replication and packaging of the virus (37).
In addition to its effects on IE and early EBV gene transcription, BZLF1 profoundly affects the host cell environment. For example, our research group has shown that BZLFl activates the host cell stress map kinase signaling pathway (1), inhibits host cell gamma interferon signaling (45), and interacts with the tumor suppressor protein p53 (82). These effects of BZLF1 on the host cell environment presumably serve to enhance the efficiency of lytic virus replication.
Many viruses have been shown to manipulate the host cell cycle progression in order to create the optimal conditions for viral replication. Small DNA tumor viruses, which use the host cell DNA polymerase and are completely dependent upon the host cell for nucleotide substrates, drive cells into S phase through the expression of viral proteins which inhibit the functions of p53 and pRb (70). However, the effects of the larger herpesviruses on cell cycle progression are more complex. While the lytic form of herpes simplex virus (HSV) infection induces a G1 block (21, 67), lytic human cytomegalovirus (HCMV) infection has been reported to either inhibit or enhance cell cycle progression (19, 32, 43, 61, 64). Given the larger genome of herpesviruses, which encodes a DNA polymerase as well as a number of DNA-modifying enzymes, the replication of these viruses is much less dependent than the smaller DNA viruses on the host cell. Instead, competition with the host cell for limiting nucleotide pools may be of greater concern for herpesviruses; thus, it could be advantageous for these viruses to prevent host cell DNA replication.
In the case of EBV, viral proteins expressed during type III latency induce cell cycle progression in B cells (9, 50, 51, 68), contributing to the transforming capacity of EBV. However, the effect of lytic EBV infection on cell cycle progression has not been well studied, in part because there is no cell culture system at present that supports efficient primary lytic EBV infection. Using Burkitt's lymphoma lines treated with various inducing agents (which convert a portion of the cells from the latent to lytic form of infection), one group reported that BZLF1-positive cells are primarily in the G0/G1 or G2/M phases of the cell cycle (59), whereas another group found BZLF1-positive cells distributed throughout the cell cycle (31). A potentially confounding variable in these previous studies is that the agents used to induce lytic EBV infection (anti-immunoglobulin M [anti-IgM], phorbol esters, and transforming growth factor β) are quite toxic and produce cell cycle effects and/or apoptosis even in the absence of EBV. In addition, previous studies examining the effects of lytic EBV infection were performed in tumor cell lines, which are likely deficient in healthy cell cycle control mechanisms.
The cell cycle effects of lytic HSV and HCMV infection are primarily attributable to expression of the viral IE proteins (6, 10, 29, 35, 42, 46, 79). Assuming that this is also the case with EBV, we have been examining the effects of BRLF1 and BZLF1 expression on cell cycle progression in various cell types. We previously reported that BRLF1 induces S-phase progression in normal human fibroblasts (71), which is likely due to its abilities to bind pRb, and increases the level of cellular E2F-1 (71, 80). In contrast, expression of the IE protein BZLF1 has been reported by another group to cause a G0/G1 arrest in several epithelial cell tumor lines, with an associated increase in p21, p27, and hypophosphorylated pRb (11, 12, 58). To date, however, the cell cycle effect of BZLF1 has not been examined in primary cell lines.
In this report, we demonstrate that BZLF1 activates expression of a number of cellular proteins associated with G1/S progression in primary human keratinocytes (including E2F-1, cyclin E, and SLBP), as well as in telomerase-immortalized human keratinocytes. In contrast, BZLF1 does not activate E2F-1 expression in normal human fibroblasts. While BZLF1 expression in primary human fibroblasts results in a profound G0/G1 block, it does not significantly inhibit cell cycle progression in telomerase-immortalized, or primary, keratinocytes. Furthermore, we find that the BZLF1-positive population in gastric carcinoma cells infected with EBV contains an increased number of cells in S phase. Our results indicate that BZLF1 has cell type-dependent effects on cell cycle progression. The ability of lytic EBV infection to induce an S-phase-like environment in certain epithelial cell types correlates well with the hyperproliferative nature of OHL.
MATERIALS AND METHODS
Cell culture.
Telomerase-immortalized human keratinocytes (clone 22) were originally derived from human neonatal foreskin as previously described (23). Telomerase-immortalized human keratinocytes were maintained in Keratinocyte-SFM medium (Gibco-BRL) with epidermal growth factor and bovine pituitary extract added. The AGS-EBV cell line (a generous gift from Lindsey Hutt-Fletcher) was obtained by G418 selection of AGS cells (a gastric carcinoma cell line) that were infected with a recombinant Akata virus in which a neomycin resistance cassette had been inserted into the nonessential BDLF3 open reading frame. Both the control AGS cell line and the AGS-EBV cell line were maintained in Ham's F12 medium, supplemented with 10% fetal bovine serum (FBS). Normal human fibroblasts were derived from neonatal foreskin as previously described (34). Normal human fibroblasts were maintained in Eagle's minimal essential medium, supplemented with 10% FBS and nonessential amino acids. All media contained penicillin (100 U/ml) and streptomycin (100 μg/ml). The cells were maintained at 37°C in a humidified atmosphere containing 10% CO2.
Isolation of primary human oropharyngeal epithelial cells.
Human oropharyngeal mucosa from surgical specimens (tonsillectomy and uvulectomy) was used as a source of primary keratinocytes. Surgical specimens were collected in Dulbecco's modified Eagle medium (DMEM) containing 10% FBS and supplemented with penicillin (50 μg/ml) and streptomycin (50 μg/ml) at 4°C. Primary cultures were established by a modified version of the method developed for the cultivation of epidermal keratinocytes (56). By using surgical instruments, 2 to 3 cm2 of mucosa from the anterior tonsillar pillar or uvula was dissected away from underlying tissue. Mucosa was then disinfected by immersion in a solution of 1% povidone iodine for 5 min followed by serial rinses in phosphate-buffered saline (PBS). Mucosa was incubated in 10 ml of 0.25% trypsin (Gibco-BRL) at 4°C for 12 h with agitation. The contents of the digest were added to keratinocyte serum-free medium (KSFM; Gibco-BRL) with 10% FBS, and larger tissue fragments remaining were removed, cut cross-wise with a scalpel, and resuspended. After vigorous pipetting, the sample was passed through a 100-μm-pore-size nylon cell strainer (Falcon 2360), centrifuged, and resuspended in KSFM with 10% FBS. Cells were plated onto collagen-coated plates (0.1 mg of collagen IV per ml in PBS) with a feeder layer of mitomycin C-treated NIH 3T3 fibroblasts (72) and grown at 37°C in KSFM containing 10% FBS supplemented with epidermal growth factor (5 ng/ml), bovine pituitary extract (50 μg/ml), penicillin (100 U/ml), streptomycin (100 μg/ml), ceftazidime (100 μg/ml), tobramycin (40 μg/ml), and amphotericin (1 μg/ml). The medium was changed every 2 days, and when cells were nearly confluent, KSFM containing 10% FBS was replaced by KSFM with no serum added. When there were sufficient cells for experimentation, detachment was accomplished using 0.05% trypsin-EDTA (Gibco-BRL) followed by plating onto collagen-coated plastic tissue culture plates in the absence of a feeder layer.
Adenoviral vectors and infections.
An E1/E3-deficient adenovirus 5 vector expressing the EBV IE protein BZLF1 cDNA (under the control of a HCMV promoter) was made using the recombinant Cre-lox-mediated recombination system as previously described (AdBZLF1) (77). The control adenovirus vector (AdLacZ) is identical to AdBZLF1, except that it contains the bacterial β-galactosidase gene in place of the BZLF1 cDNA. All adenovirus preparations used in this manuscript were confirmed to be free of any detectable wild-type virus and did not produce any detectable E1A after infection of cells.
Adenovirus infections were performed as follows. Telomerase-immortalized human keratinocytes were plated at a density of 2 × 106 cells per 150-mm-diameter plate and infected at an multiplicity of infection (MOI) of 20. The MOIs of various preparations was determined by plaque formation in 293 cells. Primary tonsil keratinocytes were plated at a density of 105 cells per 60-mm-diameter plate and infected at an MOI of 30. AGS and AGS-EBV cells were plated at a cell density of 2 × 106 per 150-mm-diameter plate and infected at an MOI of 5. Normal human fibroblasts were plated at a cell density of 107 per 150-mm-diameter plates, serum starved for 3 days, infected at an MOI of 250 in the absence of serum, 24 h later split 1:5, and replated in the presence of 10% serum. The adenovirus MOI required for each cell type was determined by using an adenovirus expressing the green fluorescence protein or by performing immunofluorescence on AdBZLF1-infected cells and determining the minimal dose required to infect the majority of cells.
Affymetrix GeneChip analysis.
Telomerase-immortalized human keratinocytes were plated at a cell density of 2 × 107 per 150-mm-diameter dish and then either mock infected or infected with adenovirus expressing LacZ or BZLF1 at an MOI of 17. The cells were harvested 48 h later, and total RNA was obtained using the RNAeasy kit (Qiagen). From each condition, cDNA was then synthesized using a T7-(dT)24 primer (cDNA kit from Life Technologies). Biotinylated cRNA was then generated from the cDNA reaction mixture using the BioArray high-yield RNA transcript kit. The cRNA was then fragmented in fragmentation buffer (5× fragmentation buffer consists of 200 mM Tris-acetate [pH 8.1], 500 mM potassium acetate, and 150 mM magnesium acetate) at 94°C for 35 min before the chip hybridization. Fifteen micrograms of fragmented cRNA was then added to a hybridization cocktail. The hybridization cocktail contains the following ingredients: 0.05 μg of fragmented cRNA per μl; 50 pM control oligonucleotide B2; BioB, BioC, BioD, and cre hybridization controls; 0.1 mg of herring sperm DNA per ml; 0.5 mg of acetylated bovine serum albumin (BSA) per ml; 100 mM morpholineethanesulfonic acid (MES); 1 M Na+; 20 mM EDTA; 0.01% Tween 20; and the GeneChip HuGeneFL Array, which provides gene expression data for approximately 5,000 full-length human sequences. Arrays were hybridized for 16 h in the GeneChip Fluidics Station 400 and were washed and scanned with the Hewlett-Packard GeneArray Scanner. During the washing, the cRNA probe was labeled with R-phycoerythrin streptavidin. Affymetrix GeneChip Microarray Suite 4.0 software was used for washing, scanning, and basic analysis. Sample quality was assessed by examination of 3′ to 5′ intensity ratios of certain genes.
Cell cycle analysis.
Cells were infected as described above. Forty-eight hours after infection, cells were incubated with 10 μM bromodeoxyuridine (BrdU) for 60 min at 37°C. Cells were then trypsinized, washed once in 1× PBS, fixed in 1.5 ml of cold 1× PBS plus 3.5 ml of ice-cold 95% ethanol, and incubated at 20°C overnight. After fixation, cells were pelleted, resuspended in 3 ml of 0.8% pepsin in 0.1 N HCl, and incubated for 20 min at 37°C. Lysates were centrifuged, nuclear pellets were suspended in 1.5 ml of 2N HCl, and the suspensions were incubated at 37°C for 20 min. Subsequently 3 ml of 0.1 M sodium borate was added, and the lysates were repelleted. The nuclear pellets were washed in indirect immunofluorescence assay buffer (IFA) (10 mM HEPES [pH 7.4], 150 mM NaCl, 4% FBS, 0.1% sodium azide) containing 0.5% Tween 20. They were then stained with anti-BrdU antibody (1:50; Becton Dickinson) in IFA at room temperature (RT) for 1 h, washed with IFA containing Tween 20, counterstained with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (whole molecule) (diluted 1:100) (Sigma) in IFA at RT for 1 h, and washed with IFA containing Tween 20. Nuclear pellets were suspended in IFA containing 500 μg of RNase A and 50 μg of propidium iodide per ml and incubated at 37°C for 15 min. Fluorescence was measured with a fluorescence-activated cell sorter (FACS; Becton Dickinson).
To compare the number of cells in S phase in BZLF1-positive versus BZLF1-negative AGS-EBV cells, trypsinized AGS-EBV cells were washed once in 1× PBS, fixed in 1.5 ml of cold 1× PBS plus 3.5 ml of ice-cold 95% ethanol, and incubated at 20°C overnight. Cells were then washed with 1× PBS-0.5% bovine serum albumin (BSA), stained with anti-BZLF1 antibody (diluted 1:100) (Argene) in PBS-0.5% BSA for 60 min, washed with 1× PBS-0.5% BSA, and counterstained with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (whole molecule) (diluted 1:100) in 1× PBS-0.5% BSA for 60 min. Cells were then washed, resuspended in 1× PBS containing 500 μg of RNase A per ml and 50 μg of propidium iodide per ml, and incubated at 37°C for 15 min. Fluorescence was measured with a FACS. Cells which were BZLF1 positive (approximately 4% of the total AGS-EBV population) were identified using the BZLF1-specific antibody and by comparison between the AGS-EBV-positive and AGS-EBV-negative cell lines. The percentage of BZLF1-positive versus BZLF1-negative AGS-EBV cells in S phase was then determined by comparing the propidium iodine staining of each population, using Mod-Fit LT analysis. At least 3,000 BZLF1-positive AGS-EBV cells in each of three different experiments were counted to determine the cell cycle distribution of BZLF1-positive cells.
Immunoblotting.
Cell extracts were prepared as previously described (1). Proteins (50 to 100 μg) were separated by polyacrylamide gel electrophoresis and blotted onto a nitrocellulose membrane. The membranes were then blocked in blocking buffer (1× PBS, 0.1% Tween 20, 5% milk) at RT for 60 min and then incubated at RT for 60 min with primary antibody in blocking buffer. The primary antibodies included anti-E2F-1 rabbit polyclonal antibody (diluted 1:500; C20 [Santa Cruz]), anti-cyclin E mouse monoclonal antibody (1:500; BF683 [Santa Cruz]), Cdc25A mouse monoclonal antibody (1:500; F-6 [Santa Cruz]), SLBP rabbit polyclonal antibody (1:2,000; a generous gift of William Marzluff, University of North Carolina at Chapel Hill), and anti-BZLF1 mouse monoclonal antibody (1:200; AZ-69 [Argene]). The membranes were washed in wash buffer (1× PBS, 0.1% Tween 20) and incubated with horseradish peroxidase-conjugated secondary antibody (1:10,000) (Promega) at RT for 60 min. The membranes were washed in wash buffer, and proteins were detected by using an enhanced chemiluminescence (ECL) kit (Amersham).
Apoptosis assay.
Cellular apoptosis was quantitated as suggested by the manufacturer using the ApoAlert Annexin V kit (Clontech), which measures phosphatidylserine on the cell membrane.
RESULTS
Microarray analysis of AdBZLF1-infected telomerase-immortalized human keratinocytes.
To examine the effect of BZLF1 on host cell gene expression, telomerase-immortalized keratinocytes were either mock infected or infected with adenovirus vectors expressing LacZ (AdLacZ) or BZLF1 (AdBZLF1). At 2 days postinfection, cells were harvested and cellular RNA was analyzed using the Affymetrix GeneChip. Interestingly, infection of keratinocytes with the BZLF1 adenovirus vector induced expression of a number of cellular genes, including E2F-1, DP-1, cyclin A, Cdc25A, and cyclin E, which are important for entry into S phase (3, 33, 63), as well as genes (including thymidine kinase and thymidylate kinase) that are expressed preferentially in S phase (41) (Table 1). Although BZLF1 was previously shown to induce a G0/G1 block in HeLa cells (11, 12), the results of the microarray experiment in telomerase-immortalized human keratinocyte cells suggested that BZLF1 is unlikely to induce a G0/G1 cell cycle block in this cell type and instead may be activating cell cycle progression.
TABLE 1.
Representative cellular genes activated by BZLF1 expressiona
| Gene | Fold activation |
|---|---|
| E2F-1 | 16 |
| DP-1 | 43 |
| Cyclin A | 15 |
| Cyclin E | 35 |
| Cdc25A | 18 |
| Thymidine kinase | 5 |
| Thymidylate kinase | 21 |
RNA from telomerase-immortalized human keratinocytes, mock infected or infected with AdLacZ or AdBZLF1, was analyzed by microarray analysis using the Affymetrix GeneChip HuGeneFL Array.
BZLF1 increases the levels of E2F-1, cyclin E, SLBP, and Cdc25A in keratinocytes.
To confirm the results of the microarray experiment, we performed immunoblot analyses to examine the effects of BZLF1 on the levels of E2F-1, cyclin A, Cdc25A, and SLBP in telomerase-immortalized human keratinocytes, primary tonsil keratinocytes, and the AGS gastric carcinoma line. The E2F-1 protein is known to be both essential and sufficient for S-phase entry (38, 47) and activates expression of both cyclin E and Cdc25A (18, 69, 75). Cyclin E is essential for progression from early to late G1 (62), whereas the Cdc25A tyrosine phosphatase is essential for activation of cyclin-dependent kinases in G1 (14, 27). SLBP, which is important for histone mRNA processing, is primarily expressed during cellular S phase (78).
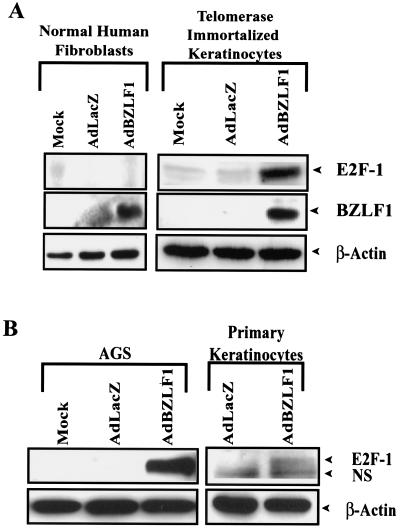
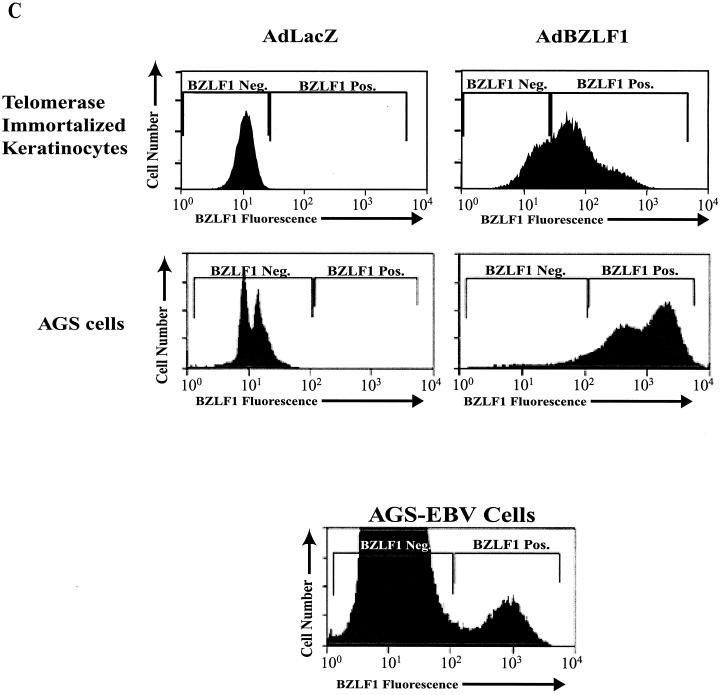
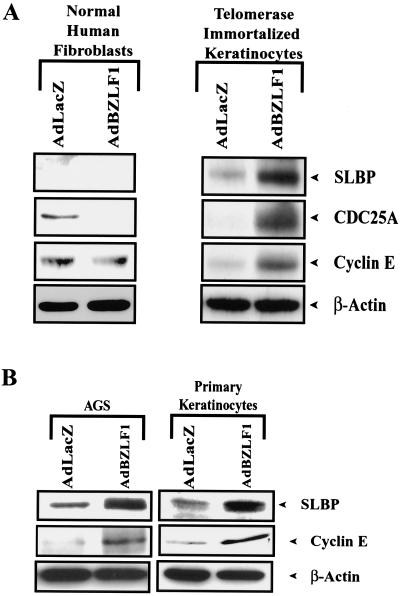
As shown in Fig. 1, BZLF1 increased the level of cellular E2F-1 in telomerase-immortalized human keratinocytes, primary tonsil keratinocytes, and AGS cells, whereas it had no effect on the level of β-actin. In contrast, BZLF1 did not increase the level of E2F-1 in normal human fibroblasts, even though it was expressed at a similar level in these cells. Consistent with its ability to induce E2F-1 in telomerase-immortalized keratinocytes, BZLF1 also enhanced expression of cyclin E, CDC25, and SLBP in these cells (Fig. 2A). Similarly, BZLF1 enhanced expression of the SLBP in primary keratinocytes and AGS cells (Fig. 2B), but not in normal human fibroblasts (Fig. 2A). Infection of AGS cells and telomerase-immortalized keratinocytes with the BZLF1 adenovirus vector at the MOI used in these experiments resulted in a level of cellular BZLF1 expression similar to that of the BZLF1-positive population of AGS cells infected with the intact virus (Fig. 1C). These results suggest that BZLF1 induces E2F-1 expression, and consequently activation of E2F-1-regulated genes, in a cell-specific manner.
FIG. 1.
BZLF1 induces E2F-1 in a cell type-dependent manner. (A) Telomerase-immortalized human keratinocytes and normal human fibroblasts were infected with AdLacZ or AdBZLF1. At 48 h postinfection, the levels of E2F-1, BZLF1, and β-actin were quantitated by immunoblot analysis. (B) AGS gastric carcinoma cells and primary tonsil keratinocytes were infected as in panel A, and the level of E2F-1 was determined by immunoblot analysis. NS, nonspecific cross-reacting band. (C) FACS analysis was performed to compare the level of BZLF1 expression in Ad-BZLF1-infected AGS cells or telomerase-immortalized keratinocytes to that in the constitutively BZLF1-positive population of EBV-AGS cells. Neg., negative; Pos., positive.
FIG. 2.
BZLF1 increases the expression of E2F-1-responsive genes in some cell types. (A) Telomerase-immortalized keratinocytes or normal human fibroblasts were mock infected or infected with the AdLacZ or AdBZLF1 vector. Immunoblot analysis was performed 2 days later to quantitate expression of cyclin E, SLBP, or Cdc25A, as indicated. (B) AGS and primary tonsil keratinocytes were infected with the AdLacZ or AdBZLF1 vector, and immunoblot analysis was performed 2 days later to quantitate expression of cyclin E and SLBP as indicated.
BZLF1 regulates the host cell cycle in a cell type-dependent manner.
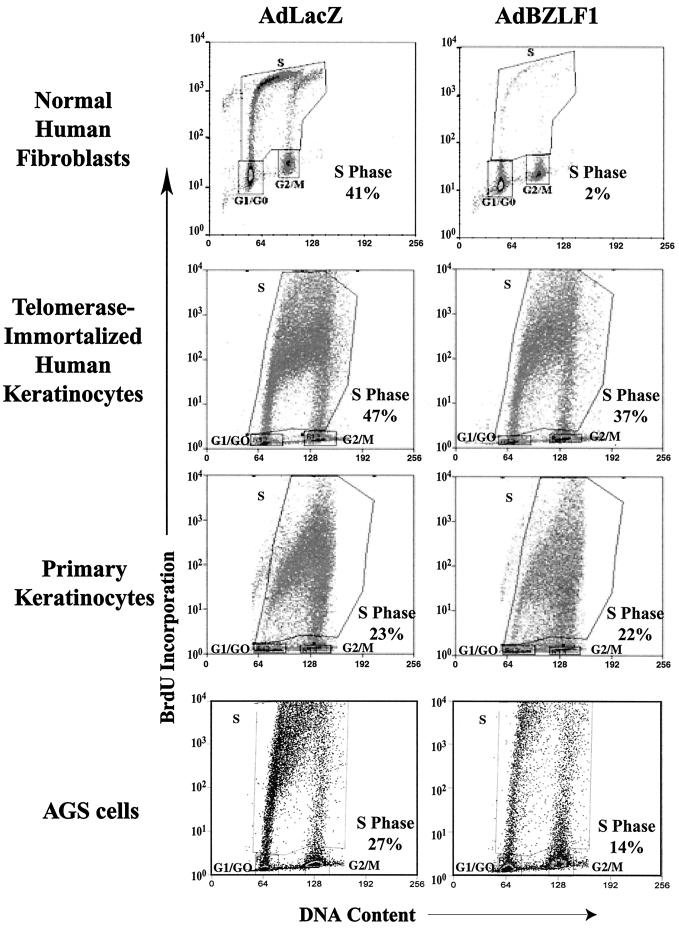
Telomerase-immortalized human keratinocytes, primary keratinocytes, and normal human fibroblasts were mock infected or infected with the AdLacZ or AdBZLF1 vectors, and the effect on cell cycle progression was analyzed by FACS analysis. Infection of serum-starved normal human fibroblasts with the AdBZLF1 vector completely inhibited the ability of these cells to enter S phase following serum stimulation (Fig. 3). AdBZLF1 infection also dramatically reduced the number of healthy human fibroblast cells in S phase in asynchronously growing cultures (data not shown). In contrast, BZLF1 expression produced only a mild G1/S block in the telomerase-immortalized human keratinocytes and AGS cells and did not significantly affect cell cycle progression in primary keratinocytes. Thus, similar to its effects on E2F-1 activation, BZLF1 produces very different effects on host cell cycle progression in telomerase-immortalized and primary human keratinocytes versus normal human fibroblasts.
FIG. 3.
BZLF1 has cell type-dependent effects on cell cycle progression. Normal human fibroblasts, primary keratinocytes, telomerase-immortalized keratinocytes, or AGS cells were infected with the AdLacZ or AdBZLF1 vector. Two days later, the cells were labeled for 1 h with 10 μM BrdU, harvested, fixed, and stained with propidium iodide. BrdU incorporation was quantitated by FACS analysis using a BrdU monoclonal antibody and a fluorescein isothiocyanate-labeled secondary antibody.
The BZLF1 effect on p53 is also dependent on the cell type.
Many viruses regulate cell cycle progression through their effects on the p53 tumor suppressor protein. p53 transcriptionally activates p21, which inhibits the cyclin-dependent kinases. We have previously shown that BZLF1 interacts with and stabilizes the p53 protein (82). There is some evidence that stabilization of p53 may contribute to the ability of BZLF1 to induce cell cycle block in some cell types (12), although we have found that BZLF1 inhibits the transcriptional function of p53 (82).
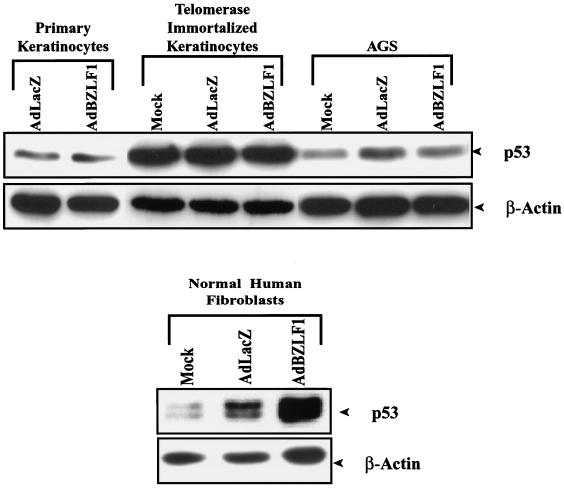
To determine if the effect of BZLF1 expression on p53 is also dependent on the cell type, normal human fibroblasts, telomerase-immortalized or primary human keratinocytes, and AGS cells were mock infected or infected with the AdLacZ or AdBZLF1 vector, and immunoblot analysis for p53 was performed on cell extracts 2 days later. Each of these lines has wild-type p53. As shown in Fig. 4, BZLF1 expression increased the level of cellular p53 in normal human fibroblasts, whereas it had little effect on the level of p53 in keratinocytes and AGS cells. Thus, the ability of BZLF1 to stabilize p53 also appears to be cell type dependent.
FIG. 4.
BZLF1 affects p53 level in a cell type-dependent manner. Primary or telomerase-immortalized keratinocytes and AGS cells or normal human fibroblasts were mock infected or infected with AdLacZ or AdBZLF1. Immunoblot analysis was performed 48 h after infection to quantitate the level of cellular p53.
BZLF1 does not induce significant apoptosis in telomerase-immortalized human keratinocytes.
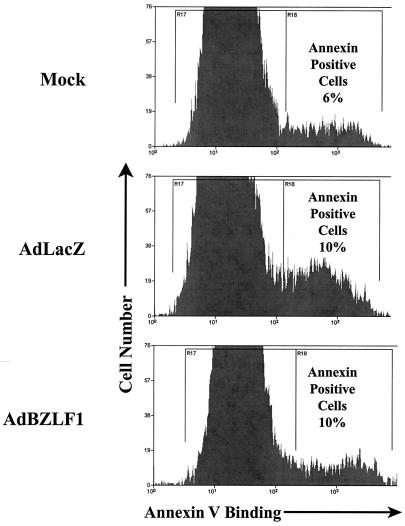
Overexpression of E2F-1 has been shown to induce apoptosis in many cell types (20, 52). We therefore investigated whether BZLF1 enhances apoptosis in keratinocytes. Annexin V binding to mock-infected or AdLacZ- and AdBZLF1-infected telomerase-immortalized human keratinocytes was quantitated by FACS analysis, as shown in Fig. 5. Annexin V binding was not significantly enhanced in the BZLF1-positive cells versus the AdLacZ-infected cells. Similarly, we have previously reported that BZLF1 does not induce apoptosis in normal human fibroblasts (77). Thus, BZLF1 induction of E2F-1 expression is apparently not accompanied by significant apoptosis, possibly due to the previously noted ability of BZLF1 to inhibit p53 function.
FIG. 5.
BZLF1 induction of E2F-1 is not associated with apoptosis. Telomerase-immortalized human keratinocytes were mock infected or infected with AdLacZ or AdBZLF1. At 48 h postinfection, the binding of Annexin V-fluorescein isothiocyanate was quantitated by FACS analysis.
The BZLF1-positive subset of AGS-EBV cells has an increased number of cells in S phase.
The above results suggest that BZLF1 expression in keratinocytes activates expression of some cellular proteins normally associated with cell cycle progression but is not sufficient by itself to induce full S-phase progression. We previously reported that the other EBV IE protein, BRLF1, activates S-phase progression in normal human fibroblasts. Therefore, BZLF1 and BRLF1 together might be sufficient to induce S-phase progression during lytic EBV infection in certain cell types.
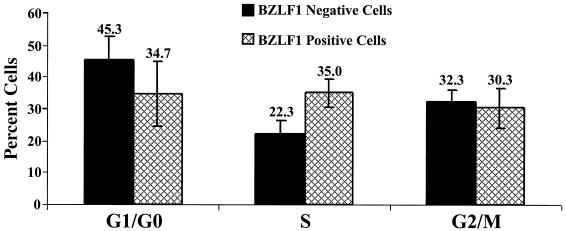
To determine whether lytic EBV infection is associated with S-phase progression in the context of normal EBV infection, we compared the cell cycle distributions of the BZLF1-positive and BZLF1-negative subsets of AGS cells superinfected with EBV. Approximately 4% of AGS-EBV cells express BZLF1 constitutively (Fig. 2C), allowing us to avoid the use of toxic chemical agents that are required for the induction of BZLF1 expression in most other EBV-positive cell lines. The percentage of BZLF1-positive and BZLF1-negative cells in each stage of the cell cycle (from three separate experiments) is shown in Fig. 6; the cell cycle distribution of at least 3,000 BZLF1-positive AGS cells was determined in each experiment. The BZLF1-positive cells had significantly more cells in S phase than the BZLF1-negative cells. Similar results were obtained in the presence of acyclovir, which inhibits the lytic form of EBV replication and prevents expression of viral late genes (data not shown) (37, 57). These results suggest that the two EBV IE proteins together (BZLF1 and BRLF1) induce S-phase progression within the context of normal lytic EBV infection in AGS cells.
FIG. 6.
The BZLF1-positive subset of AGS-EBV cells has an increase in cells in S phase. The cell cycle stages of the BZLF1-positive cells and BZLF1-negative AGS-EBV cells were compared using propidium iodine staining and Mod Fit LT analysis. The percentages of BZLF1-positive and BZLF1-negative AGS-EBV cells in each stage of the cell cycle are shown (average of three separate experiments). The cell cycle distribution of at least 3,000 BZLF1-positive AGS cells was examined in each experiment.
DISCUSSION
Many viruses manipulate the host cell environment in order to optimize the conditions for viral replication. HSV and HCMV have both been shown to affect cell cycle regulation during the lytic form of infection, suggesting that lytic EBV infection might likewise regulate the host cell cycle progression. In this study, we have investigated the cell cycle effects of the EBV IE protein BZLF1 in primary (or telomerase-immortalized) keratinocytes versus normal human fibroblasts. Although it has previously been reported that BZLF1 inhibits cell cycle progression in a variety of tumor cell lines (11, 12, 59), the effect of BZLF1 on cell cycle progression in primary cells has not been previously studied. Somewhat surprisingly, we show here that the cell cycle effects of BZLF1 are dramatically cell type dependent. Thus, while BZLF1 inhibits cell cycle progression in normal human fibroblasts (a cell type not normally infected by EBV), it does not significantly inhibit S-phase entry in primary and telomerase-immortalized keratinocytes (a natural target cell for the lytic form of EBV infection) and instead enhances expression of several cellular proteins normally associated with cell cycle progression. The cell cycle effects of BZLF1 in primary keratinocytes correlate with the known ability of lytic EBV infection to induce a hyperproliferative epithelial lesion, OHL.
Our results show that the ability of BZLF1 to induce expression of the critical cell cycle regulator E2F-1 is cell type dependent. E2F-1 overexpression by itself is often sufficient to activate entry into S phase, although there is usually concomitant apoptosis (20, 38, 47, 52). E2F-1 transcriptionally activates many genes associated with DNA synthesis and S-phase progression, in particular cyclins E and A and Cdc25A (18, 69, 75). A number of stimuli, including DNA damage and activation of the ATM kinase, have recently been shown to induce cellular E2F-1 expression by increasing E2F-1 protein stability (40). However, given the results of the microarray assay in telomerase-immortalized human keratinocytes (in which E2F-1 RNA level was increased 16-fold in AdBZLF1-infected cells), the effect of BZLF1 on E2F-1 is likely to be mediated at least partially at the RNA level, rather than the protein level.
The cell type-dependent nature of the BZLF1 cell cycle effect correlates with its ability to enhance the level of E2F-1 in a cell type-specific manner. At this point, we are uncertain why BZLF1 activates E2F-1 in some cell types, but not others. Assuming that BZLF1 transcriptionally activates the E2F-1 promoter, BZLF1 may not be able to activate the E2F-1 promoter in certain cell types due to promoter binding of negatively regulating cellular factors, methylation, or inaccessible chromatin. Alternatively, we have previously shown that the ability of BZLF1 to transcriptionally activate certain early EBV promoters is also cell type dependent (30). Thus, cell type-dependent modification of the BZLF1 protein may alter its ability to activate or bind to some promoters. Consistent with this, we previously showed that mutation of BZLF1 residue 186, which is known to be phosphorylated in vivo (4), inhibits its ability to bind to the viral BRLF1 IE promoter but not the early BMRF1 promoter (2).
Our findings that BZLF1 expression by itself is apparently not sufficient to activate full S-phase progression in keratinocytes or AGS cells whereas the lytically infected population of EBV-positive AGS cells has an increased number of cells in S phase suggest that BZLF1 may cooperate with other lytic EBV proteins to activate S-phase progression in certain cell types. We previously reported that the other EBV IE protein, BRLF1, also induces E2F-1 expression in a variety of cell types, including healthy human fibroblasts and HeLa cells, although the exact mechanism for this effect has not been defined (71). Interestingly, microarray analysis of telomerase-immortalized human keratinocytes infected with a BRLF1 adenovirus vector also showed increased expression of many cellular genes associated with cell cycle progression but did not indicate enhanced expression of the E2F-1 gene itself (A. Mauser and S. Kenney, unpublished data). In contrast, immunoblot analysis of AdBRLF1-infected telomerase-immortalized human keratinocytes confirmed that BRLF1 expression does increase the level of E2F-1 protein (Mauser and Kenney, unpublished). Thus, BZLF1 and BRLF1 activate E2F-1 through two different mechanisms in keratinocytes: BZLF1 increases the level of E2F-1 RNA, whereas BRLF1 increases E2F-1 through a posttranscriptional mechanism. The combined expression of BZLF1 and BRLF1 may activate S-phase progression in certain cell types such as AGS cells. Alternatively, in other cell types, it remains possible that BZLF1- and BRLF1-induced activation of E2F-1 results in the expression of host cell genes normally restricted to the late G1- or S-phase portions of the cell cycle but does not actually result in concomitant cell cycle progression.
In some respects, our results here are reminiscent of previous studies examining the cell cycle effects of the HCMV IE proteins. HCMV infection has been shown to upregulate the expression of a variety of cellular proteins normally involved in cell cycle progression, including c-myc, c-fos, c-jun, and cyclin E (5, 32), (44) and induces hyperphosphorylation of Rb (32). HCMV replication has been shown to require cyclin E/cdk2 (7), and thus, it would clearly be advantageous for the virus to induce cell cycle progression in quiescent host cells that normally do not have activated cdk2. Whether HCMV-infected cells actually enter S phase, versus accumulate in late G1, is somewhat controversial. One group reported that HCMV infection of irreversibly arrested cells induces cell cycle progression into early S phase (64), whereas other groups have shown that HCMV infection blocks cells in late G1 (8, 32, 43, 61). As is the case with EBV, both of the HCMV IE proteins, the72-kDa IE1 protein and the 86-kDa IE2 protein, appear to contribute to the cell cycle effects of HCMV. IE1 binds and inactivates p107 (53), while IE2 binds and inactivates pRb (28), thus increasing E2F transcriptional activity. IE2 also upregulates cyclin E through a direct mechanism (6). Both IE proteins have also been shown to induce the cell cycle in serum-starved cells (10, 46, 79).
In contrast, HSV infection has been associated only with the induction of a G1 cell cycle block (21, 67), and the expression of the ICP0 HSV IE protein by itself causes cell cycle arrest in both the G1 and G2/M components of the cell cycle (29, 42). Whether these differences in cell cycle effects reflect a fundamental difference in HCMV and EBV pathogenesis on the one hand and HSV pathogenesis on the other hand remains to be determined. In support of this, both EBV and HCMV infection are associated with hyperproliferative lesion in patients (OHL and coronary artery restenosis), while this is not the case for HSV. However, as shown by the data presented here, misleading results may be obtained by studying the cell cycle effects of herpesviruses in vitro using cell lines which are not the normal target cells for viral infection in vivo.
Clearly, it will be important to determine the effects of the EBV IE proteins on cell cycle control in primary B cells. All previous studies examining BZLF1 cell cycle effects in B cells have been done using Burkitt's lymphoma lines (in which c-myc expression is obviously dysregulated), as well as toxic agents which are required to induce the lytic form of infection (31, 59). Thus, the results of such studies may not be applicable to the effect of BZLF1 in primary resting B cells, the normal target for EBV infection in humans. Unfortunately, as primary B cells are not readily transfectable with DNA plasmids or infectible with adenovirus vectors, the answer to this question may not be forthcoming in the near future. If lytic EBV infection activates cell cycle progression during primary infection of B cells, this effect could contribute to hyperproliferation of B cells during primary mononucleosis.
REFERENCES
- 1.Adamson, A. L., D. Darr, E. Holley-Guthrie, R. A. Johnson, A. Mauser, J. Swenson, and S. Kenney. 2000. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J. Virol. 74:1224-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson, A. L., and S. C. Kenney. 1998. Rescue of the Epstein-Barr virus BZLF1 mutant, Z(S186A), early gene activation defect by the BRLF1 gene product. Virology 251:187-197. [DOI] [PubMed] [Google Scholar]
- 3.Bartek, J., and J. Lukas. 2001. Pathways governing G1/S transition and their response to DNA damage. FEBS Lett. 490:117-122. [DOI] [PubMed] [Google Scholar]
- 4.Baumann, M., H. Mischak, S. Dammeier, W. Kolch, O. Gires, D. Pich, R. Zeidler, H. J. Delecluse, and W. Hammerschmidt. 1998. Activation of the Epstein-Barr virus transcription factor BZLF1 by 12-O-tetradecanoylphorbol-13-acetate-induced phosphorylation. J. Virol. 72:8105-8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boldogh, I., S. AbuBakar, and T. Albrecht. 1990. Activation of proto-oncogenes: an immediate early event in human cytomegalovirus infection. Science 247:561-564. [DOI] [PubMed] [Google Scholar]
- 6.Bresnahan, W. A., T. Albrecht, and E. A. Thompson. 1998. The cyclin E promoter is activated by human cytomegalovirus 86-kDa immediate early protein. J. Biol. Chem. 273:22075-22082. [DOI] [PubMed] [Google Scholar]
- 7.Bresnahan, W. A., I. Boldogh, P. Chi, E. A. Thompson, and T. Albrecht. 1997. Inhibition of cellular Cdk2 activity blocks human cytomegalovirus replication. Virology 231:239-247. [DOI] [PubMed] [Google Scholar]
- 8.Bresnahan, W. A., I. Boldogh, E. A. Thompson, and T. Albrecht. 1996. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology 224:150-160. [DOI] [PubMed] [Google Scholar]
- 9.Cannell, E. J., P. J. Farrell, and A. J. Sinclair. 1996. Epstein-Barr virus exploits the normal cell pathway to regulate Rb activity during the immortalisation of primary B-cells. Oncogene 13:1413-1421. [PubMed] [Google Scholar]
- 10.Castillo, J. P., A. D. Yurochko, and T. F. Kowalik. 2000. Role of human cytomegalovirus immediate-early proteins in cell growth control. J. Virol. 74:8028-8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cayrol, C., and E. Flemington. 1996. G0/G1 growth arrest mediated by a region encompassing the basic leucine zipper (bZIP) domain of the Epstein-Barr virus transactivator Zta. J. Biol. Chem. 271:31799-31802. [DOI] [PubMed] [Google Scholar]
- 12.Cayrol, C., and E. K. Flemington. 1996. The Epstein-Barr virus bZIP transcription factor Zta causes G0/G1 cell cycle arrest through induction of cyclin-dependent kinase inhibitors. EMBO J. 15:2748-2759. [PMC free article] [PubMed] [Google Scholar]
- 13.Chang, Y. N., D. L. Dong, G. S. Hayward, and S. D. Hayward. 1990. The Epstein-Barr virus Zta transactivator: a member of the bZIP family with unique DNA-binding specificity and a dimerization domain that lacks the characteristic heptad leucine zipper motif. J. Virol. 64:3358-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, X., and R. Prywes. 1999. Serum-induced expression of the cdc25A gene by relief of E2F-mediated repression. Mol. Cell. Biol. 19:4695-4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chevallier-Greco, A., E. Manet, P. Chavrier, C. Mosnier, J. Daillie, and A. Sergeant. 1986. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 5:3243-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Countryman, J., and G. Miller. 1985. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc. Natl. Acad. Sci. USA 82:4085-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox, M. A., J. Leahy, and J. M. Hardwick. 1990. An enhancer within the divergent promoter of Epstein-Barr virus responds synergistically to the R and Z transactivators. J. Virol. 64:313-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeGregori, J., T. Kowalik, and J. R. Nevins. 1995. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol. Cell. Biol. 15:4215-4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dittmer, D., and E. S. Mocarski. 1997. Human cytomegalovirus infection inhibits G1/S transition. J. Virol. 71:1629-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dyson, N. 1998. The regulation of E2F by pRB-family proteins. Genes Dev. 12:2245-2262. [DOI] [PubMed] [Google Scholar]
- 21.Ehmann, G. L., T. I. McLean, and S. L. Bachenheimer. 2000. Herpes simplex virus type 1 infection imposes a G1/S block in asynchronously growing cells and prevents G1 entry in quiescent cells. Virology 267:335-349. [DOI] [PubMed] [Google Scholar]
- 22.Farrell, P. J., D. T. Rowe, C. M. Rooney, and T. Kouzarides. 1989. Epstein-Barr virus BZLF1 trans-activator specifically binds to a consensus AP-1 site and is related to c-fos. EMBO J. 8:127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farwell, D. G., K. A. Shera, J. I. Koop, G. A. Bonnet, C. P. Matthews, G. W. Reuther, M. D. Coltrera, J. K. McDougall, and A. J. Klingelhutz. 2000. Genetic and epigenetic changes in human epithelial cells immortalized by telomerase. Am. J. Pathol. 156:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faulkner, G. C., A. S. Krajewski, and D. H. Crawford. 2000. The ins and outs of EBV infection. Trends Microbiol. 8:185-189. [DOI] [PubMed] [Google Scholar]
- 25.Flemington, E., and S. H. Speck. 1990. Autoregulation of Epstein-Barr virus putative lytic switch gene BZLF1. J. Virol. 64:1227-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flemington, E. K., A. M. Borras, J. P. Lytle, and S. H. Speck. 1992. Characterization of the Epstein-Barr virus BZLF1 protein transactivation domain. J. Virol. 66:922-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu, Y., J. Rosenblatt, and D. O. Morgan. 1992. Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 11:3995-4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagemeier, C., R. Caswell, G. Hayhurst, J. Sinclair, and T. Kouzarides. 1994. Functional interaction between the HCMV IE2 transactivator and the retinoblastoma protein. EMBO J. 13:2897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hobbs, W. E., and N. A. DeLuca. 1999. Perturbation of cell cycle progression and cellular gene expression as a function of herpes simplex virus ICP0. J. Virol. 73:8245-8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holley-Guthrie, E. A., E. B. Quinlivan, E. C. Mar, and S. Kenney. 1990. The Epstein-Barr virus (EBV) BMRF1 promoter for early antigen (EA-D) is regulated by the EBV transactivators, BRLF1 and BZLF1, in a cell-specific manner. J. Virol. 64:3753-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inman, G. J., U. K. Binne, G. A. Parker, P. J. Farrell, and M. J. Allday. 2001. Activators of the Epstein-Barr virus lytic program concomitantly induce apoptosis, but lytic gene expression protects from cell death. J. Virol. 75:2400-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jault, F. M., J. M. Jault, F. Ruchti, E. A. Fortunato, C. Clark, J. Corbeil, D. D. Richman, and D. H. Spector. 1995. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb, and p53, leading to cell cycle arrest. J. Virol. 69:6697-6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones, S. M., and A. Kazlauskas. 2001. Growth factor-dependent signaling and cell cycle progression. FEBS Lett. 490:110-116. [DOI] [PubMed] [Google Scholar]
- 34.Kaufmann, W. K., J. L. Schwartz, J. C. Hurt, L. L. Byrd, D. A. Galloway, E. Levedakou, and R. S. Paules. 1997. Inactivation of G2 checkpoint function and chromosomal destabilization are linked in human fibroblasts expressing human papillomavirus type 16 E6. Cell Growth Differ. 8:1105-1114. [PubMed] [Google Scholar]
- 35.Kawaguchi, Y., C. Van Sant, and B. Roizman. 1997. Herpes simplex virus 1 alpha regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J. Virol. 71:7328-7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kenney, S., E. Holley-Guthrie, E. C. Mar, and M. Smith. 1989. The Epstein-Barr virus BMLF1 promoter contains an enhancer element that is responsive to the BZLF1 and BRLF1 transactivators. J. Virol. 63:3878-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kieff, E. 1996. Epstein-Barr virus and its replication, p. 2343-2395. In B. N. Fields, D. N. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott-Raven Publishers, Philadelphia, Pa.
- 38.Kowalik, T. F., J. DeGregori, J. K. Schwarz, and J. R. Nevins. 1995. E2F1 overexpression in quiescent fibroblasts leads to induction of cellular DNA synthesis and apoptosis. J. Virol. 69:2491-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lieberman, P. M., J. M. Hardwick, and S. D. Hayward. 1989. Responsiveness of the Epstein-Barr virus NotI repeat promoter to the Z transactivator is mediated in a cell-type-specific manner by two independent signal regions. J. Virol. 63:3040-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin, W. C., F. T. Lin, and J. R. Nevins. 2001. Selective induction of E2F1 in response to DNA damage, mediated by ATM-dependent phosphorylation. Genes Dev. 15:1833-1844. [PMC free article] [PubMed] [Google Scholar]
- 41.Lipson, K. E., S. T. Chen, J. Koniecki, D. H. Ku, and R. Baserga. 1989. S-phase-specific regulation by deletion mutants of the human thymidine kinase promoter. Proc. Natl. Acad. Sci. USA 86:6848-6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lomonte, P., and R. D. Everett. 1999. Herpes simplex virus type 1 immediate-early protein Vmw110 inhibits progression of cells through mitosis and from G1 into S phase of the cell cycle. J. Virol. 73:9456-9467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu, M., and T. Shenk. 1996. Human cytomegalovirus infection inhibits cell cycle progression at multiple points, including the transition from G1 to S. J. Virol. 70:8850-8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monick, M. M., L. J. Geist, M. F. Stinski, and G. W. Hunninghake. 1992. The immediate early genes of human cytomegalovirus upregulate expression of the cellular genes myc and fos. Am. J. Respir. Cell Mol. Biol. 7:251-256. [DOI] [PubMed] [Google Scholar]
- 45.Morrison, T., A. Mauser, A. Wong, J. Ting, and S. Kenney. 2001. Inhibition of IFN-γ signaling by an Epstein-Barr virus immediate-early protein. Immunity 15:787-799. [DOI] [PubMed] [Google Scholar]
- 46.Murphy, E. A., D. N. Streblow, J. A. Nelson, and M. F. Stinski. 2000. The human cytomegalovirus IE86 protein can block cell cycle progression after inducing transition into the S phase of permissive cells. J. Virol. 74:7108-7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nevins, J. R. 1998. Toward an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 9:585-593. [PubMed] [Google Scholar]
- 48.Niedobitek, G., N. Meru, and H. J. Delecluse. 2001. Epstein-Barr virus infection and human malignancies. Int. J. Exp. Pathol. 82:149-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Packham, G., A. Economou, C. M. Rooney, D. T. Rowe, and P. J. Farrell. 1990. Structure and function of the Epstein-Barr virus BZLF1 protein. J. Virol. 64:2110-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parker, G. A., T. Crook, M. Bain, E. A. Sara, P. J. Farrell, and M. J. Allday. 1996. Epstein-Barr virus nuclear antigen (EBNA)3C is an immortalizing oncoprotein with similar properties to adenovirus E1A and papillomavirus E7. Oncogene 13:2541-2549. [PubMed] [Google Scholar]
- 51.Parker, G. A., R. Touitou, and M. J. Allday. 2000. Epstein-Barr virus EBNA3C can disrupt multiple cell cycle checkpoints and induce nuclear division divorced from cytokinesis. Oncogene 19:700-709. [DOI] [PubMed] [Google Scholar]
- 52.Phillips, A. C., M. K. Ernst, S. Bates, N. R. Rice, and K. H. Vousden. 1999. E2F-1 potentiates cell death by blocking antiapoptotic signaling pathways. Mol. Cell 4:771-781. [DOI] [PubMed] [Google Scholar]
- 53.Poma, E. E., T. F. Kowalik, L. Zhu, J. H. Sinclair, and E. S. Huang. 1996. The human cytomegalovirus IE1-72 protein interacts with the cellular p107 protein and relieves p107-mediated transcriptional repression of an E2F-responsive promoter. J. Virol. 70:7867-7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quinlivan, E. B., E. A. Holley-Guthrie, M. Norris, D. Gutsch, S. L. Bachenheimer, and S. C. Kenney. 1993. Direct BRLF1 binding is required for cooperative BZLF1/BRLF1 activation of the Epstein-Barr virus early promoter, BMRF1. Nucleic Acids Res. 21:1999-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ragoczy, T., and G. Miller. 1999. Role of the Epstein-Barr virus RTA protein in activation of distinct classes of viral lytic cycle genes. J. Virol. 73:9858-9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rheinwald, J. G., and H. Green. 1975. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6:331-343. [DOI] [PubMed] [Google Scholar]
- 57.Rickinson, A. B., and E. Kieff. 1996. Epstein-Barr virus, p. 2397-2446. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott-Raven Publishers, Philadelphia, Pa.
- 58.Rodriguez, A., M. Armstrong, D. Dwyer, and E. Flemington. 1999. Genetic dissection of cell growth arrest functions mediated by the Epstein-Barr virus lytic gene product, Zta. J. Virol. 73:9029-9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodriguez, A., E. J. Jung, and E. K. Flemington. 2001. Cell cycle analysis of Epstein-Barr virus-infected cells following treatment with lytic cycle-inducing agents. J. Virol. 75:4482-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rooney, C. M., D. T. Rowe, T. Ragot, and P. J. Farrell. 1989. The spliced BZLF1 gene of Epstein-Barr virus (EBV) transactivates an early EBV promoter and induces the virus productive cycle. J. Virol. 63:3109-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salvant, B. S., E. A. Fortunato, and D. H. Spector. 1998. Cell cycle dysregulation by human cytomegalovirus: influence of the cell cycle phase at the time of infection and effects on cyclin transcription. J. Virol. 72:3729-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sherr, C. J. 1993. Mammalian G1 cyclins. Cell 73:1059-1065. [DOI] [PubMed] [Google Scholar]
- 63.Sherr, C. J. 2000. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 60:3689-3695. [PubMed] [Google Scholar]
- 64.Sinclair, J., J. Baillie, L. Bryant, and R. Caswell. 2000. Human cytomegalovirus mediates cell cycle progression through G1 into early S phase in terminally differentiated cells. J. Gen. Virol. 81(Part 6):1553-1565. [DOI] [PubMed] [Google Scholar]
- 65.Sixbey, J. W., S. M. Lemon, and J. S. Pagano. 1986. A second site for Epstein-Barr virus shedding: the uterine cervix. Lancet 2:1122-1124. [DOI] [PubMed] [Google Scholar]
- 66.Sixbey, J. W., J. G. Nedrud, N. Raab-Traub, R. A. Hanes, and J. S. Pagano. 1984. Epstein-Barr virus replication in oropharyngeal epithelial cells. N. Engl. J. Med. 310:1225-1230. [DOI] [PubMed] [Google Scholar]
- 67.Song, B., J. J. Liu, K. C. Yeh, and D. M. Knipe. 2000. Herpes simplex virus infection blocks events in the G1 phase of the cell cycle. Virology 267:326-334. [DOI] [PubMed] [Google Scholar]
- 68.Spender, L. C., E. J. Cannell, M. Hollyoake, B. Wensing, J. M. Gawn, M. Brimmell, G. Packham, and P. J. Farrell. 1999. Control of cell cycle entry and apoptosis in B lymphocytes infected by Epstein-Barr virus. J. Virol. 73:4678-4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spitkovsky, D., A. Schulze, B. Boye, and P. Jansen-Durr. 1997. Down-regulation of cyclin A gene expression upon genotoxic stress correlates with reduced binding of free E2F to the promoter. Cell Growth Differ. 8:699-710. [PubMed] [Google Scholar]
- 70.Swanton, C., and N. Jones. 2001. Strategies in subversion: de-regulation of the mammalian cell cycle by viral gene products. Int. J. Exp. Pathol. 82:3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swenson, J. J., A. E. Mauser, W. K. Kaufmann, and S. C. Kenney. 1999. The Epstein-Barr virus protein BRLF1 activates S-phase entry through E2F1 induction. J. Virol. 73:6540-6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taichman, L., S. Reilly, and P. R. Garant. 1979. In-vitro cultivation of human oral keratinocytes. Arch. Oral Biol. 24:335-341. [DOI] [PubMed] [Google Scholar]
- 73.Takada, K., N. Shimizu, S. Sakuma, and Y. Ono. 1986. trans Activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J. Virol. 57:1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Urier, G., M. Buisson, P. Chambard, and A. Sergeant. 1989. The Epstein-Barr virus early protein EB1 activates transcription from different responsive elements including AP-1 binding sites. EMBO J. 8:1447-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vigo, E., H. Muller, E. Prosperini, G. Hateboer, P. Cartwright, M. C. Moroni, and K. Helin. 1999. Cdc25A phosphatase is a target of E2F and is required for efficient E2F-induced S phase. Mol. Cell. Biol. 19:6379-6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Webster-Cyriaque, J., J. Middeldorp, and N. Raab-Traub. 2000. Hairy leukoplakia: an unusual combination of transforming and permissive Epstein-Barr virus infections. J. Virol. 74:7610-7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Westphal, E. M., A. Mauser, J. Swenson, M. G. Davis, C. L. Talarico, and S. C. Kenney. 1999. Induction of lytic Epstein-Barr virus (EBV) infection in EBV-associated malignancies using adenovirus vectors in vitro and in vivo. Cancer Res. 59:1485-1491. [PubMed] [Google Scholar]
- 78.Whitfield, M. L., L. X. Zheng, A. Baldwin, T. Ohta, M. M. Hurt, and W. F. Marzluff. 2000. Stem-loop binding protein, the protein that binds the 3′ end of histone mRNA, is cell cycle regulated by both translational and posttranslational mechanisms. Mol. Cell. Biol. 20:4188-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wiebusch, L., and C. Hagemeier. 2001. The human cytomegalovirus immediate early 2 protein dissociates cellular DNA synthesis from cyclin-dependent kinase activation. EMBO J. 20:1086-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zacny, V. L., J. Wilson, and J. S. Pagano. 1998. The Epstein-Barr virus immediate-early gene product, BRLF1, interacts with the retinoblastoma protein during the viral lytic cycle. J. Virol. 72:8043-8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zalani, S., E. Holley-Guthrie, and S. Kenney. 1996. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc. Natl. Acad. Sci. USA 93:9194-9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang, Q., D. Gutsch, and S. Kenney. 1994. Functional and physical interaction between p53 and BZLF1: implications for Epstein-Barr virus latency. Mol. Cell. Biol. 14:1929-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]