Abstract
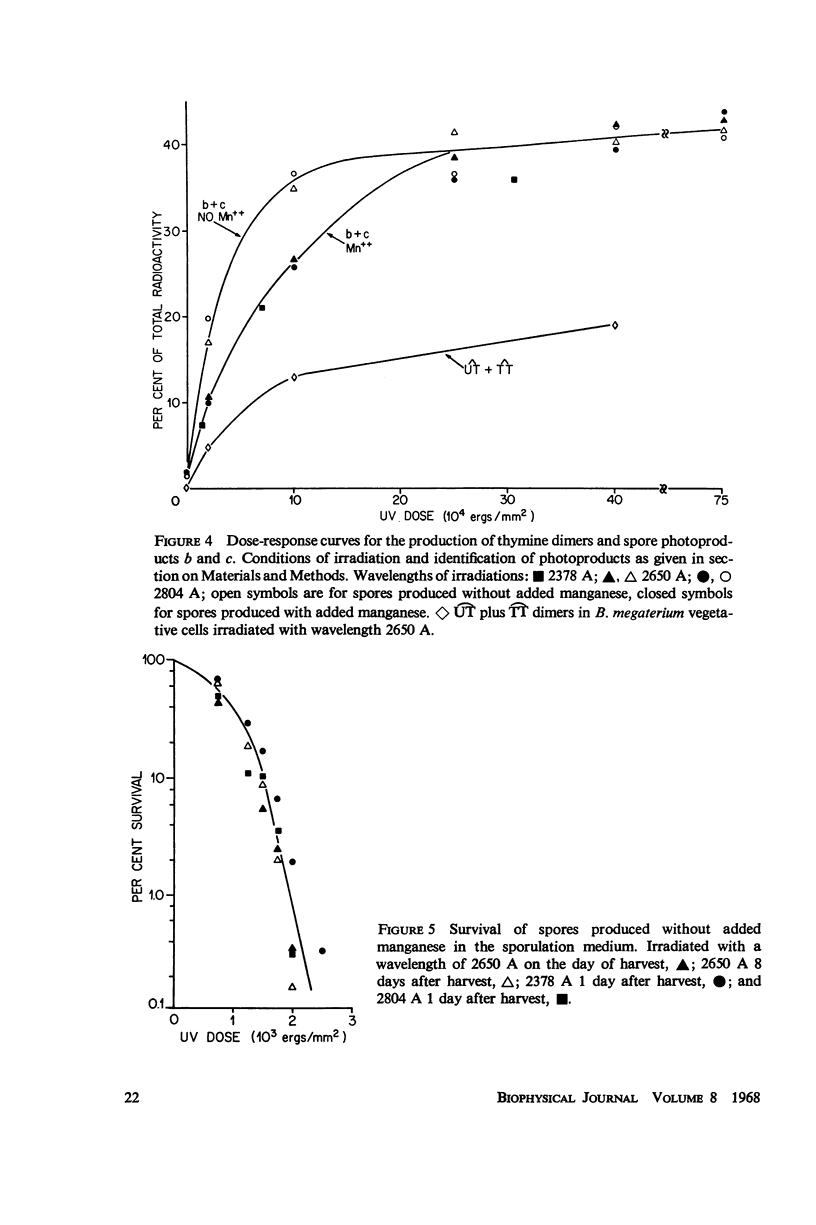
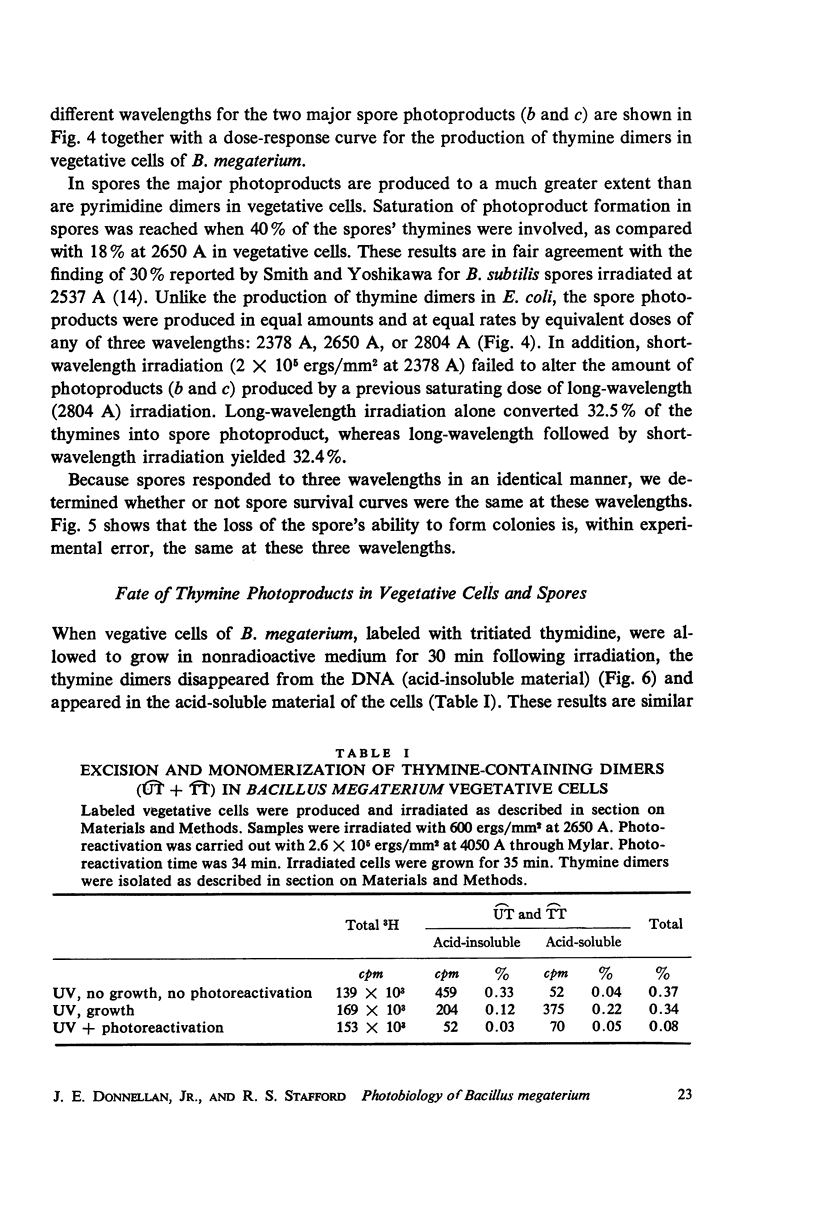
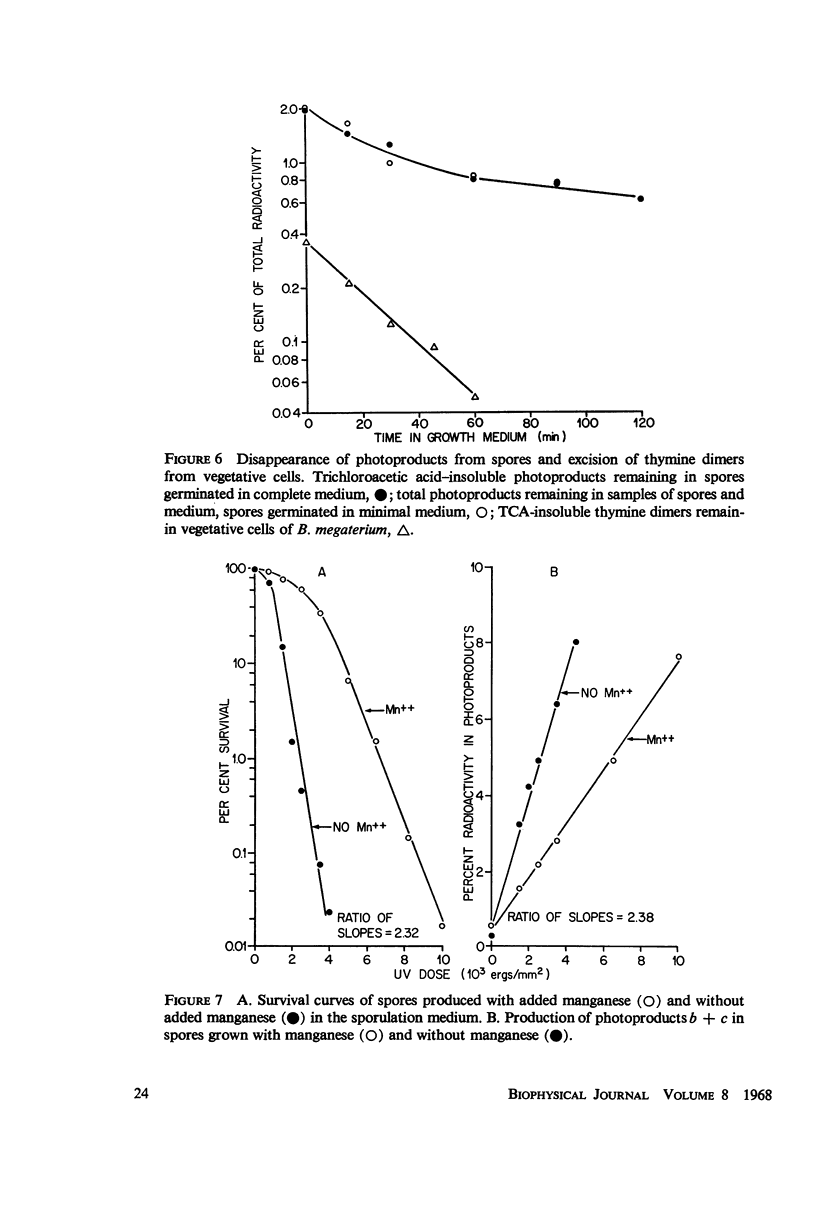
The ultraviolet (UV) photochemistry and photobiology of spores and vegetative cells of Bacillus megaterium have been studied. The response of vegetative cells of B. megaterium appears qualitatively similar to those of Escherichia coli, Micrococcus radiodurans, and Bacillus subtilis with respect to photoproduct formation and repair mechanisms. UV irradiation, however, does not produce cyclobutane-type thymine dimers in the DNA of spores, although other thymine photo-products are produced. The photoproducts do not disappear after photoreactivation, but they are eliminated from the DNA by a dark-repair mechanism different from that found for dimers in vegetative cells. Irradiations performed at three wavelengths produce the same amounts of spore photoproduct and give the same survival curves. Variation of the sporulation medium before irradiation results in comparable alterations in the rate of spore photoproduct production and in survival.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. H. Growth Requirements of Virus-Resistant Mutants of Escherichia Coli Strain "B". Proc Natl Acad Sci U S A. 1946 May;32(5):120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boling M. E., Setlow J. K. The resistance of Micrococcus radiodurans to ultraviolet radiation. 3. A repair mechanism. Biochim Biophys Acta. 1966 Jul 20;123(1):26–33. doi: 10.1016/0005-2787(66)90155-9. [DOI] [PubMed] [Google Scholar]
- Cook J. S. Direct demonstration of the monomerization of thymine-containing dimers in U.V.-irradiated DNA by yeast photoreactivating enzyme and light. Photochem Photobiol. 1967 Feb;6(2):97–101. doi: 10.1111/j.1751-1097.1967.tb08794.x. [DOI] [PubMed] [Google Scholar]
- Donnellan J. E., Jr, Setlow R. B. Thymine Photoproducts but not Thymine Dimers Found in Ultraviolet-Irradiated Bacterial Spores. Science. 1965 Jul 16;149(3681):308–310. doi: 10.1126/science.149.3681.308. [DOI] [PubMed] [Google Scholar]
- JAGGER J., STAFFORD R. S. EVIDENCE FOR TWO MECHANISMS OF PHOTOREACTIVATION IN ESCHERICHIA COLI B. Biophys J. 1965 Jan;5:75–88. doi: 10.1016/s0006-3495(65)86703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNS H. E., RAPAPORT S. A., DELBRUECK M. Photochemistry of thymine dimers. J Mol Biol. 1962 Feb;4:104–114. doi: 10.1016/s0022-2836(62)80042-4. [DOI] [PubMed] [Google Scholar]
- Rahn R. O. Pyrimidine dimers: effect of temperature on photoinduction. Science. 1966 Oct 28;154(3748):503–504. doi: 10.1126/science.154.3748.503. [DOI] [PubMed] [Google Scholar]
- Riklis E. Studies on mechanism of repair of ultraviolet-irradiated viral and bacterial DNAin vivo and in vitro. Can J Biochem. 1965 Jul;43(7):1207–1219. doi: 10.1139/o65-132. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETLOW R. B., SETLOW J. K. Evidence that ultraviolet-induced thymine dimers in DNA cause biological damage. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1250–1257. doi: 10.1073/pnas.48.7.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETLOW R. B., SWENSON P. A., CARRIER W. L. THYMINE DIMERS AND INHIBITION OF DNA SYNTHESIS BY ULTRAVIOLET IRRADIATION OF CELLS. Science. 1963 Dec 13;142(3598):1464–1466. doi: 10.1126/science.142.3598.1464. [DOI] [PubMed] [Google Scholar]
- Setlow J. K. Photoreactivation. Radiat Res. 1966;(Suppl):141+–141+. [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L. Pyrimidine dimers in ultraviolet-irradiated DNA's. J Mol Biol. 1966 May;17(1):237–254. doi: 10.1016/s0022-2836(66)80105-5. [DOI] [PubMed] [Google Scholar]
- Setlow R. B. Cyclobutane-type pyrimidine dimers in polynucleotides. Science. 1966 Jul 22;153(3734):379–386. doi: 10.1126/science.153.3734.379. [DOI] [PubMed] [Google Scholar]
- Shuster R. C. Fate of thymine-containing dimers in the deoxyribonucleic acid of ultravioletirradiated Bacillus subtilis. J Bacteriol. 1967 Mar;93(3):811–815. doi: 10.1128/jb.93.3.811-815.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. C., Yoshikawa H. Variation in the photochemical reactivity of thymine in the DNA of B. subtilis spores, vegetative cells and spores germinated in chloramphenicol. Photochem Photobiol. 1966 Oct;5(10):777–786. doi: 10.1111/j.1751-1097.1966.tb05773.x. [DOI] [PubMed] [Google Scholar]
- Strauss B., Searashi T., Robbins M. Repair of DNA studied with a nuclease specific for UV-induced lesions. Proc Natl Acad Sci U S A. 1966 Sep;56(3):932–939. doi: 10.1073/pnas.56.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]


