Abstract
The amount of radioactivity incorporated into completed polypeptides and into nascent polypeptides bound to polyribosomes was measured for rapidly dividing, cultured hamster embryo cells incubated in the presence of labelled amino acids. Using a mathematical analysis which took into account variations in the specific activity of the intracellular amino acid pool, these measurements yielded a value for the length of time required for the synthesis of a primary polypeptide of average length, the “transit time.” Subject to a number of reasonable assumptions in the mathematical analysis which were, however, only approximately verified, the transit time was found to be 23 ± 2 sec in four independent experiments. Regardless of the absolute accuracy of this measurement, the method is considered a useful measure of translation independent of transcription.
Full text
PDF


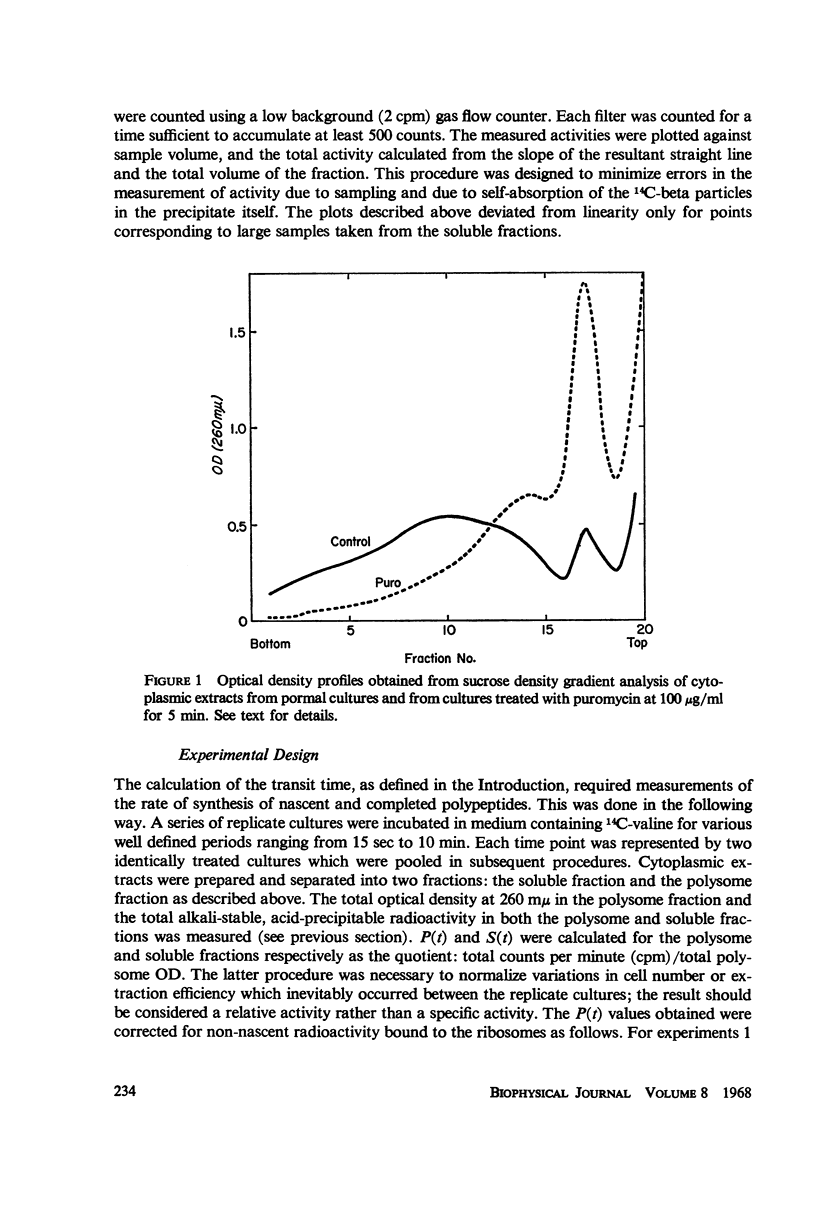
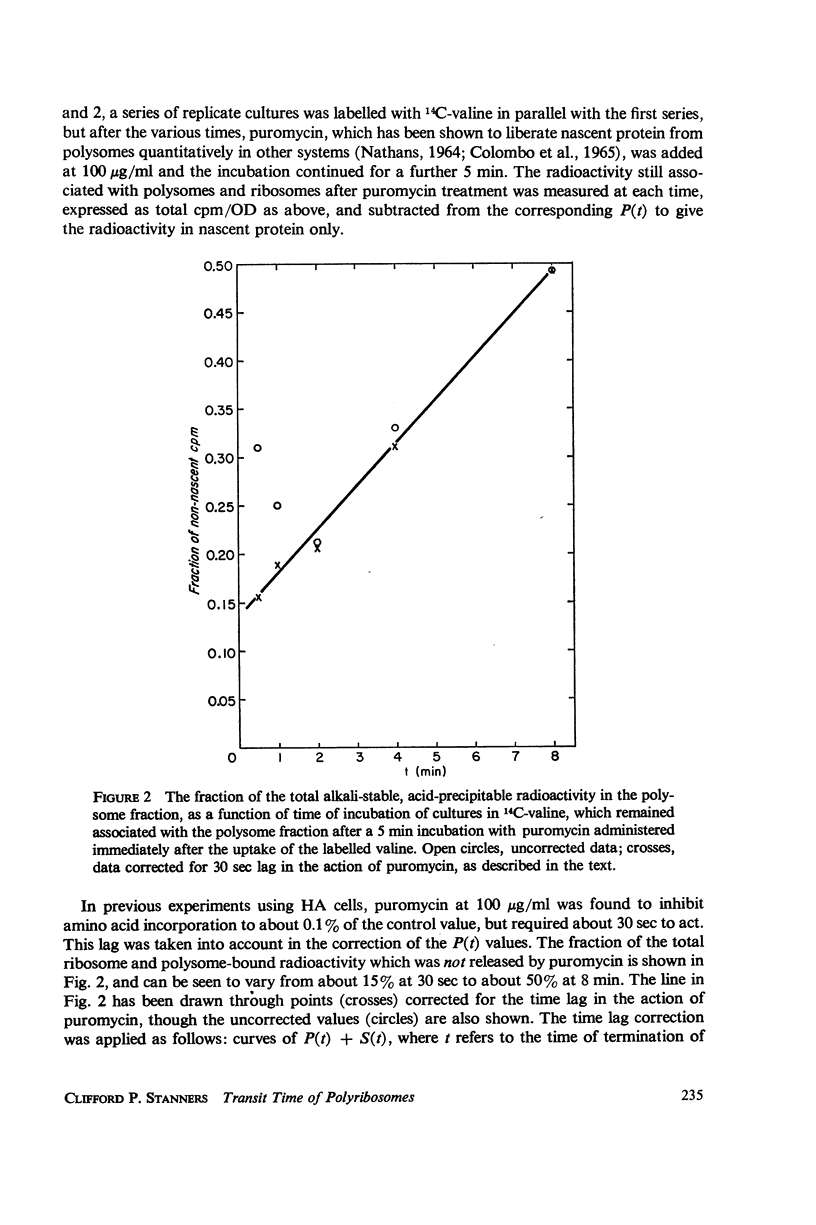
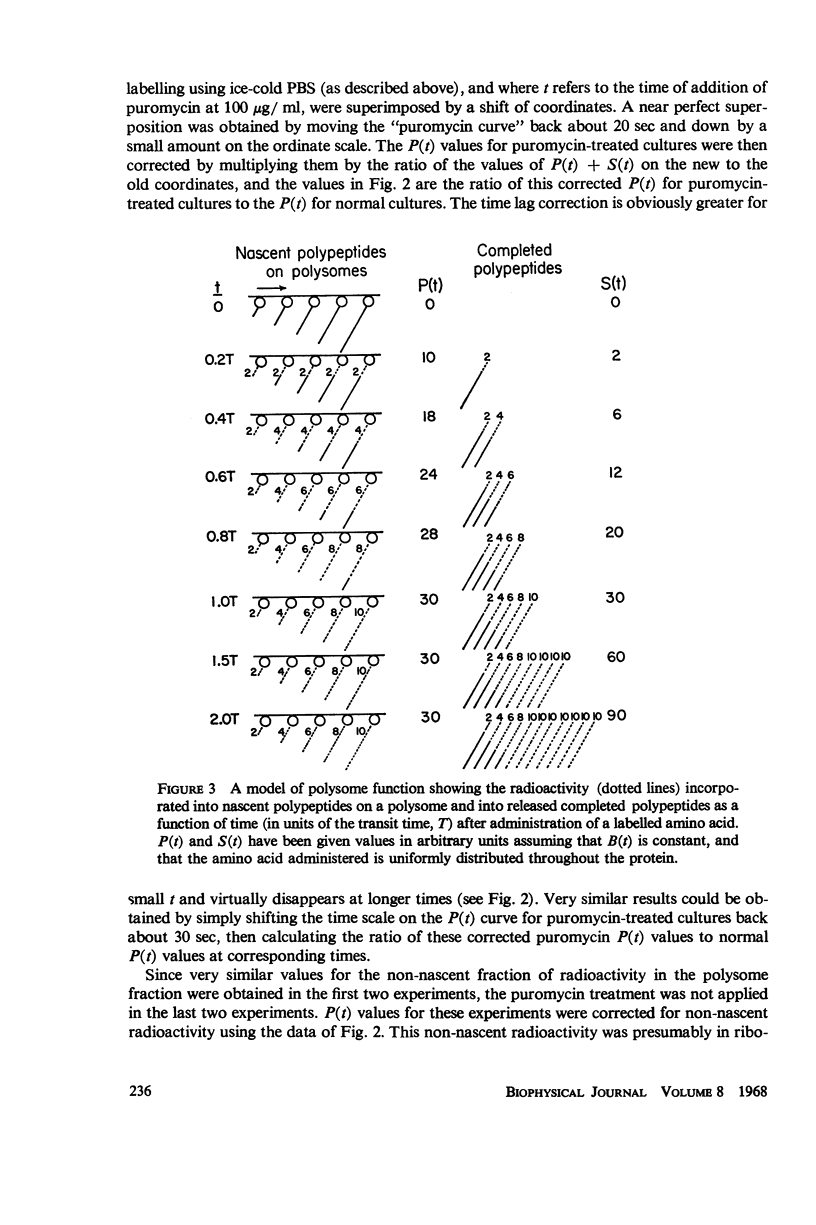

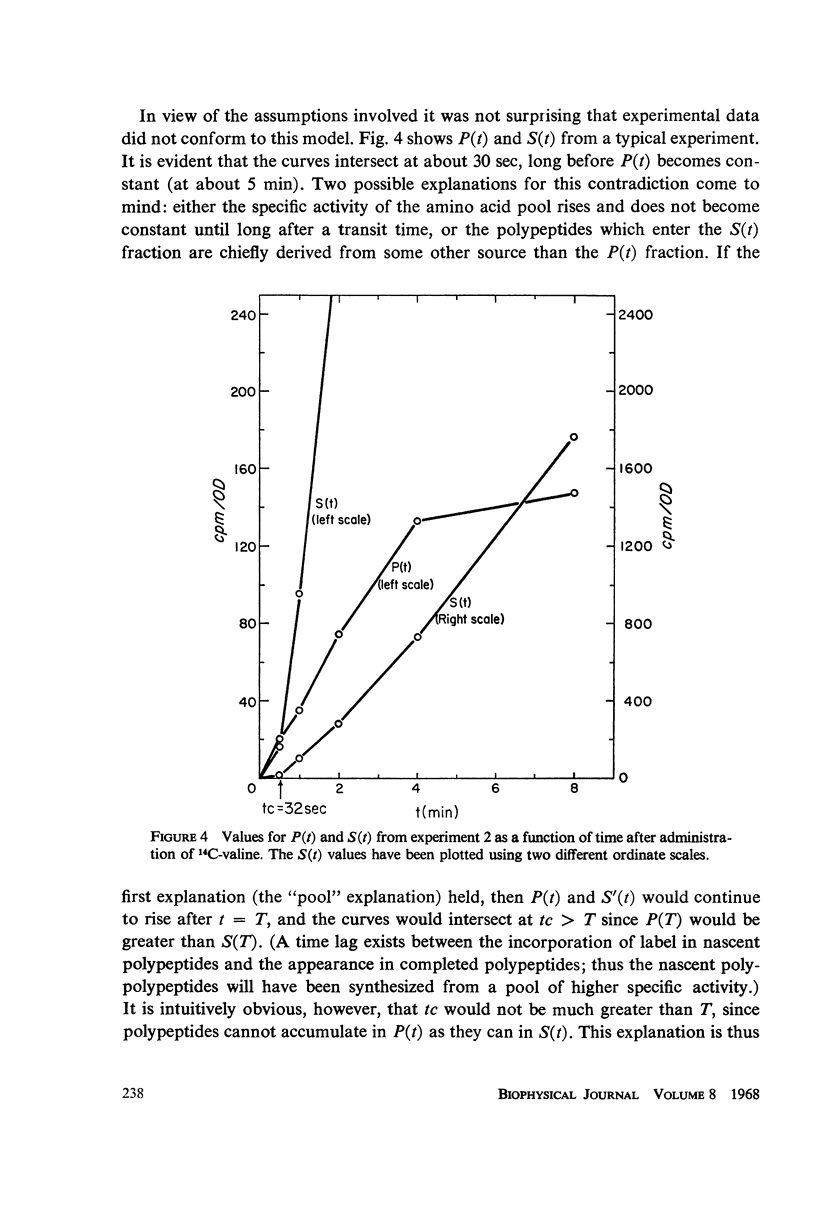
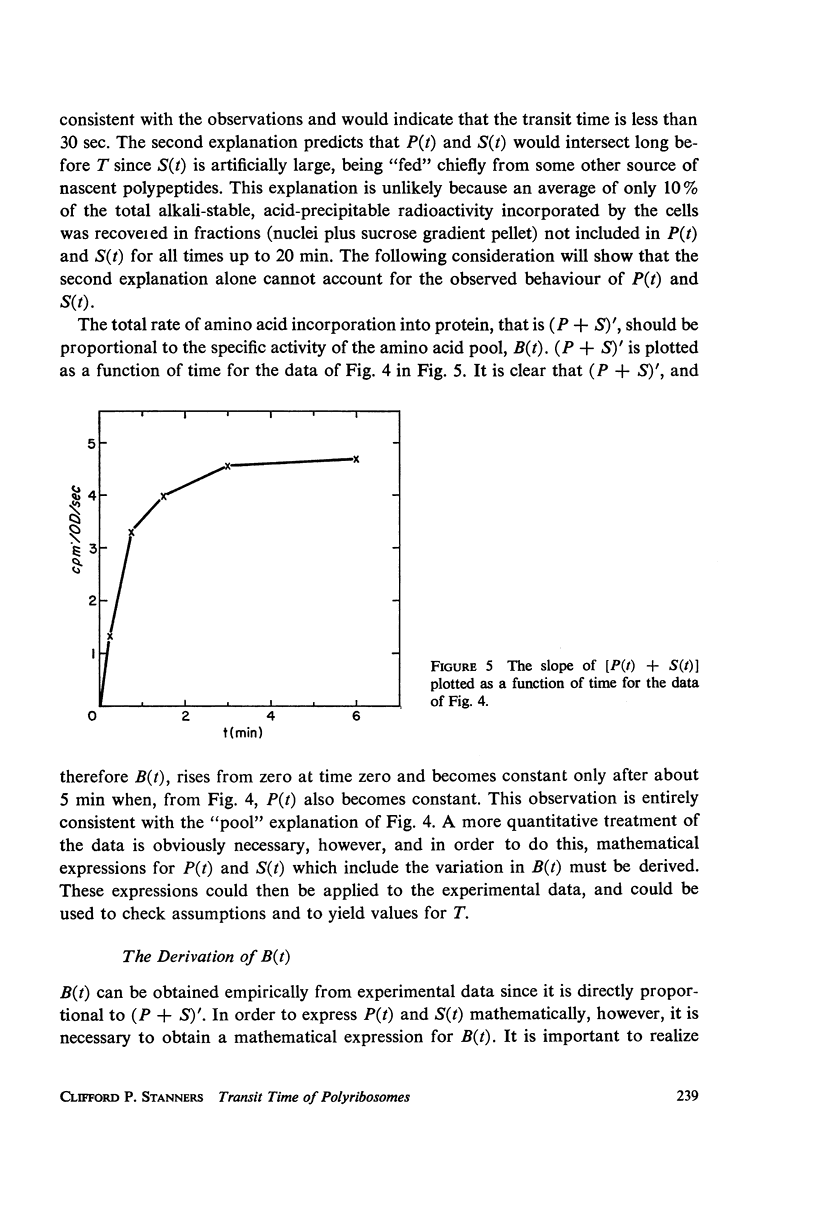

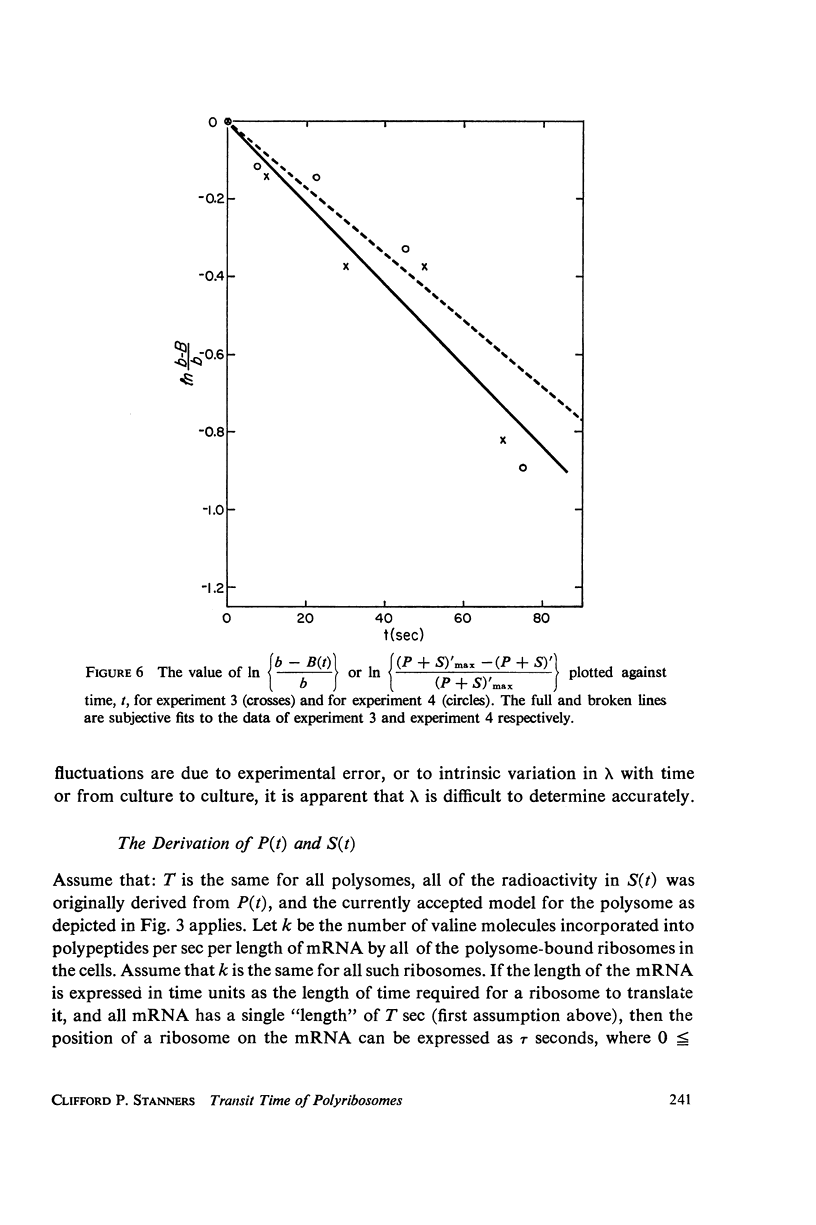




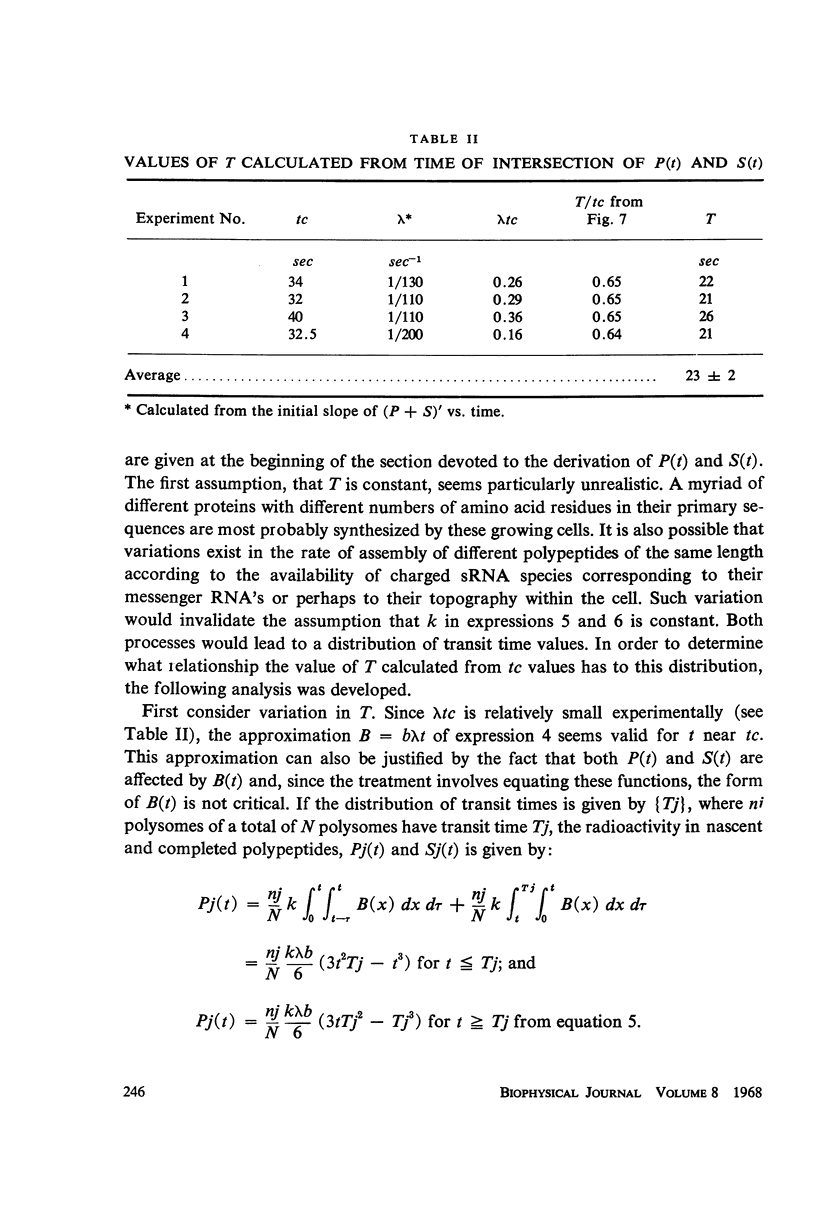





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERCROMBIE M., HEAYSMAN J. E. Observations on the social behaviour of cells in tissue culture. II. Monolayering of fibroblasts. Exp Cell Res. 1954 May;6(2):293–306. doi: 10.1016/0014-4827(54)90176-7. [DOI] [PubMed] [Google Scholar]
- Bautz E. K., Kasai T., Reilly E., Bautz F. A. Gene-specific mRNA. II. Regulation of mRNA synthesis in E. coli after infection with bacteriophage T4. Proc Natl Acad Sci U S A. 1966 May;55(5):1081–1088. doi: 10.1073/pnas.55.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLOMBO B., FELICETTI L., BAGLIONI C. INHIBITION OF PROTEIN SYNTHESIS BY CYCLOHEXIMIDE IN RABBIT RETICULOCYTES. Biochem Biophys Res Commun. 1965 Feb 3;18:389–395. doi: 10.1016/0006-291x(65)90719-9. [DOI] [PubMed] [Google Scholar]
- Conconi F. M., Bank A., Marks P. A. Polyribosomes and control of protein synthesis: effects of sodium fluoride and temperature of reticulocytes. J Mol Biol. 1966 Aug;19(2):525–540. doi: 10.1016/s0022-2836(66)80020-7. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN A., GOLDSTEIN D. B., LOWNEY L. I. PROTEIN SYNTHESIS OF 0 DEGREES C IN ESCHERICHIA COLI. J Mol Biol. 1964 Jul;9:213–235. doi: 10.1016/s0022-2836(64)80102-9. [DOI] [PubMed] [Google Scholar]
- GROSS P. R., MALKIN L. I., MOYER W. A. TEMPLATES FOR THE FIRST PROTEINS OF EMBRYONIC DEVELOPMENT. Proc Natl Acad Sci U S A. 1964 Mar;51:407–414. doi: 10.1073/pnas.51.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano-Sueoka T., Sueoka N. Modification of leucyl-sRNA after bacteriophage infection. J Mol Biol. 1966 Sep;20(1):183–209. doi: 10.1016/0022-2836(66)90124-0. [DOI] [PubMed] [Google Scholar]
- MCAUSLAN B. R. THE INDUCTION AND REPRESSION OF THYMIDINE KINASE IN THE POXVIRUS-INFECTED HELA CELL. Virology. 1963 Nov;21:383–389. doi: 10.1016/0042-6822(63)90199-5. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Burka E. R., Conconi F. M., Perl W., Rifkind R. A. Polyribosome dissociation and formation in intact reticulocytes with conservation of messenger ribonucleic acid. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1437–1443. doi: 10.1073/pnas.53.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NATHANS D. PUROMYCIN INHIBITION OF PROTEIN SYNTHESIS: INCORPORATION OF PUROMYCIN INTO PEPTIDE CHAINS. Proc Natl Acad Sci U S A. 1964 Apr;51:585–592. doi: 10.1073/pnas.51.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S., Scherrer K., Becker Y., Darnell J. E. POLYRIBOSOMES IN NORMAL AND POLIOVIRUS-INFECTED HELA CELLS AND THEIR RELATIONSHIP TO MESSENGER-RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):654–662. doi: 10.1073/pnas.49.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader R. W., Stanners C. P. On the significance of ribosome dimers in extracts of animal cells. J Mol Biol. 1967 Sep 14;28(2):211–223. doi: 10.1016/s0022-2836(67)80004-4. [DOI] [PubMed] [Google Scholar]
- Salb J. M., Marcus P. I. Translational inhibition in mitotic HeLa cells. Proc Natl Acad Sci U S A. 1965 Nov;54(5):1353–1358. doi: 10.1073/pnas.54.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Stanners C. P. A note on the incorporation of amino acids by hamster embryo cells in monolayer culture. Biochim Biophys Acta. 1967 May 30;138(3):627–630. doi: 10.1016/0005-2787(67)90566-7. [DOI] [PubMed] [Google Scholar]
- Vogt M., Dulbecco R. VIRUS-CELL INTERACTION WITH A TUMOR-PRODUCING VIRUS. Proc Natl Acad Sci U S A. 1960 Mar;46(3):365–370. doi: 10.1073/pnas.46.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARNER J. R., KNOPF P. M., RICH A. A multiple ribosomal structure in protein synthesis. Proc Natl Acad Sci U S A. 1963 Jan 15;49:122–129. doi: 10.1073/pnas.49.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]


