Abstract
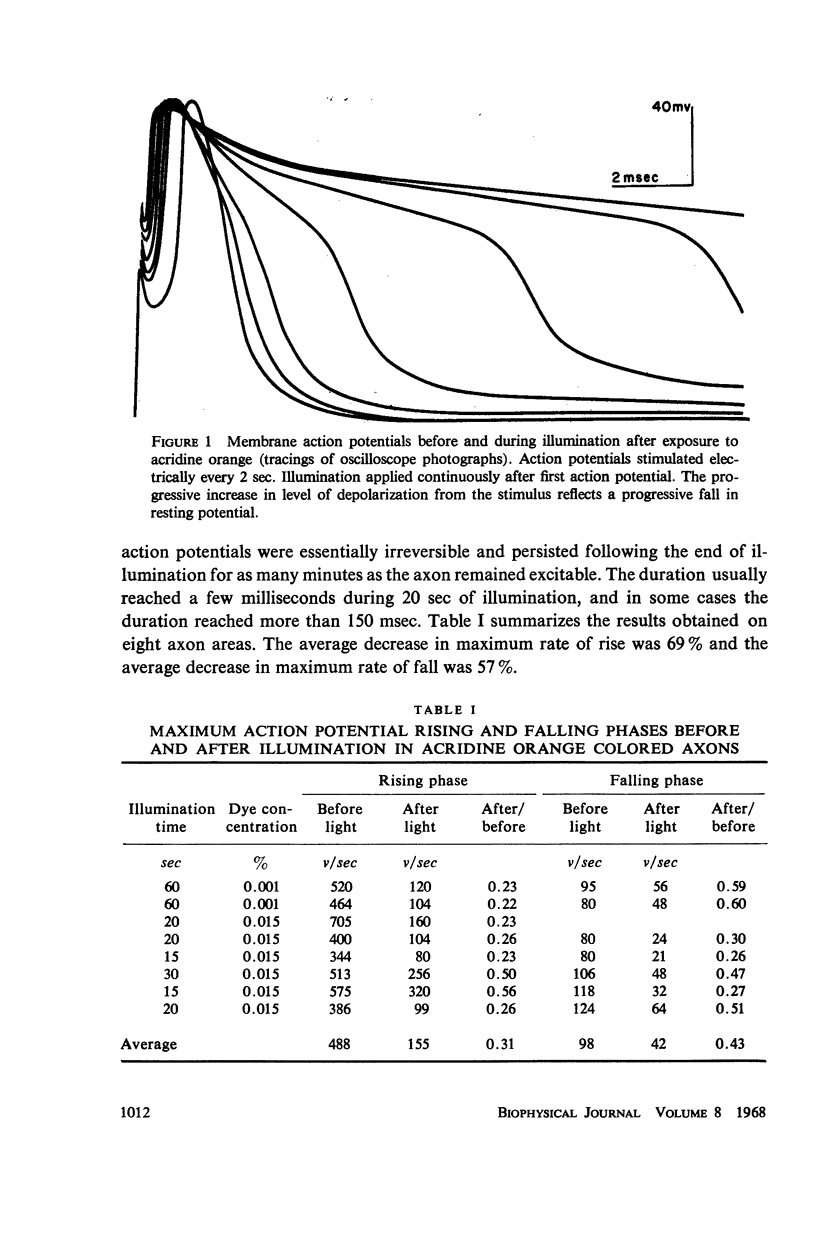
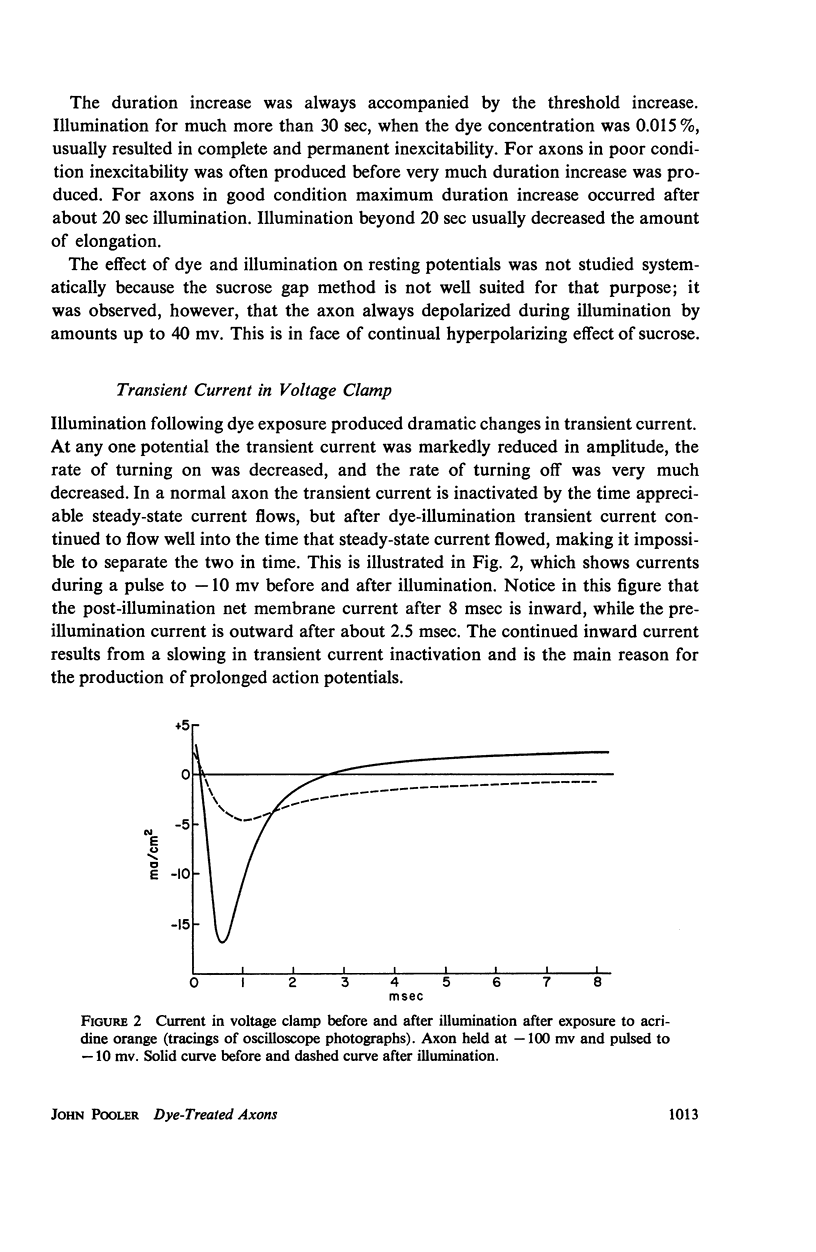
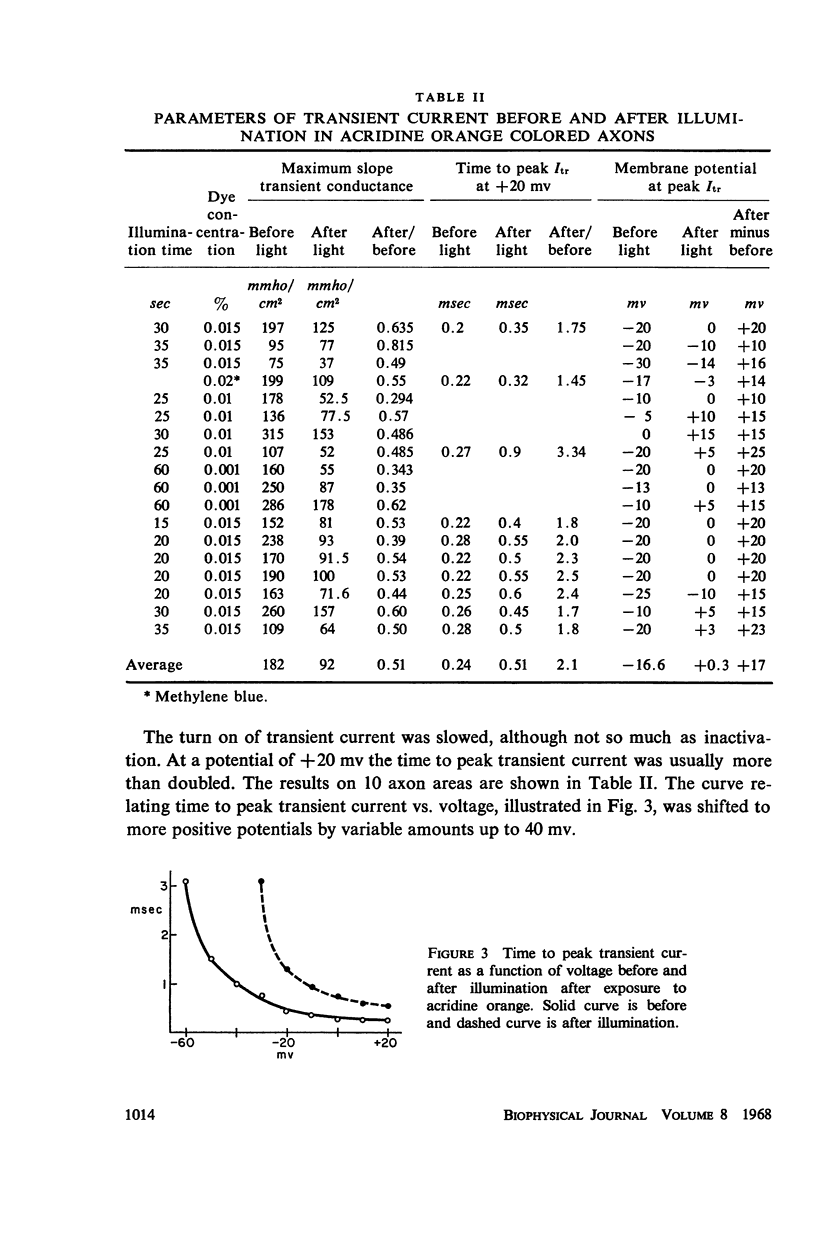
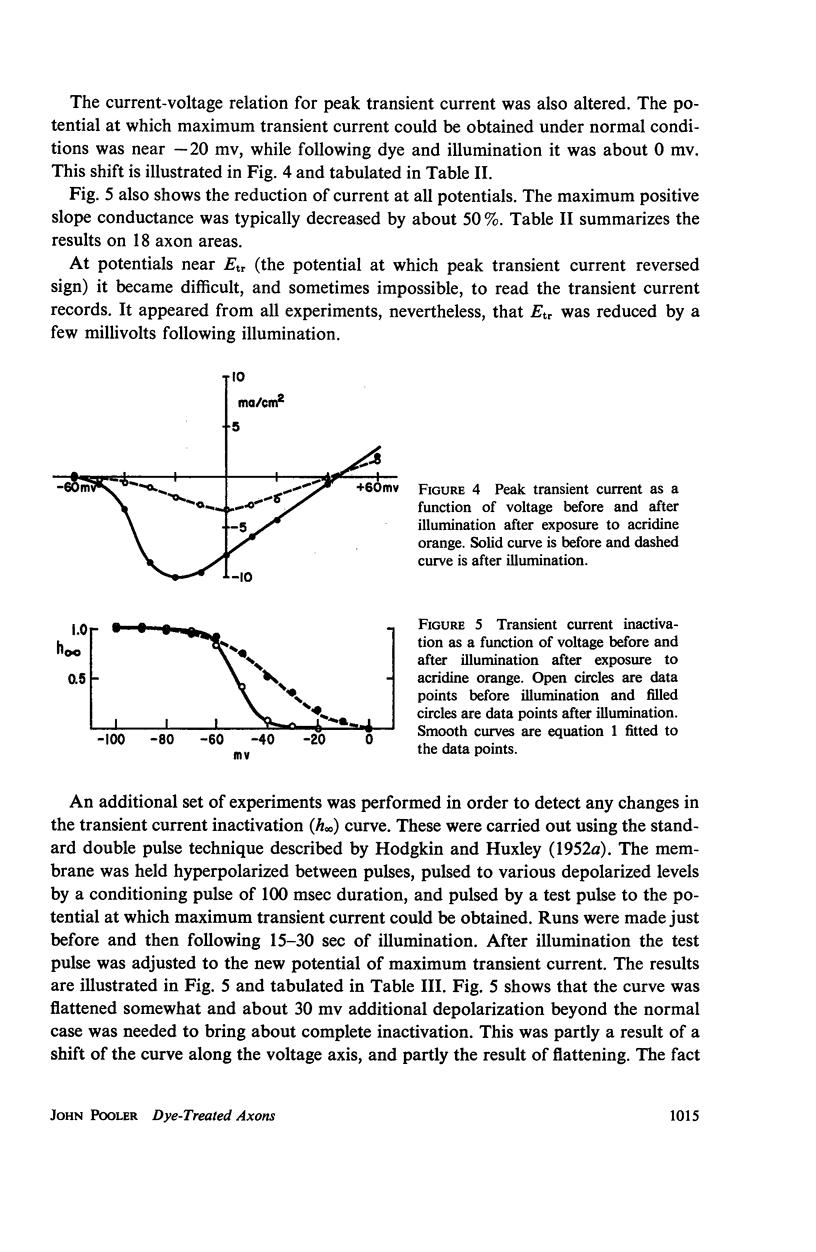
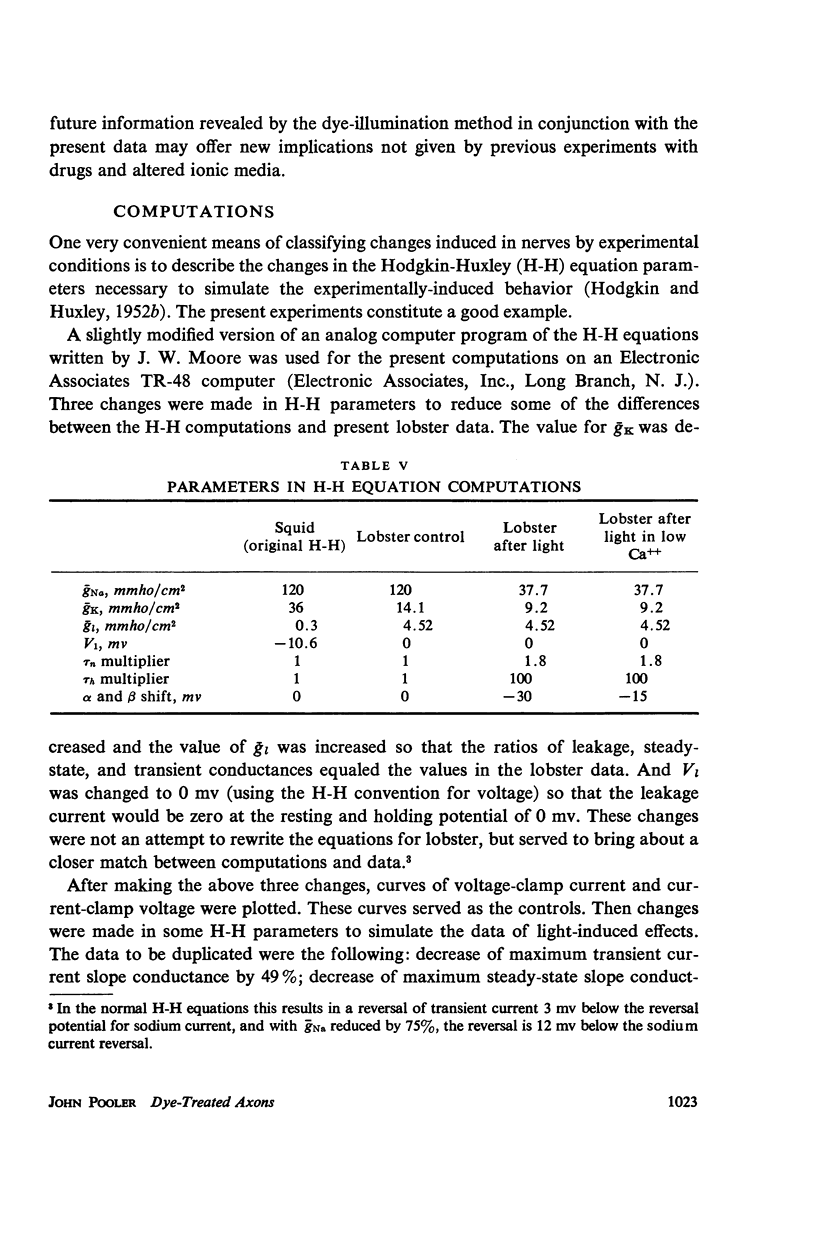
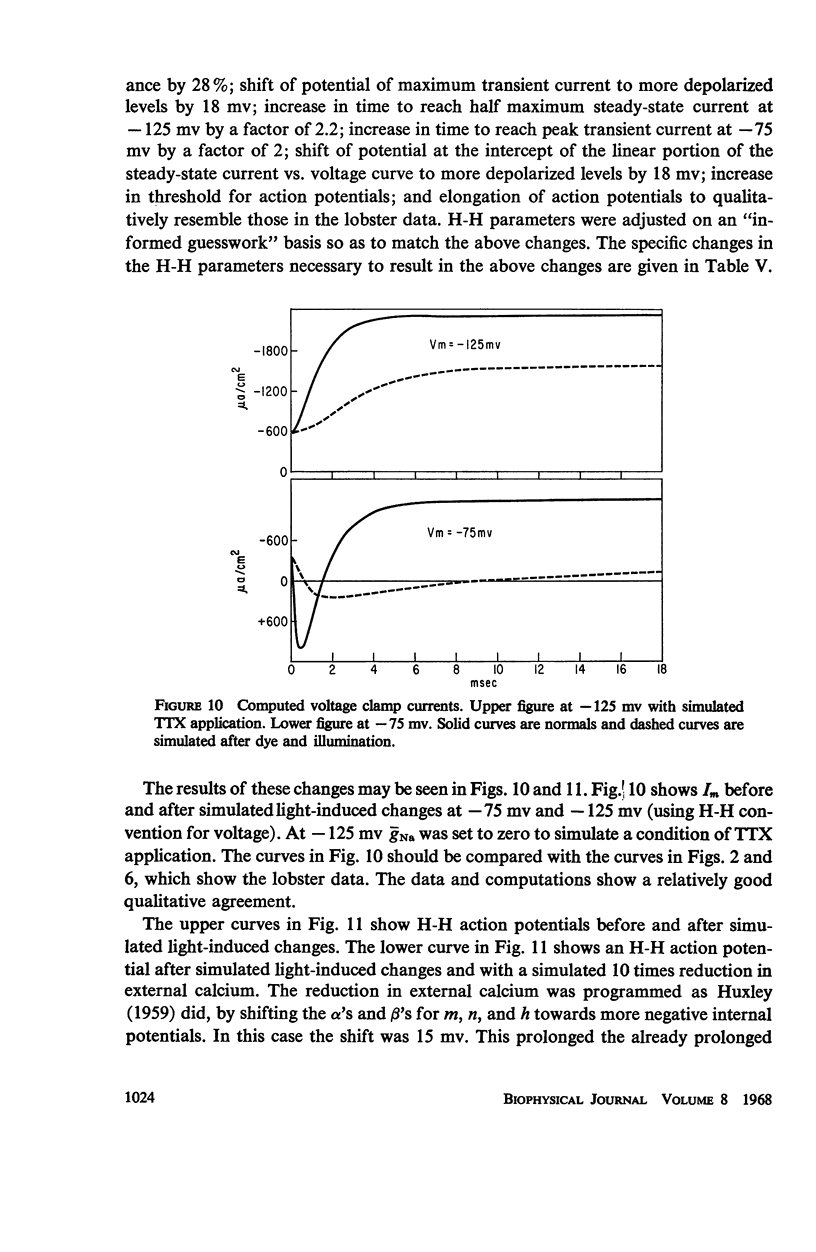
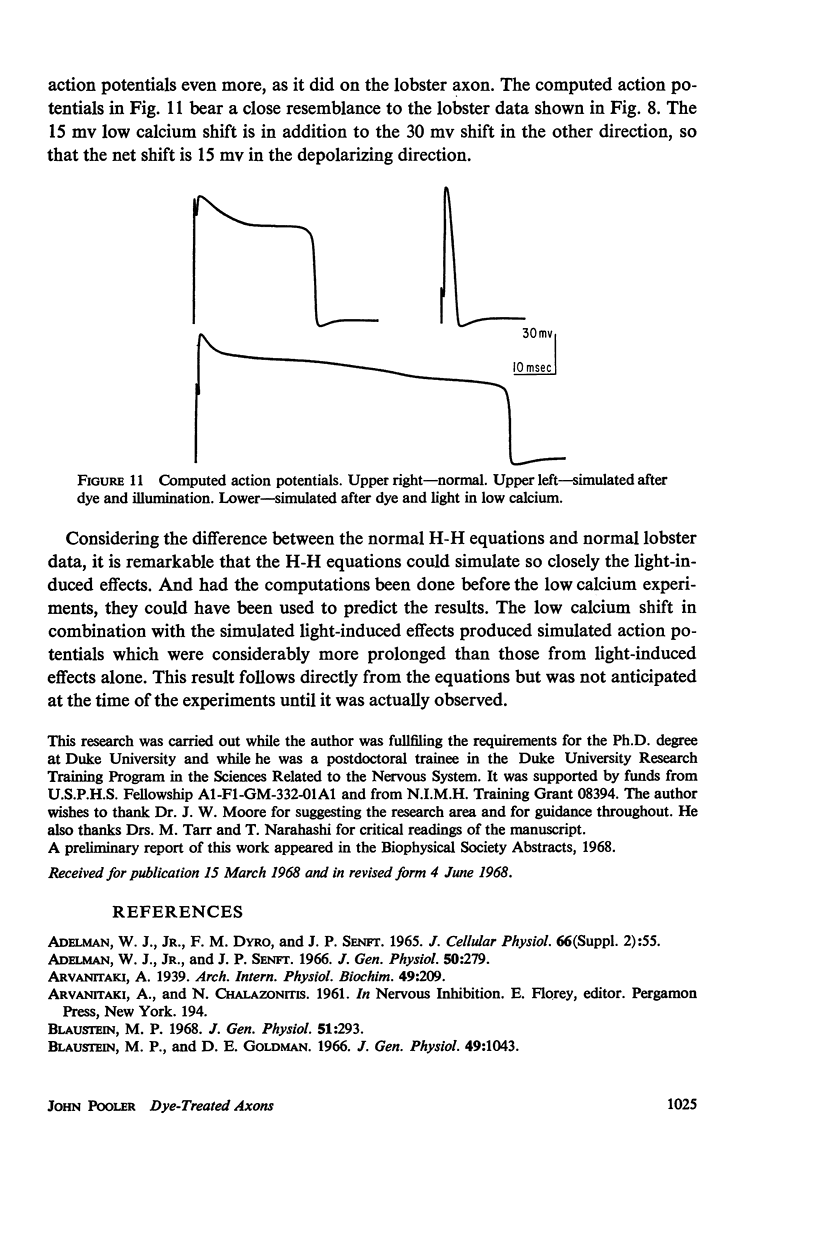
Single giant axons from the lobster circumesophageal connective were studied using the sucrose gap voltage-clamp technique. The axon area in the gap was bathed in acridine orange for several minutes and then rinsed for several minutes. Subsequent illumination resulted in progressive prolongation of electrically stimulated action potentials to durations of 150 msec. The prolongation was accompanied by an increase in threshold. Currents in voltage clamp were altered such that transient current inactivation was greatly slowed. The turn on of transient current was somewhat slowed, the voltage at which peak transient current could be obtained was shifted to more positive internal potentials, and transient current at all potentials was decreased. Steady-state current was similarly affected. Low calcium following illumination partially counteracted some of the changes, but not the slowing of inactivation. Low calcium increased the duration of prolonged action potentials. Selective alteration of parameters in the Hodgkin-Huxley equations brought about a qualitative match between computations and data.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman W. J., Jr, Senft J. P. Voltage clamp studies on the effect of internal cesium ion on sodium and potassium currents in the squid giant axon. J Gen Physiol. 1966 Nov;50(2):279–293. doi: 10.1085/jgp.50.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Goldman D. E. Competitive action of calcium and procaine on lobster axon. A study of the mechanism of action of certain local anesthetics. J Gen Physiol. 1966 May;49(5):1043–1063. doi: 10.1085/jgp.49.5.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Goldman D. E. The action of certain polyvalent cations on the voltage-clamped lobster axon. J Gen Physiol. 1968 Mar;51(3):279–291. doi: 10.1085/jgp.51.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALTON J. C. Effects of external ions on membrane potentials of a lobster giant axon. J Gen Physiol. 1958 Jan 20;41(3):529–542. doi: 10.1085/jgp.41.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Ion movements during nerve activity. Ann N Y Acad Sci. 1959 Aug 28;81:221–246. doi: 10.1111/j.1749-6632.1959.tb49311.x. [DOI] [PubMed] [Google Scholar]
- Hille B. Pharmacological modifications of the sodium channels of frog nerve. J Gen Physiol. 1968 Feb;51(2):199–219. doi: 10.1085/jgp.51.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JULIAN F. J., MOORE J. W., GOLDMAN D. E. Current-voltage relations in the lobster giant axon membrane under voltage clamp conditions. J Gen Physiol. 1962 Jul;45:1217–1238. doi: 10.1085/jgp.45.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JULIAN F. J., MOORE J. W., GOLDMAN D. E. Membrane potentials of the lobster giant axon obtained by use of the sucrose-gap technique. J Gen Physiol. 1962 Jul;45:1195–1216. doi: 10.1085/jgp.45.6.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W., Anderson N., Blaustein M., Takata M., Lettvin J. Y., Pickard W. F., Bernstein T., Pooler J. Alkali cation selectivity of squid axon membrane. Ann N Y Acad Sci. 1966 Jul 14;137(2):818–829. doi: 10.1111/j.1749-6632.1966.tb50202.x. [DOI] [PubMed] [Google Scholar]
- Moore J. W., Blaustein M. P., Anderson N. C., Narahashi T. Basis of tetrodotoxin's selectivity in blockage of squid axons. J Gen Physiol. 1967 May;50(5):1401–1411. doi: 10.1085/jgp.50.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T., Haas H. G. Interaction of DDT with the components of lobster nerve membrane conductance. J Gen Physiol. 1968 Feb;51(2):177–198. doi: 10.1085/jgp.51.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T., Wright E. B. A study of the crustacean axon repetitive response. I. The effect of membrane potential and resistance. J Cell Physiol. 1965 Apr;65(2):195–209. doi: 10.1002/jcp.1030650207. [DOI] [PubMed] [Google Scholar]



