Abstract
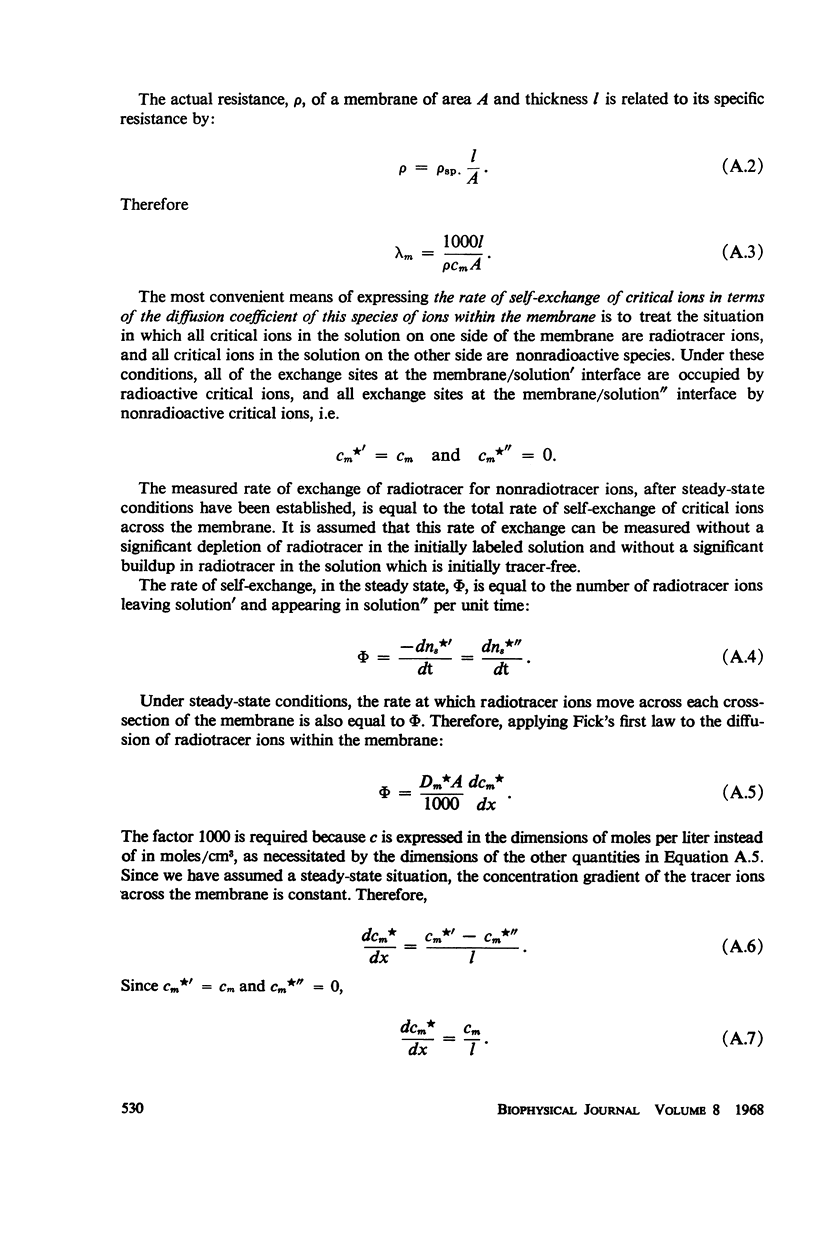
The electrical resistances and rates of self-exchange of univalent critical ions across several types of collodion matrix membranes of high ionic selectivity were studied over a wide range of conditions. The relationship which was observed between these quantities with membranes of a certain type, namely those activated with poly-2-vinyl-N-methyl pyridinium bromide, cannot be explained on the basis of current concepts of the movement of ions across ion exchange membranes. Rates of self-exchange across these membranes were several times greater than those calculated from the electrical resistances of the membranes on the basis of an expression derived by the use of the Nernst-Einstein equation. The magnitude of the discrepancy was greatest at low concentrations of the ambient electrolyte solution and was independent of the species of both critical and noncritical ions. The data obtained with other types of collodion matrix membranes were, at least approximately, in agreement with the predictions based on the Nernst-Einstein equation. Self-exchange rates across the anion permeable protamine collodion membranes, and across the cation permeable polystyrene sulfonic acid collodion membranes, were about 20% less than those calculated from the electrical resistances. The direction and magnitude of these differences, also observed by other investigators, are qualitatively understood as an electroosmotic effect. With cation permeable membranes prepared by the oxidation of preformed collodion membranes, almost exact agreement was obtained between measured and calculated self-exchange rates; the cause of the apparent absence of an electroosmotic effect with these membranes is unknown.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kedem O., Essig A. Isotope flows and flux ratios in biological membranes. J Gen Physiol. 1965 Jul;48(6):1047–1070. doi: 10.1085/jgp.48.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USSING H. H. Some aspects of the application of tracers in permeability studies. Adv Enzymol Relat Subj Biochem. 1952;13:21–65. doi: 10.1002/9780470122587.ch2. [DOI] [PubMed] [Google Scholar]



