Abstract
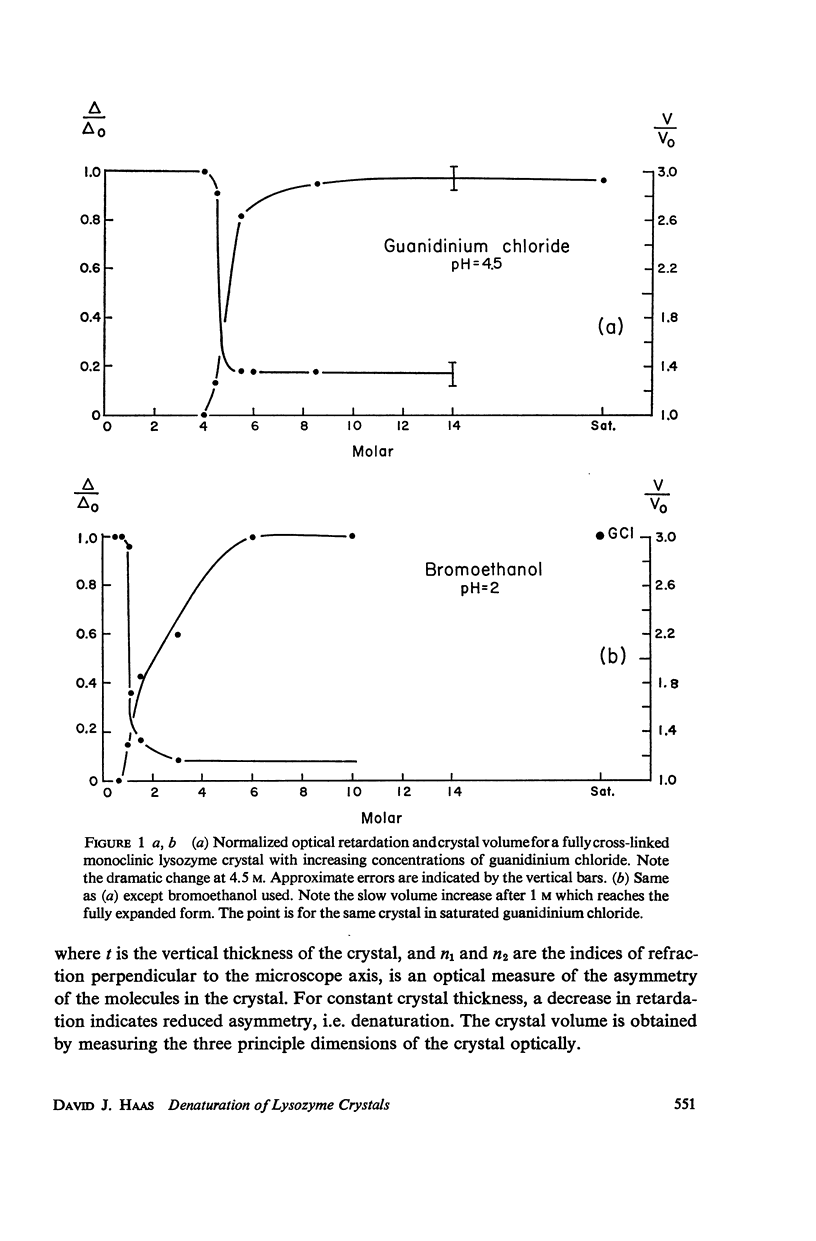
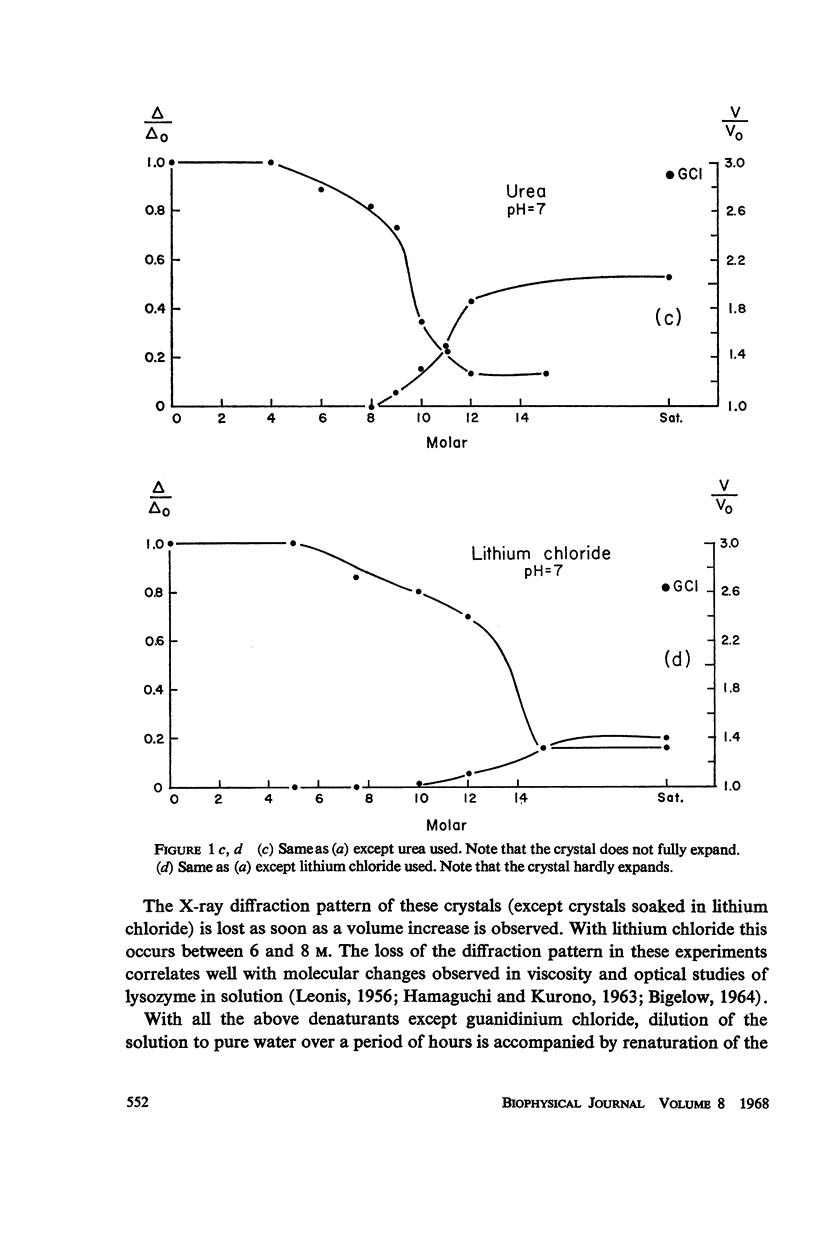
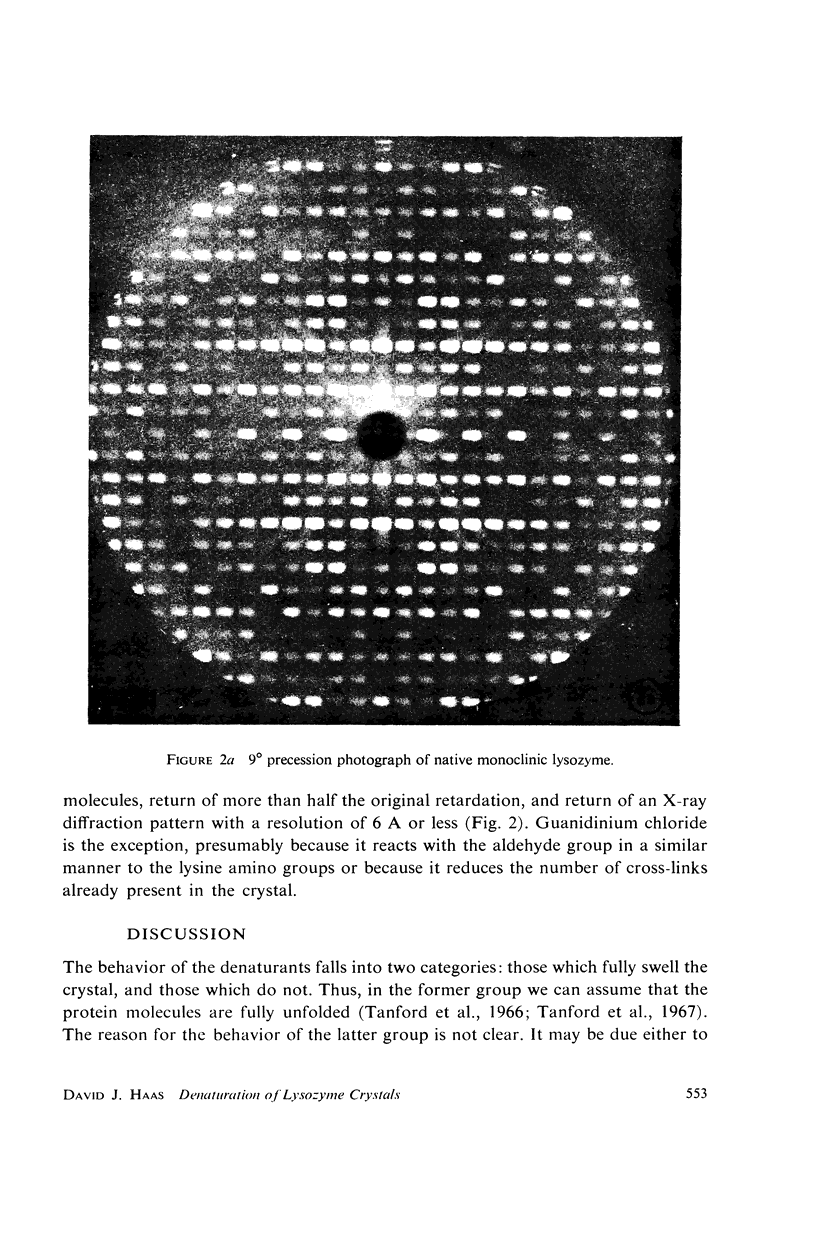
When monoclinic lysozyme crystals are fully cross-linked with glutaraldehyde, and then the protein molecules are denatured while in the crystalline state, a single crystal-gel is formed which is a jelly-like crystal of a denatured protein molecule. It is highly disordered, but has crystalline optical and morphological properties and can be renatured to a cross-linked crystal resembling the original crystal as determined from the X-ray diffraction pattern. Experiments with the following denaturants are described: guanidinium chloride, bromoethanol, urea, and lithium chloride.
Full text
PDF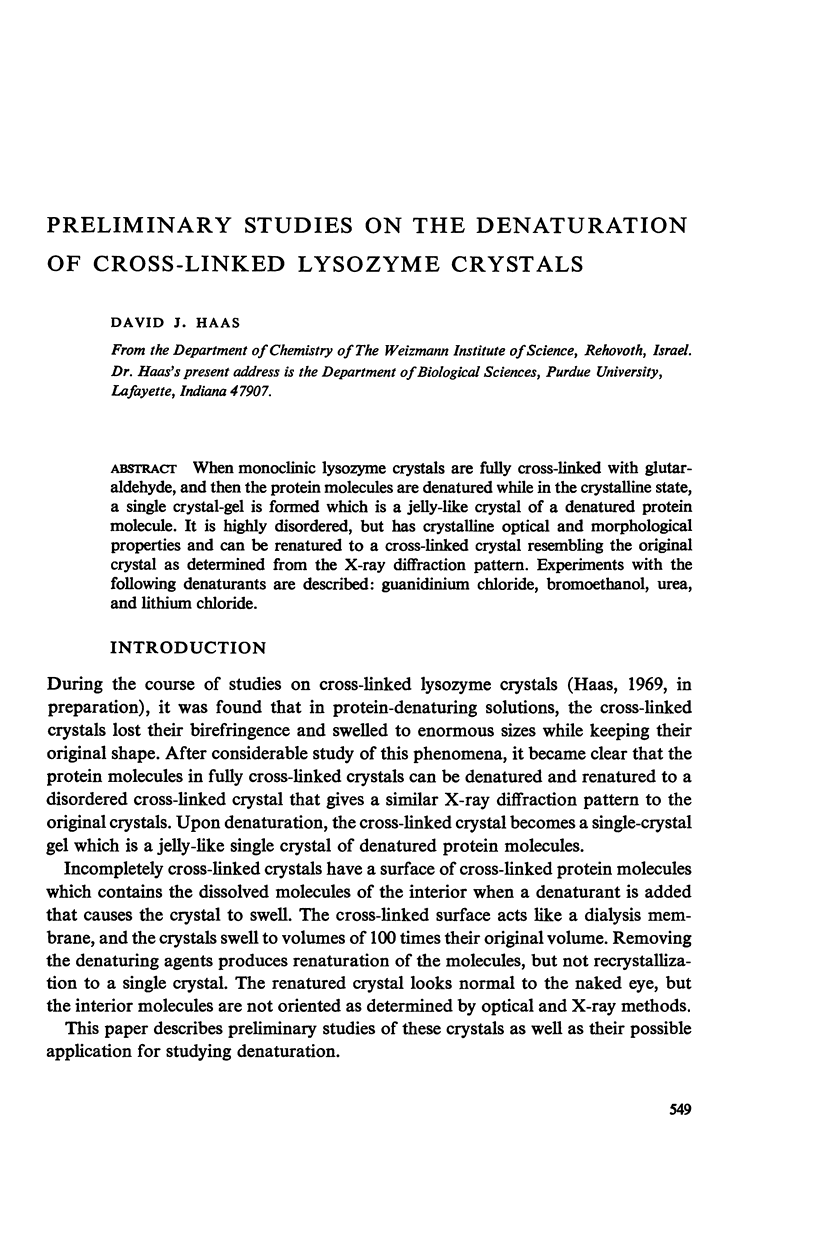


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIGELOW C. C. THE DENATURED STATES OF RIBONUCLEASE. J Mol Biol. 1964 May;8:696–701. doi: 10.1016/s0022-2836(64)80118-2. [DOI] [PubMed] [Google Scholar]
- HAMAGUCHI K., KURONO A. STRUCTURE OF MURAMIDASE (LYSOZYME). I. THE EFFECT OF GUANIDINE HYDROCHLORIDE ON MURAMIDASE. J Biochem. 1963 Aug;54:111–122. [PubMed] [Google Scholar]
- LEONIS J. Preliminary investigation of the behavior of lysozyme in urea solutions. Arch Biochem Biophys. 1956 Nov;65(1):182–193. doi: 10.1016/0003-9861(56)90186-2. [DOI] [PubMed] [Google Scholar]
- Tanford C., Pain R. H., Otchin N. S. Equilibrium and kinetics of the unfolding of lysozyme (muramidase) by guanidine hydrochloride. J Mol Biol. 1966 Feb;15(2):489–504. doi: 10.1016/s0022-2836(66)80123-7. [DOI] [PubMed] [Google Scholar]




