Abstract
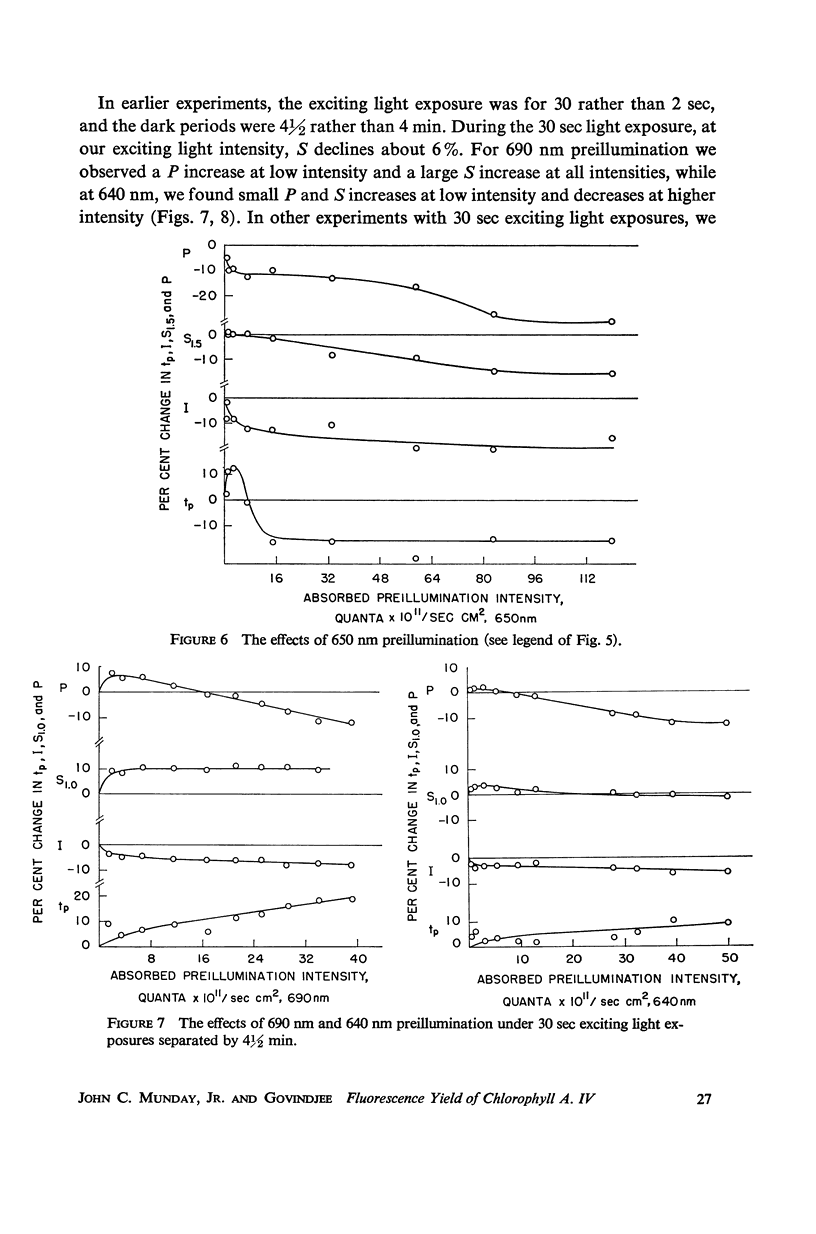
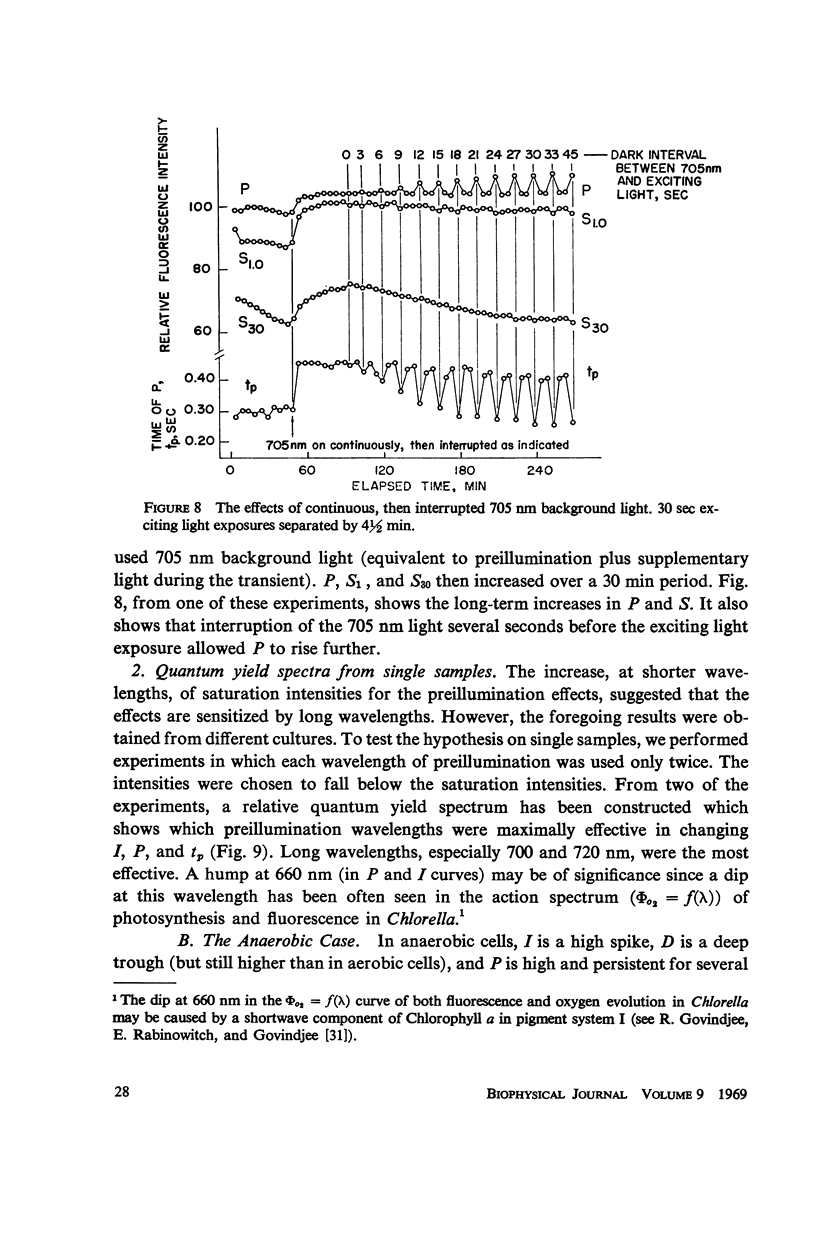
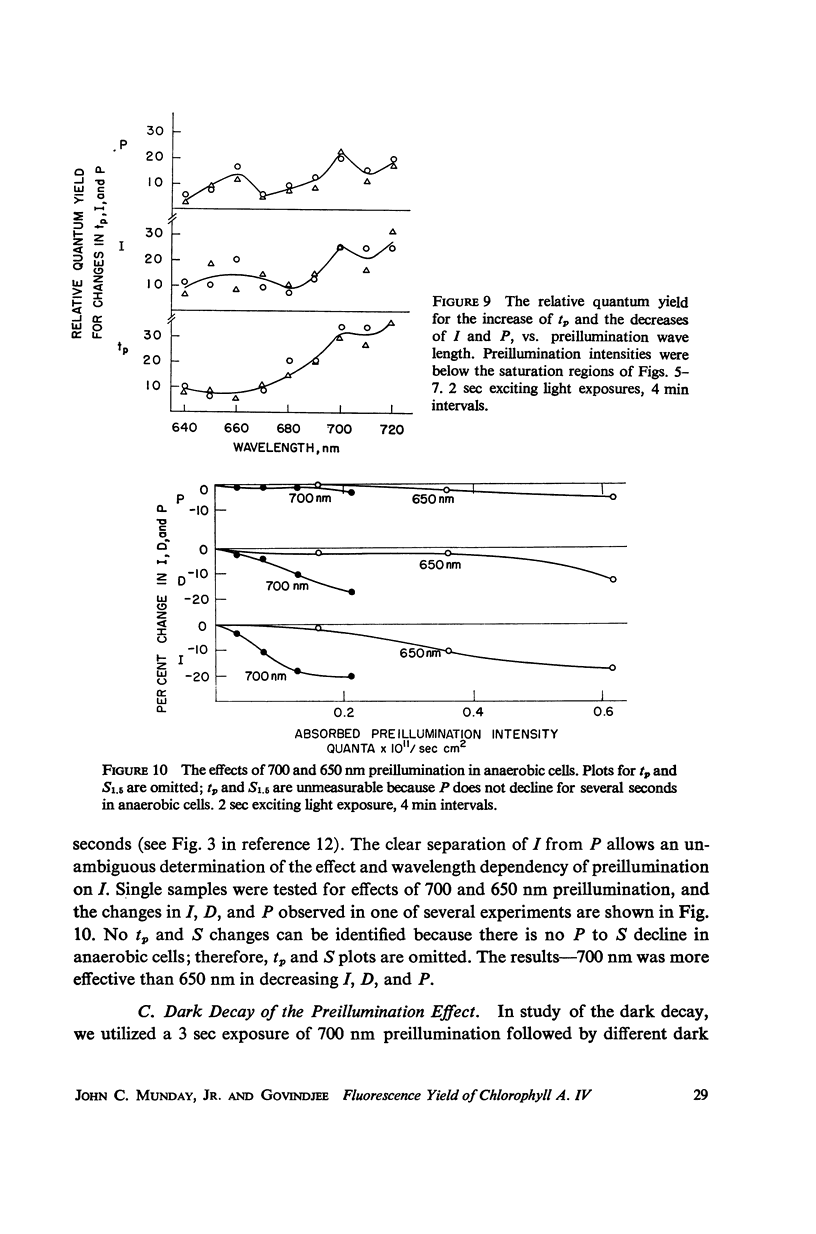
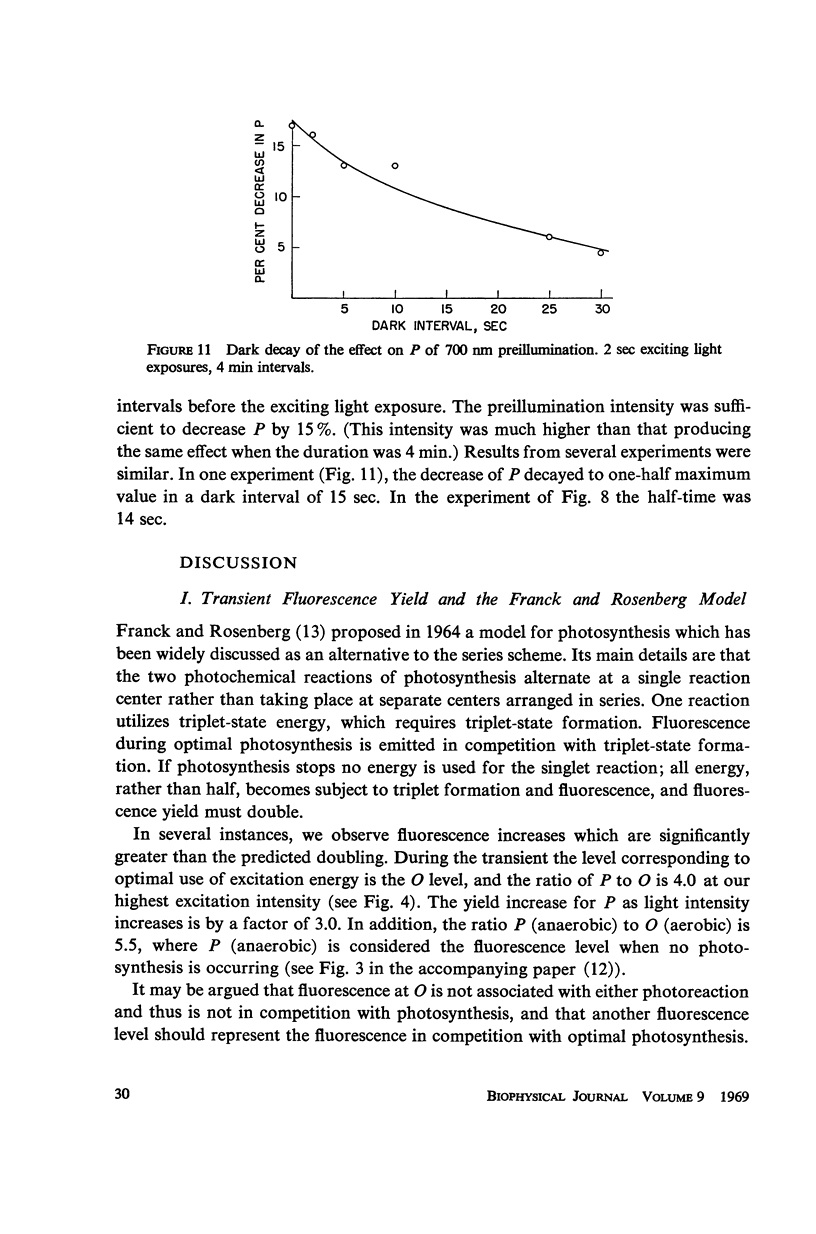
The fluorescence transient of Chlorella pyrenoidosa, excited by saturating blue light, has a base level O, hump I, dip D, peak P, and at 1.5 sec a quasi-steady level S (12). With 2 sec exciting exposures and 4 min dark periods, preillumination-1 (λ ≥ 690 nm, intensities 1-750 ergs/sec-cm2 incident), replacing the dark periods, lowers I more effectively than preillumination-2 (650 nm ≤ λ ≤ 680 nm) in both aerobic and anaerobic cells. Results indicate that the intersystem electron transport pool A as well as the primary electron acceptor of pigment system II Q (fluorescence quencher) is normally being reduced at I. Preillumination-1 lowers and delays P. Preillumination-2 (absorbed by both pigment systems) also lowers P, but delays P only at low intensity; at high intensity it hastens P. Preillumination-1 raises S while preillumination-2 lowers S. With 30 instead of 2 sec exciting light exposures, preillumination-1 causes a large S increase, and at low intensity a P increase. The S effects seem to be of a long-term nature (26-29) rather than rapid changes in the redox state of Q. As exciting light intensity increases, fluorescence yield at P increases three-fold maximally. The ratio of P (anaerobic) to O (aerobic) is 5.5. These high ratios restrict the Franck-Rosenberg model of photosynthesis (13), which is based on fluorescence yield doubling.
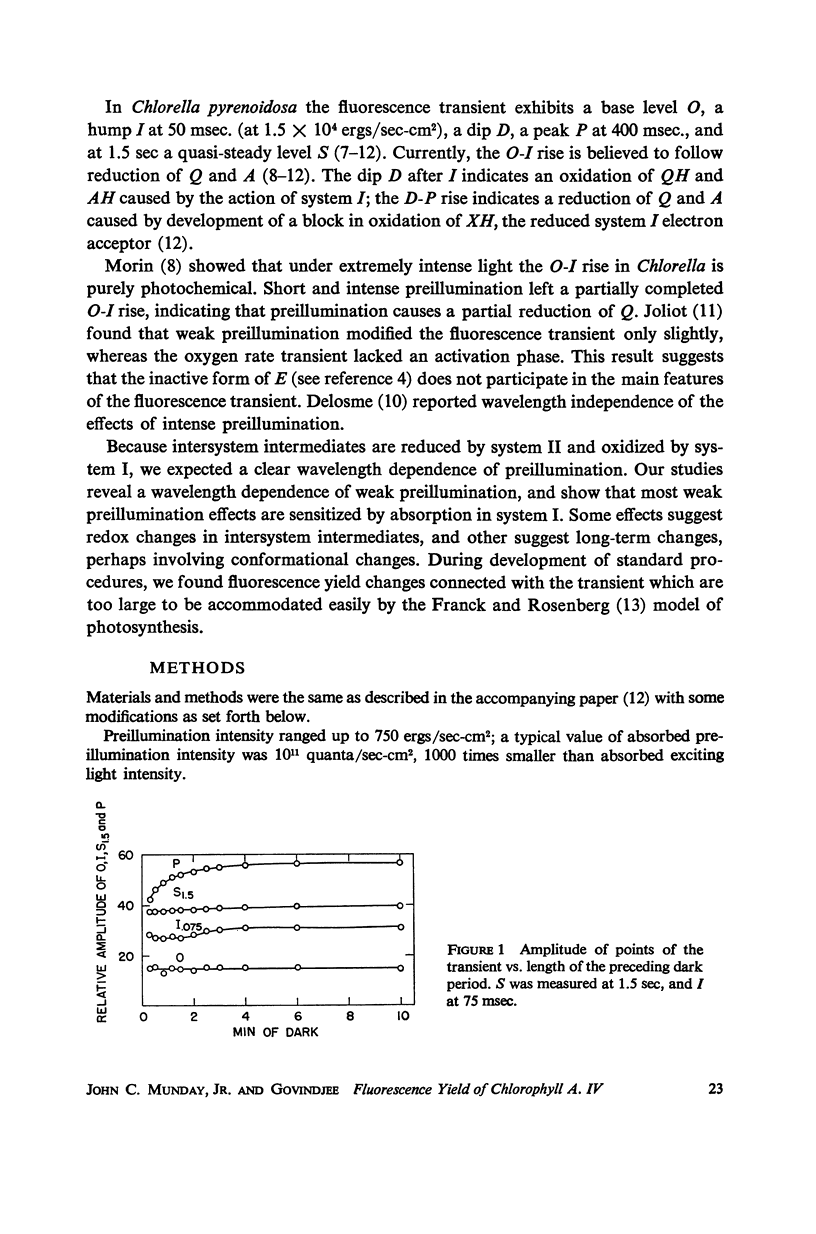
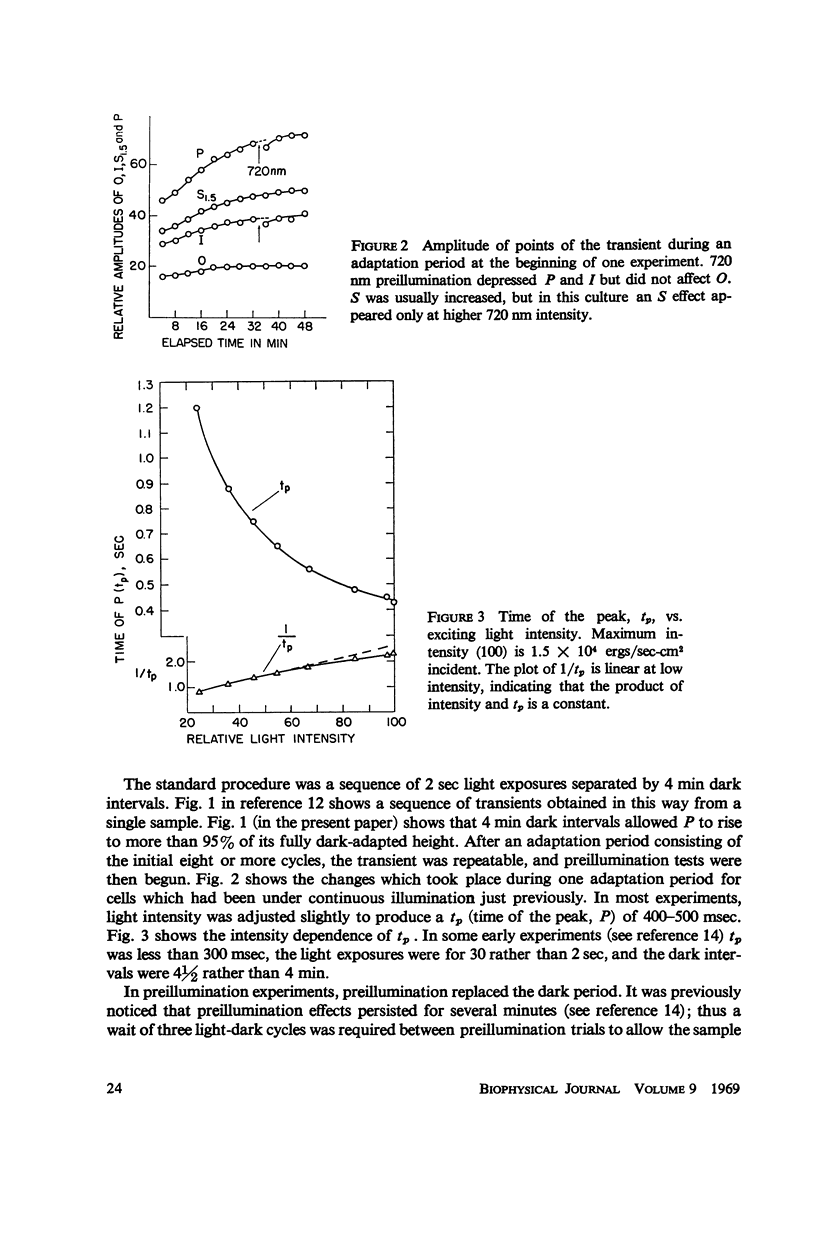
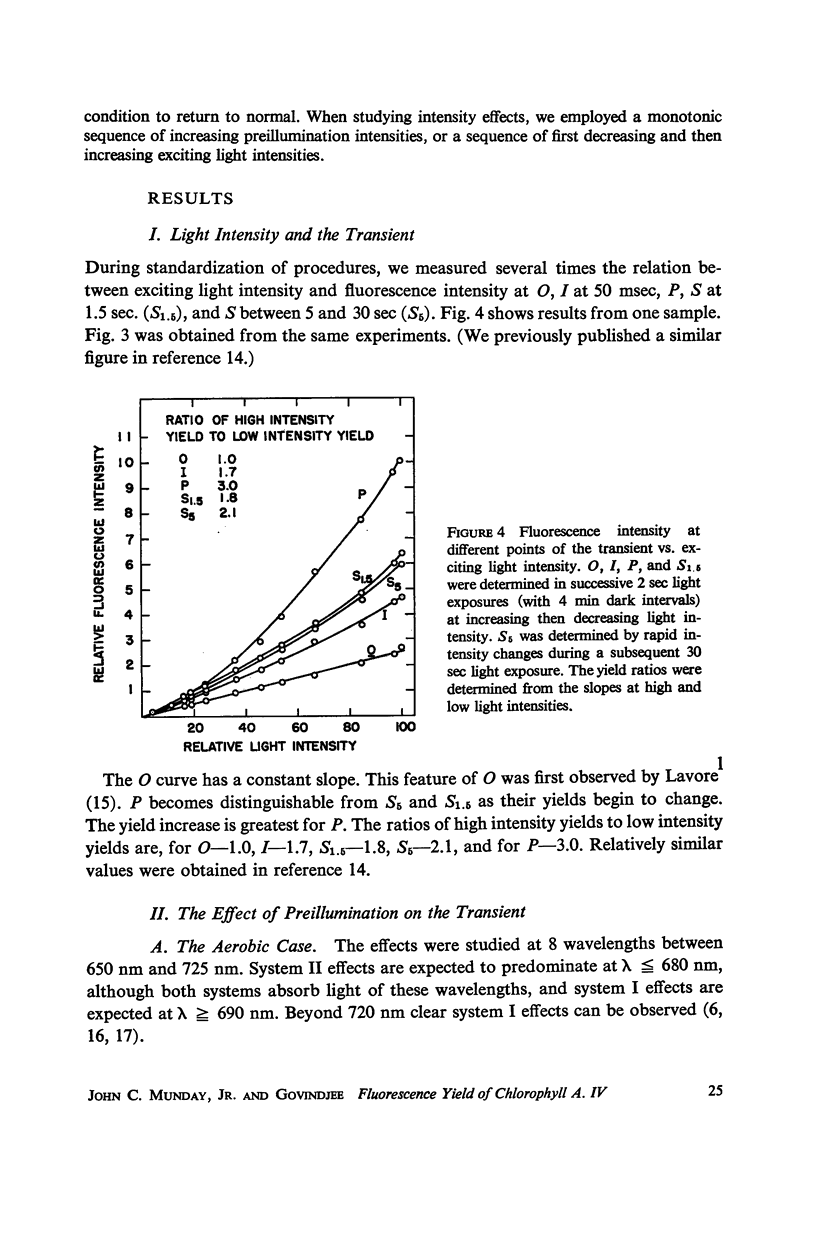
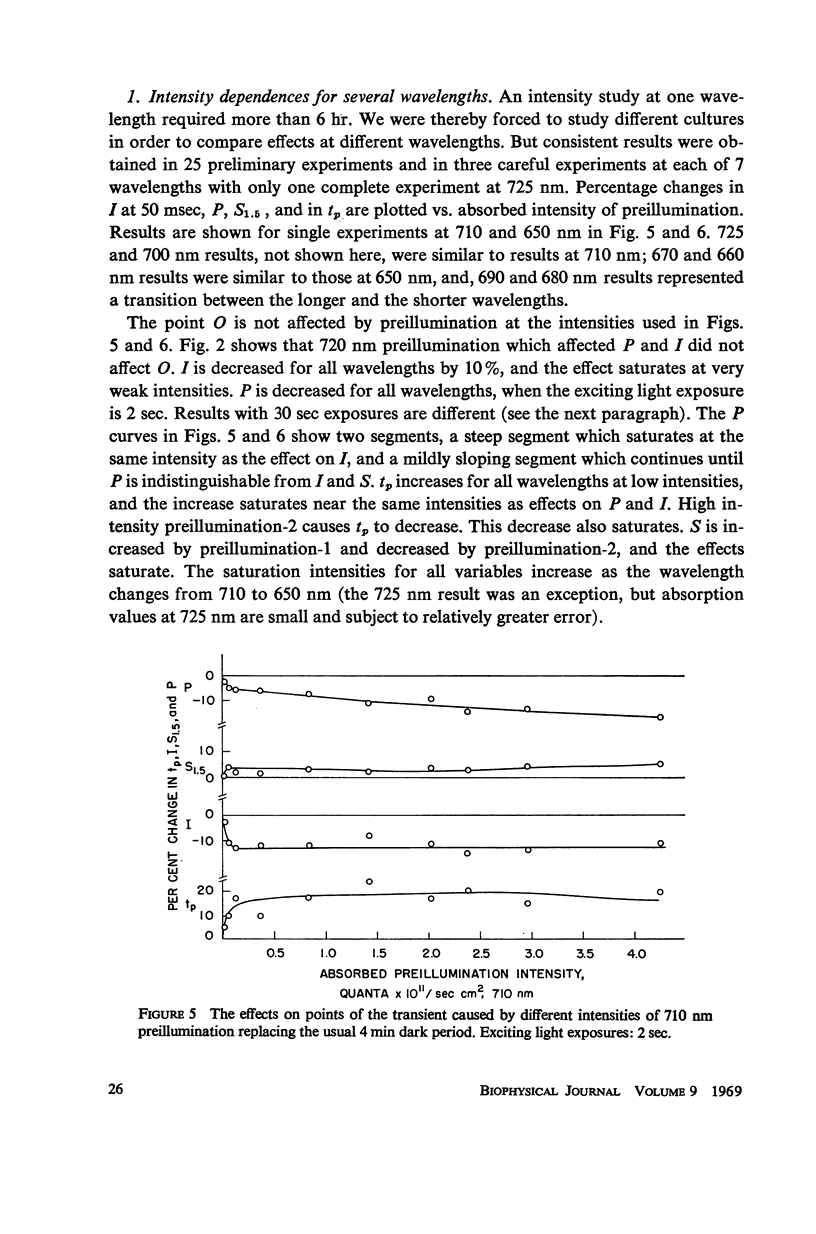
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER W. L. Effects of red and far-red light on the fluorescence yield of chlorophyll in vivo. Biochim Biophys Acta. 1962 Oct 22;64:309–317. doi: 10.1016/0006-3002(62)90739-4. [DOI] [PubMed] [Google Scholar]
- Delosme R. Etude de l'induction de fluorescence des algues vertes et des chloroplastes au début d'une illumination intense. Biochim Biophys Acta. 1967 Jul 5;143(1):108–128. doi: 10.1016/0005-2728(67)90115-6. [DOI] [PubMed] [Google Scholar]
- FRANCK J., ROSENBERG J. L. A THEORY OF LIGHT UTILIZATION IN PLANT PHOTOSYNTHESIS. J Theor Biol. 1964 Sep;7:276–301. doi: 10.1016/0022-5193(64)90073-6. [DOI] [PubMed] [Google Scholar]
- GOVINDJEE, ICHIMURA S., CEDERSTRAND C., RABINOWITCH E. Effect of combining far-red light with shorter wave light on the excitation of fluorescence in Chlorella. Arch Biochem Biophys. 1960 Aug;89:322–323. doi: 10.1016/0003-9861(60)90063-1. [DOI] [PubMed] [Google Scholar]
- Govindjee, Munday J. C., Jr, Papageorgiou G. Fluorescence studies with algae: changes with time and preillumination. Brookhaven Symp Biol. 1966;19:434–445. [PubMed] [Google Scholar]
- Govindjee R., Rabinowitch E., Govindjee Maximum quantum yield and action spectrum of photosynthesis and fluorescence in Chlorella. Biochim Biophys Acta. 1968 Nov 26;162(4):539–544. doi: 10.1016/0005-2728(68)90061-3. [DOI] [PubMed] [Google Scholar]
- JOLIOT P., LAVOREL J. LES R'EACTIONS PRIMAIRES DE LA PHOTOSYNTH'ESE. Bull Soc Chim Biol (Paris) 1964;46:1607–1626. [PubMed] [Google Scholar]
- Joliot P. Cinétiques des réactions liées a l'émission d'oxygène photosynthétique. Biochim Biophys Acta. 1965 May 25;102(1):116–134. [PubMed] [Google Scholar]
- Joliot P. Etudes simultanées des cinétiques de fluorescence et d'émission d'oxygène photosynthétique. Biochim Biophys Acta. 1965 May 25;102(1):135–148. [PubMed] [Google Scholar]
- KAUTSKY H., APPEL W., AMANN H. [Chlorophyll fluorescence and carbon assimilation. Part XIII. The fluorescence and the photochemistry of plants]. Biochem Z. 1960;332:277–292. [PubMed] [Google Scholar]
- Kok B., Malkin S., Owens O., Forbush B. Observations on the reducing side of the O2-evolving photoact. Brookhaven Symp Biol. 1966;19:446–459. [PubMed] [Google Scholar]
- Lavorel J. Induction of Fluorescence in Quinone Poisoned Chlorella Cells. Plant Physiol. 1959 May;34(3):204–209. doi: 10.1104/pp.34.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin S. Analysis of the weak light effect on the fluorescence yield in isolated chloroplasts. Biochim Biophys Acta. 1968 Jan 15;153(1):188–196. doi: 10.1016/0005-2728(68)90159-x. [DOI] [PubMed] [Google Scholar]
- Malkin S., Kok B. Fluorescence induction studies in isolated chloroplasts. I. Number of components involved in the reaction and quantum yields. Biochim Biophys Acta. 1966 Nov 8;126(3):413–432. doi: 10.1016/0926-6585(66)90001-x. [DOI] [PubMed] [Google Scholar]
- Murata N., Nishimura M., Takamiya A. Fluorescene of chlorophyll in photosynthetic systems. II. Induction of fluorescence in isolated spinach chloroplasts. Biochim Biophys Acta. 1966 May 12;120(1):23–33. doi: 10.1016/0926-6585(66)90273-1. [DOI] [PubMed] [Google Scholar]
- Papageorgiou G., Govindjee Light-induced changes in the fluorescence yield of chlorophyll a in vivo. I. Anacystis nidulans. Biophys J. 1968 Nov;8(11):1299–1315. doi: 10.1016/S0006-3495(68)86557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou G., Govindjee Light-induced changes in the fluorescence yield of chlorophyll a in vivo. II. Chlorella pyrenoidosa. Biophys J. 1968 Nov;8(11):1316–1328. doi: 10.1016/S0006-3495(68)86558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


