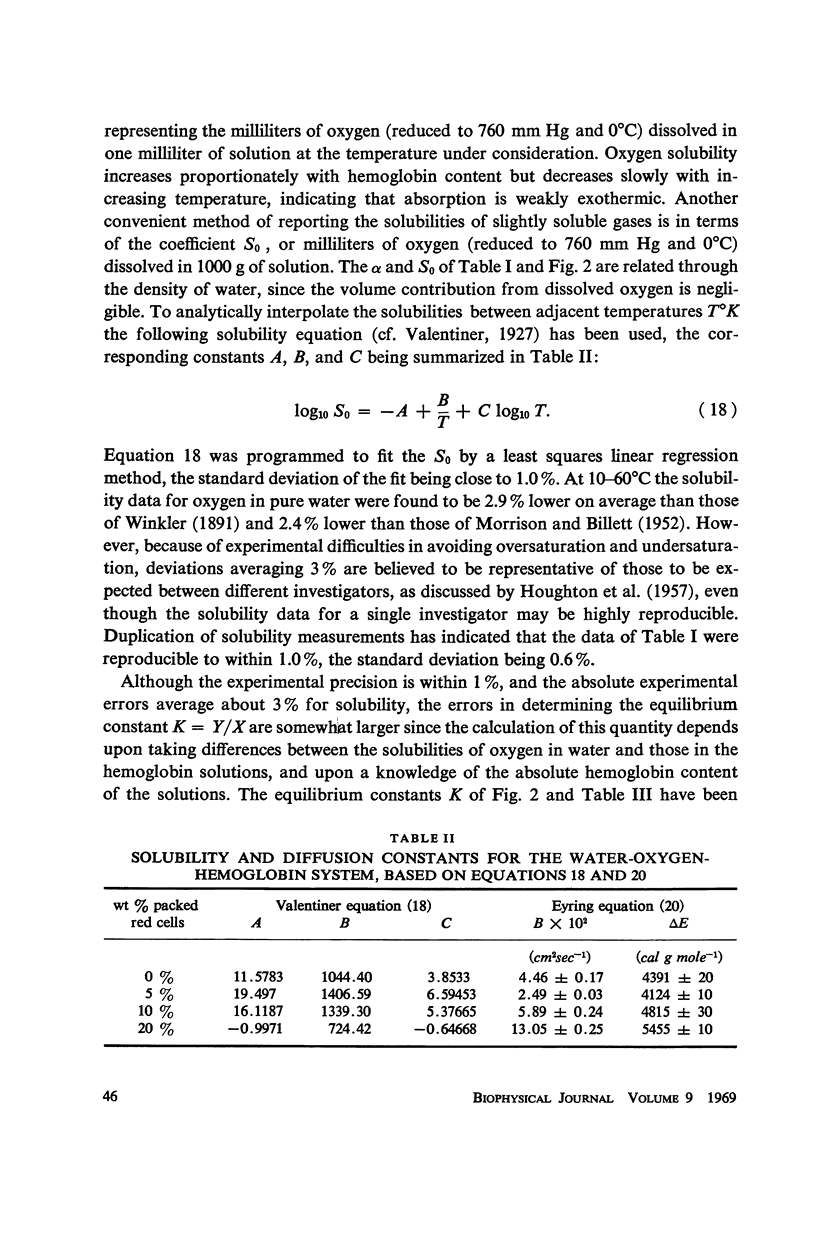
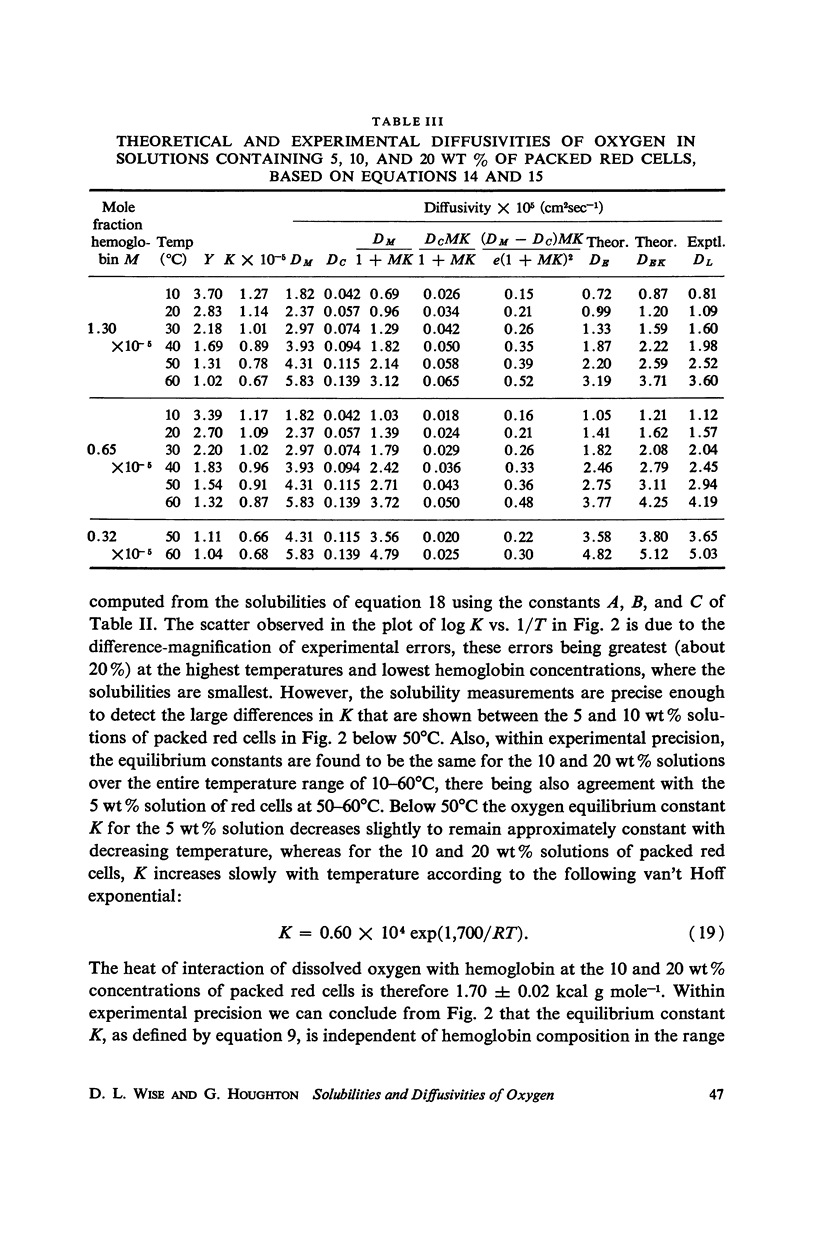
Abstract
By continuous absorption and by bubble collapse methods respectively, the solubilities and diffusion coefficients of oxygen in water and in dilute solutions of human hemoglobin (1.11, 2.22, and 4.44 wt%) have been determined at one atmosphere and 10°, 20°, 30°, 40°, 50°, and 60°C. Measured equilibrium constants, oxygen/hemoglobin ratios and isochoric heats of solution have been interpreted in terms of various mechanisms for oxygen-hemoglobin interaction. Oxygen diffusivities obtained experimentally for the hemolyzed blood solutions have been found to compare favorably with those predicted by a model of facilitated transport proposed by Houghton (1966). The diffusion measurements indicate that, while kinetic phenomena cannot be ignored, the over-all rate of exchange of oxygen with hemoglobin is not a controlling factor in facilitated diffusion. Anomalous equilibrium constants and temperature coefficients have been observed in the most dilute hemoglobin solution at the lowest temperatures.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAUNITZER G., GEHRING-MUELLER R., HILSCHMANN N., HILSE K., HOBOM G., RUDLOFF V., WITTMANN-LIEBOLD B. [The structure of normal adult human hemoglobins]. Hoppe Seylers Z Physiol Chem. 1961 Sep 20;325:283–286. doi: 10.1515/bchm2.1961.325.1.283. [DOI] [PubMed] [Google Scholar]
- ENNS T. MOLECULAR COLLISION-EXCHANGE TRANSPORT OF OXYGEN BY HEMOGLOBIN. Proc Natl Acad Sci U S A. 1964 Feb;51:247–252. doi: 10.1073/pnas.51.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTTER F. J., SOBER H. A., PETERSON E. A. The effect of mercaptoethanol and urea on the molecular weight of hemoglobin. Arch Biochem Biophys. 1956 Jun;62(2):427–434. doi: 10.1016/0003-9861(56)90141-2. [DOI] [PubMed] [Google Scholar]
- HEMMINGSEN E. A. ENHANCEMENT OF OXYGEN TRANSPORT BY MYOGLOBIN. Comp Biochem Physiol. 1963 Nov;10:239–244. doi: 10.1016/0010-406x(63)90037-9. [DOI] [PubMed] [Google Scholar]
- HEMMINGSEN E. Accelerated exchange of oxygen-18 through a membrane containing oxygen-saturated hemoglobin. Science. 1962 Mar 2;135(3505):733–734. doi: 10.1126/science.135.3505.733. [DOI] [PubMed] [Google Scholar]
- HEMMINGSEN E., SCHOLANDER P. F. Specific transport of oxygen through hemoglobin solutions. Why is this transport abolished when opposed by a slight back pressure of oxygen? Science. 1960 Nov 11;132(3437):1379–1381. doi: 10.1126/science.132.3437.1379. [DOI] [PubMed] [Google Scholar]
- HOUGHTON G. THE ADDITIVITY OF RATE AND DIFFUSION PHENOMENA IN CONTINUOUS CHROMATOGRAPHY. J Chromatogr. 1964 Jun;14:5–8. doi: 10.1016/s0021-9673(01)82733-3. [DOI] [PubMed] [Google Scholar]
- Keller K. H., Friedlander S. K. Diffusivity measurements of human methemoglobin. J Gen Physiol. 1966 Mar;49(4):681–687. doi: 10.1085/jgp.49.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAFORCE R. C., FATT I. STEADY-STATE GAS TRANSPORT THROUGH HEMOGLOBIN SOLUTIONS. Biopolym Symp. 1964;13:555–562. [PubMed] [Google Scholar]
- Lamm O., Polson A. The determination of diffusion constants of proteins by a refractometric method. Biochem J. 1936 Mar;30(3):528–541. doi: 10.1042/bj0300528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki M., Forster R. E. Diffusion of Carbon Monoxide through Thin Layers of Hemoglobin Solution. Science. 1962 Nov 23;138(3543):897–898. doi: 10.1126/science.138.3543.897. [DOI] [PubMed] [Google Scholar]
- ROUGHTON F. J., OTIS A. B., LYSTER R. L. The determination of the individual equilibrium constants of the four intermediate reactions between oxygen and sheep haemoglobin. Proc R Soc Lond B Biol Sci. 1955 Aug 16;144(914):29–54. doi: 10.1098/rspb.1955.0032. [DOI] [PubMed] [Google Scholar]
- Roughton F. J., Adair G. S., Barcroft J., Goldschmidt G., Herkel W., Hill R. M., Keys A. B., Ray G. B. The thermochemistry of the oxygen-haemoglobin reaction: Comparison of the heat as measured directly on purified haemoglobin with that calculated indirectly by the Van't Hoff Isochore. Biochem J. 1936 Nov;30(11):2117–2133. doi: 10.1042/bj0302117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughton F. J. The oxygen equilibrium of mammalian hemoglobin. Some old and new physicochemical studies. J Gen Physiol. 1965 Sep;49(1 Suppl):105–126. doi: 10.1085/jgp.49.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughton F. J. Thermochemistry of the oxygen-haemoglobin reaction: Direct measurements of the heat of reaction under various conditions. Biochem J. 1935 Nov;29(11):2604–2621. doi: 10.1042/bj0292604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOLANDER P. F. Oxygen transport through hemoglobin solutions. Science. 1960 Feb 26;131(3400):585–590. doi: 10.1126/science.131.3400.585. [DOI] [PubMed] [Google Scholar]
- Snell F. M. Facilitated transport of oxygen through solutions of hemoglobin. J Theor Biol. 1965 May;8(3):469–479. doi: 10.1016/0022-5193(65)90022-6. [DOI] [PubMed] [Google Scholar]
- Wittenberg J. B. Myoglobin-facilitated diffusion of oxygen. J Gen Physiol. 1965 Sep;49(1 Suppl):57–74. [PubMed] [Google Scholar]
- Wittenberg J. B. The molecular mechanism of hemoglobin-facilitated oxygen diffusion. J Biol Chem. 1966 Jan 10;241(1):104–114. [PubMed] [Google Scholar]


