Abstract
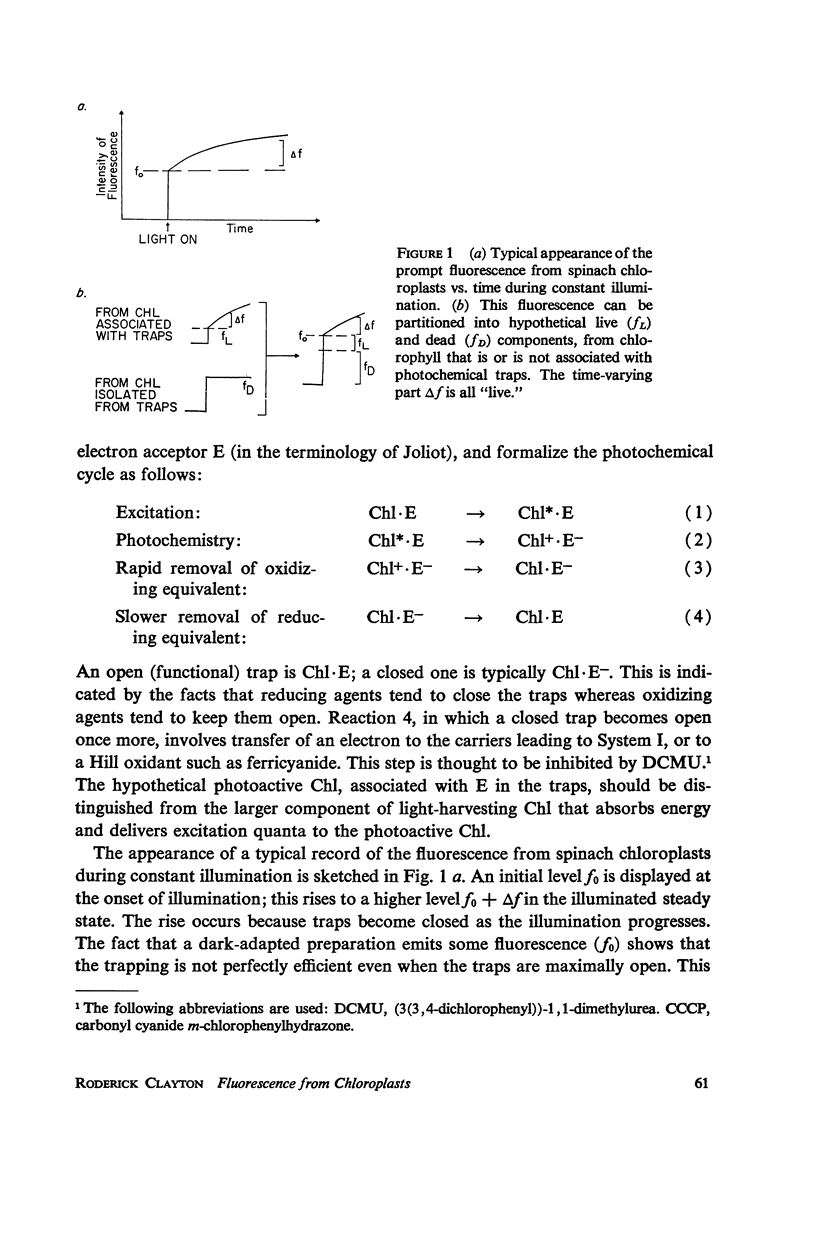
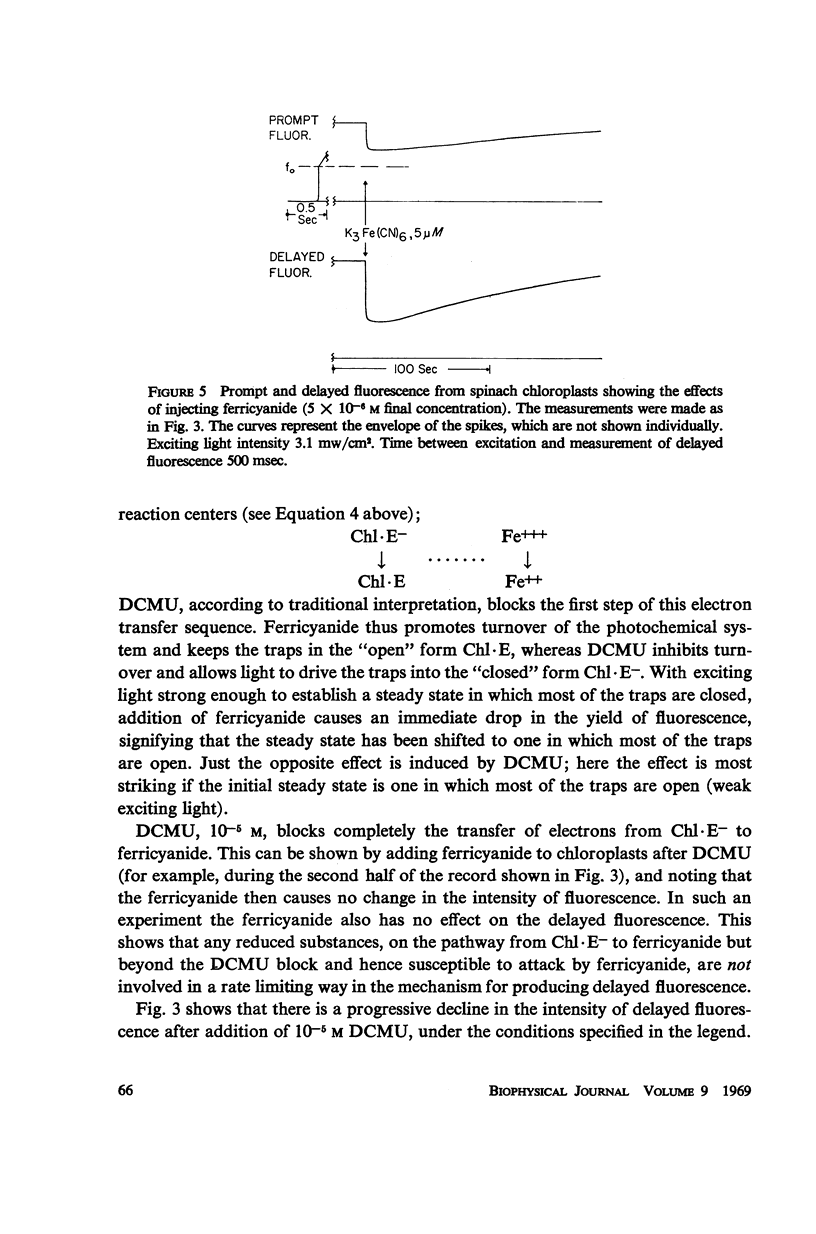
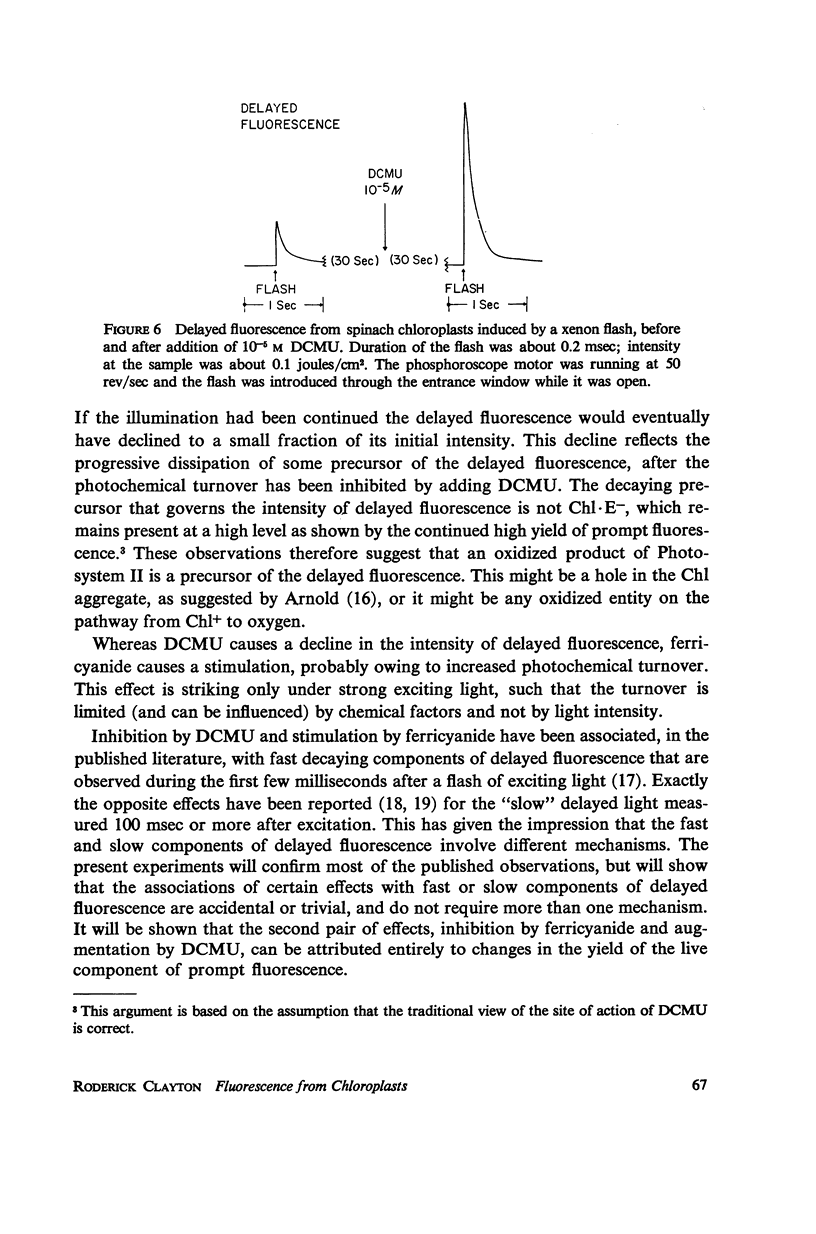
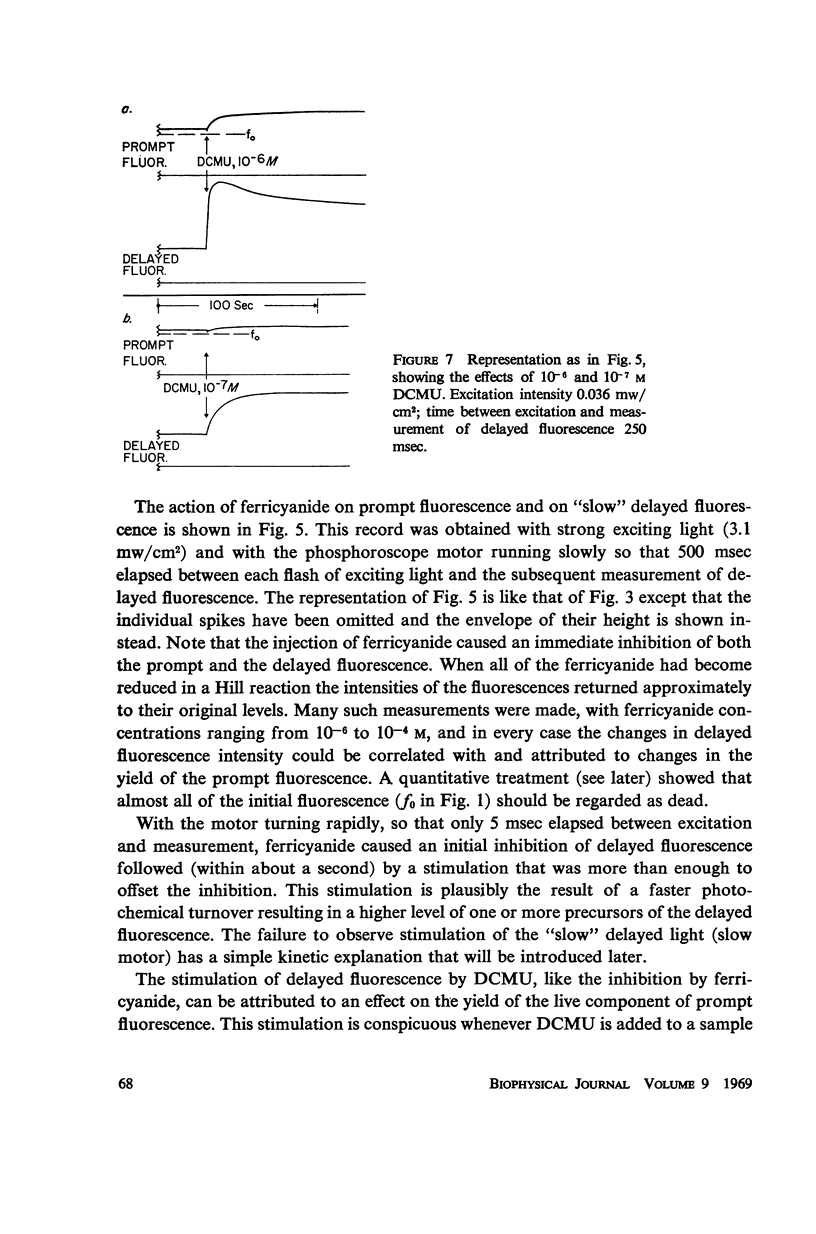
Effects of ferricyanide, dichlorophenyldimethylurea (DCMU), and uncouplers of phosphorylation on the prompt and delayed fluorescences from spinach chloroplasts are described. Any factor that affects the yield of prompt fluorescence will similarly influence the intensity of delayed fluorescence. This idea, recently investigated by Lavorel, should be expressed in terms of a “live” component of fluorescence; that is, the component from chlorophyll associated with the photochemical traps of System II. Some of the effects of ferricyanide and DCMU on delayed fluorescence can then be explained in terms of effects on the yield of prompt fluorescence. From the internal consistency of the explanation, applied to various observations, a judgment can be made that most of the prompt fluorescence observed initially when dark-adapted chloroplasts are first illuminated is “dead,” coming from chlorophyll not associated with trap II. The live fluorescence is represented almost entirely by the time-varying component that develops during illumination. The observed intensity of delayed fluorescence can be divided by the yield of live prompt fluorescence to give an intrinsic delayed fluorescence. This intrinsic delayed fluorescence is proportional to the square root of exciting light intensity (as long as the excitation is not saturating) and decays with second order kinetics. This behavior may reflect the photochemical formation and second order dissipation of an oxidized product of Photosystem II.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNOLD W. AN ELECTRON-HOLE PICTURE OF PHOTOSYNTHESIS. J Phys Chem. 1965 Mar;69:788–791. doi: 10.1021/j100887a013. [DOI] [PubMed] [Google Scholar]
- ARNOLD W., THOMPSON J. Delayed light production by blue-green algae, red algae, and purple bacteria. J Gen Physiol. 1956 Jan 20;39(3):311–318. doi: 10.1085/jgp.39.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch W., Azzi J. R., Davidson J. B. Delayed light studies on photosynthetic energy conversion. I. Identification of the oxygen-evolving photoreaction as the delayed light emitter in mutants of Scenedesmus obliquus. Biochim Biophys Acta. 1967 Jul 5;143(1):129–143. doi: 10.1016/0005-2728(67)90116-8. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. CHARACTERISTICS OF FLUORESCENCE AND DELAYED LIGHT EMISSION FROM GREEN PHOTOSYNTHETIC BACTERIA AND ALGAE. J Gen Physiol. 1965 Mar;48:633–646. doi: 10.1085/jgp.48.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton R. K. An analysis of the relations between fluorescence and photochemistry during photosynthesis. J Theor Biol. 1967 Feb;14(2):173–186. doi: 10.1016/0022-5193(67)90112-9. [DOI] [PubMed] [Google Scholar]
- Joliot P. Cinétiques des réactions liées a l'émission d'oxygène photosynthétique. Biochim Biophys Acta. 1965 May 25;102(1):116–134. [PubMed] [Google Scholar]
- Joliot P. Etudes simultanées des cinétiques de fluorescence et d'émission d'oxygène photosynthétique. Biochim Biophys Acta. 1965 May 25;102(1):135–148. [PubMed] [Google Scholar]
- Kok B., Malkin S., Owens O., Forbush B. Observations on the reducing side of the O2-evolving photoact. Brookhaven Symp Biol. 1966;19:446–459. [PubMed] [Google Scholar]
- Lavorel J. Sur une relation entre fluorescence et luminescence dans les systeèmes photosynthétiques. Biochim Biophys Acta. 1968 Apr 2;153(3):727–730. doi: 10.1016/0005-2728(68)90203-x. [DOI] [PubMed] [Google Scholar]
- Müller A., Lumry R. The relation between prompt and delayed emission in photosynthesis. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1479–1485. doi: 10.1073/pnas.54.6.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STREHLER B. L., ARNOLD W. Light production by green plants. J Gen Physiol. 1951 Jul;34(6):809–820. doi: 10.1085/jgp.34.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VREDENBERG W. J., DUYSENS L. N. Transfer of energy from bacteriochlorophyll to a reaction centre during bacterial photosynthesis. Nature. 1963 Jan 26;197:355–357. doi: 10.1038/197355a0. [DOI] [PubMed] [Google Scholar]


