Abstract
Productive entry of human immunodeficiency virus type 1 (HIV-1) into a host cell is believed to proceed via fusion of the viral envelope with the host cell's plasma membrane. Interestingly, the majority of HIV-1 particles that bind to the cell surface are taken up by the host cell via endocytosis; however, this mode of internalization generally does not result in infection. Presumably, virus particles remain trapped in the endocytic pathway and are eventually degraded. Here, we demonstrate that treatment of cells with various pharmacological agents known to elevate the pH of endosomes and lysosomes allows HIV-1 to efficiently enter and infect the host cell. Pretreatment of cells with bafilomycin A1 results in up to a 50-fold increase in the infectivity of HIV-1SF2. Similarly, pretreatment of target cells with amantadine, concanamycin A, concanamycin B, chloroquine, and ammonium chloride resulted in increases in HIV-1 infectivity ranging between 2- and 15-fold. Analysis of receptor and coreceptor expression, HIV-long terminal repeat (LTR) transactivation, and transduction with amphotropic-pseudotyped murine leukemia virus (MLV)-based vectors suggests that the increase in infectivity is not artifactual. The increased infectivity under these conditions appears to be due to the ability of HIV-1 and MLV particles to enter via the endocytic pathway when spared from degradation in the late endosomes and lysosomes. These results could have significant implications for the administration of current and future lysosmotropic agents to patients with HIV disease.
The process of viral entry involves the transport of the viral genome across host cell membranes and subsequent release of the genome into the host cell cytoplasm. Enveloped viruses accomplish the delivery of their genomes into the cytoplasm of the host cells by fusing their envelopes with host cell membranes (17). This fusion event occurs either at the plasma membrane or with endosomal membranes and is mediated by virally encoded fusion proteins incorporated into the viral envelope. Fusion at the plasma membrane occurs via a pH-independent mechanism and requires only binding of the virus to its receptor for fusion to occur. Conversely, viruses that enter the cell via the endocytic pathway require the acidification of these vesicles to trigger the fusogenic activity of their viral fusion proteins (23, 46). Historically, viruses that enter cells by the pH-dependent pathway have been identified by their sensitivity to inhibitors of endosomal/lysosomal acidification. Inhibitors of endosomal acidification fall into three groups based on their modes of action. The first class of agents is made up of the lysosomotropic weak bases, such as chloroquine, amantadine, and ammonium chloride, which diffuse across membranes in a concentration-dependent manner. These lysophilic weak bases rapidly become protonated, thereby neutralizing the acidic environment of endocytic vesicles (5). The second class of agents known to alter endosomal pH is made up of the carboxylic ionophores, such as monensin (35). These compounds exchange protons for potassium and sodium. More recently, inhibitors of vacuolar H+-ATPases (e.g., bafilomycin A1 [BFLA-1] and concanamycin A) have been used to assess the route of viral entry (8, 9, 41). Two well-characterized examples of viruses that enter via pH-dependent pathway are vesicular stomatitis virus (VSV) and influenza virus. In the case of influenza virus, inhibitors of lysosomal acidification have been shown to be effective as prophylactics and in shortening the course of disease (12, 28).
In the case of human immunodeficiency virus (HIV), electron microscopy has been used to document viral fusion with the plasma membrane. It is generally accepted that this is the main route of entry used by HIV-1 to establish a productive infection. In this scenario, virus cores are deposited into the cytoplasm of the cell at the plasma membrane and subsequently migrate, or are transported, to the nucleus. Early studies also indicated that virus particles are found in vesicles resembling endosomes. Recently, several groups have provided evidence that HIV-1 is taken up by the cell via endocytosis (33, 44). However, this route of internalization does not generally result in release of viral nucleocapsids into the cytosol of the host cell and therefore does not result in a productive infection. To further examine the fitness of the wild-type HIV-1 particles taken up via endocytosis, we evaluated the effects of five pharmacological agents capable of inhibiting the acidification of endosomes or lysosomes on the infectivity of three different HIV-1 isolates. Our results show that pretreatment of target cells with these agents results in dramatic increases in infectivity, suggesting that administration of lysosomotropic agents to HIV-infected patients has the potential to exacerbate their condition.
MATERIALS AND METHODS
Cells, constructs, and viruses.
Human 293T cells and HeLa Magi cells (kindly provided by M. Emerman) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 100 IU of penicillin, 100 μg of streptomycin per ml, 0.2 mg of glutamine per ml, and 10% fetal bovine serum (FBS). In addition, HeLa Magi cells were supplemented with G418 (0.15 mg/ml) and hygromycin B (0.1 mg/ml) as previously described (24). Cell lines were maintained at 37°C in a humidified incubator at 5% CO2. The HIV-1 isolates SF2 (kindly provided by P. Luciw), NL4-3, and LAI (kindly provided by M. Emerman) have been described previously (40). Virus supernatants were prepared by transient transfection of 293T cells, and p24 content was determined by enzyme-linked immunosorbent assay (ELISA) with a kit purchased from Coulter. Amphotropic pseudotyped viruses expressing HIV-1 Tat have been described previously (13, 15).
Infectivity assay.
HeLa Magi cells were used for the infectivity assays essentially as previously described (30), except that cells were preincubated with the indicated drugs for 2 to 3 h prior to infection with the indicated virus. These cells express both CD4 and CXCR4 and contain an integrated β-galactosidase gene under the control of the Tat-responsive HIV-1 long terminal repeat (LTR). The culture medium of cells incubated with amantadine and chloroquine was adjusted to pHs of 7.8 and 7.4, respectively, to allow maximal uptake of these compounds (23). Virus was added with DEAE-dextran (2 μM) to cells in the presence of drugs and incubated for 16 to 18 h at 37°C. Cells were washed twice with phosphate-buffered saline (PBS) and then incubated for an additional 24 h in normal culture medium. Cells were then fixed and stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). Blue cells were counted as an indicator of infected cells as previously described (24). Individual samples were evaluated in either duplicate or triplicate in at least two or three separate and independent experiments. The following compounds were used at the indicated final concentrations: BFLA-1, 1 to 100 nM; ammonium chloride, 1 to 10 mM; chloroquine, 1 to 0.001 mM; amantadine, 0.1 to 1.5 mM; concanamycin A, 20 nM (all from Sigma); and concanamycin B, 20 nM (kindly provided by H. Plough). For the experiments presented in Fig. 5, residual plasmids after transfection were removed from viral supernatants by treatment with DNase I for 30 min at 37°C.
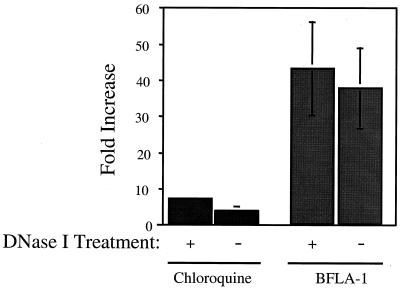
FIG. 5.
Increased infectivity observed with BFLA-1 is not affected by DNase I treatment of virus stocks. The infectivity of virus stocks pretreated with DNase I to remove contaminating DNA was determined essentially as indicated in Fig. 1 and 2. Control samples (untreated cells) were arbitrarily set to 1 to determine the fold increase in infectivity in the presence of either chloroquine (50 μM) or BFLA-1 (0.1 μM). The results represent the average of two independent experiments.
Flow cytometry analysis.
HeLa Magi cells were incubated with the indicated drug for the time specified. Following this incubation, cells were removed from plates with Versene (Gibco) and stained with phycoerythrin (PE)-conjugated monoclonal antibodies against either CD4 (Ex-alpha), CXCR4 (PharMingen), or an isotype-matched control (Ex-alpha). Samples were analyzed on a Becton Dickinson FACScan instrument equipped with LYSYS II software. All fluorescence data were collected in log mode and analyzed with CellQuest software (Becton Dickinson).
RESULTS
Inhibition of acidification of endosomes dramatically increases the infectivity of HIV-1SF2.
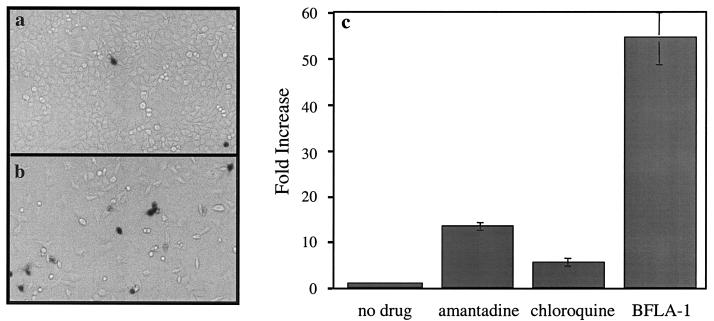
It has been shown that a significant portion of the HIV-1 viral particles that bind to the cell surface are internalized by the host cells; however, under normal infection conditions, these particles do not result in a productive infection (11, 33, 44). One possible explanation for this observation is that the majority of virus particles are defective at some stage of the entry process and therefore unable to successfully escape from endosomal/lysosomal vesicles. Alternatively, these viral particles are infectious, but the harsh environment of the endosome inhibits entry, and instead the particles are subjected to endosomal/lysosomal degradation (33). In order to examine the fitness of endocytosed HIV particles, we examined the effect of pharmacological agents capable of elevating endosomal pH on the ability of HIV-1SF2 to infect HeLa Magi indicator cells. These are CD4+ and CXCR4+ cells that contain an integrated β-galactosidase gene under the control of the HIV-1 LTR that responds to Tat expression. We chose this indicator cell line because it relies on a single round of replication and because it allows direct comparison with previous studies. Using this cell line, we have previously evaluated the effect of BFLA-1 on VSV-G (HIV-1) pseudotypes and found BFLA-1 completely inhibited infection of target cells by these pseudotyped viruses (30). These results indicated that BFLA-1 efficiently blocks entry of virus particles that rely on exposure to the acidic environment of the endosomes to trigger entry. HeLa Magi cells were incubated with BFLA-1 prior to infection with HIV-1SF2. At 36 to 40 h postinfection, the number of individual cells infected was determined by staining for β-galactosidase expression with X-Gal as a substrate. We found that pretreatment of cultures with BFLA-1 dramatically increased (up to 50-fold) the number of cells infected by HIV-1SF2 (Fig. 1a and b). In order to determine whether this increase in infectivity was a nonspecific side effect of BFLA-1 or was due to the increase in endosomal pH, we evaluated the effects of two other agents known to increase endosomal pH: amantadine and chloroquine. We found that amantadine and chloroquine also increased the infectivity of HIV-1SF2 by 15- and 6-fold, respectively (Fig. 1c). In addition, we examined the effects of concanamycin A and ammonium chloride on the infectivity of HIV-1SF2. We found that these drugs produced 5- and 2.5-fold increases in infectivity, respectively (data not shown). In summary, all agents tested resulted in an increase in the infectivity of HIV-1SF2, confirming that the enhancement in infectivity observed is not due to a side effect of a particular drug, but rather to the ability of the individual drug used to inhibit acidification and/or transport of the endosomal or lysosomal vesicles. Nonetheless, the magnitude of the enhancement effect was clearly dependent on the drug used. These data indicate that HIV-1SF2 does not require exposure to an acidic environment in order to infect the host cells, and in fact, neutralization of the endosome's acidic environment greatly enhances the infectivity of HIV-1SF2.
FIG. 1.
Inhibitors of endosome/lysosome acidification increase the infectivity of HIV-1SF2. The effects of amantadine, chloroquine, and BFLA-1 in the infectivity of the HIV-1SF2 were evaluated with HeLa Magi indicator cells. Cells were preincubated with the specific drug for 2 to 3 h prior to addition of virus. Individual infected cells were identified by staining with X-Gal and counted by visual inspection. (a and b) Comparison of the infectivity of HIV-1SF2 in control (a [untreated]) and BFLA-1-treated (b) cells. (c) Quantification of the increase in the infectivity of HIV-1SF2 (over untreated controls) by amantadine (1 mM), chloroquine (50 μM), and BFLA-1 (0.1 μM). Triplicate determinations were made in parallel. Note that in order to facilitate the manual counting of the individual infected cells, the amount of virus added to the treated cells was two- to fivefold less than that added to the control samples.
Examination of the effect of BFLA-1 on other HIV-1 strains.
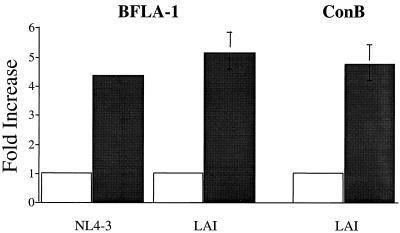
To determine if the increase in infectivity observed with HIV-1SF2 in response to BFLA-1 pretreatment reflects a unique property of this isolate, we evaluated the effect of BFLA-1 on two additional HIV-1 isolates. We chose HIV-1NL4-3 and HIV-1LAI because they represent two well-characterized, fully functional isolates, allowing direct comparison of our results with those previously published from other laboratories (11, 44). The relative infectivity of HIVNL4-3 was increased fourfold when target cells were pretreated with BFLA-1 (Fig. 2). Similarly, the infectivity of HIV-1LAI was increased fivefold in the presence of BFLA-1 (Fig. 2). We confirmed the results obtained with HIV-1LAI by testing the effect of concanamycin B on its infectivity. Concanamycin B increased the infectivity of HIV-1LAI fourfold (Fig. 2). These results demonstrate that the observed increase in the infectivity of HIV-1, which was caused by agents that elevate endosomal pH, is not limited to one particular strain of HIV.
FIG. 2.
Increase in infectivity of HIV-1NL4-3 and HIV-1LAI in the presence of inhibitors of endosomal/lysosomal acidification. Infectivity of HIV-1NL4-3 and HIV-1LAI in control (untreated) cells and cells pretreated with either BFLA-1 (0.1 μM) or concanamycin B (20 μM) for 2 h. Samples were analyzed essentially as indicated in the legend to Fig. 1. To facilitate comparisons, the infectivity in untreated samples was set to 1.
BFLA-1 increases the infectivity of amphotropic pseudotyped MLV.
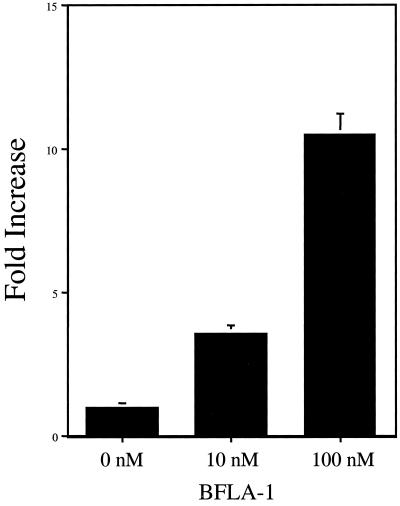
Our previous results prompted us to begin to examine the effects of these agents on the infectivity of other retroviruses. We began by examining a well-characterized type of murine leukemia virus (MLV)-based vector previously used in HIV research. This particular vector expresses the Tat protein and therefore scores in the same way as a wild-type HIV-1 in the HeLa Magi cell assay. As shown in Fig. 3, pretreatment of target cells with BFLA-1 resulted in a concentration-dependent increase in infectivity (up to 11-fold). These results suggest that the infectivity enhancement effect observed when target cells are pretreated with lysosomotropic agents is not exclusive to HIV-1 and that this aspect of the infection process is shared between these two viruses.
FIG. 3.
Pretreatment of target cells with BFLA-1 increases the infectivity of amphotropic pseudotyped MLV. HeLa Magi cells were infected with a Tat-expressing MLV-based vector (LtatSN) pseudotyped with an amphotropic envelope. Target cells were pretreated with two different concentrations of BFLA-1 as indicated. Results from a representative experiment show the average of determinations made in triplicate. Error bars show deviations from the mean.
Amantadine and chloroquine increase the infectivity of HIV-1 in a dose-dependent manner.
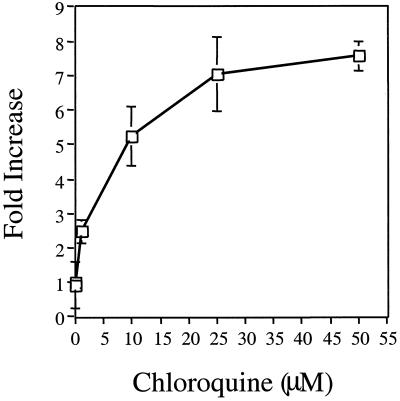
Both amantadine and chloroquine have several clinical applications that overlap with the clinical management of HIV infection (3, 6, 12, 20, 27, 28, 31, 34, 36). Since administration of these drugs to HIV-infected patients could have negative consequences, we evaluated the dose dependence of the increased infectivity observed with each of them. As indicated in Fig. 4, the increase in infectivity is dependent on the dose of drug used. In the case of chloroquine, the maximal increase in infectivity was obtained when a concentration of 50 μM was used, but doses as low as 1 μM show a reproducible increase in HIV-1 infectivity. Amantadine had no effect on infectivity at concentrations lower than 0.25 mM, but also showed a concentration-dependent increase in infectivity over a smaller range of concentrations (0.1 to 1 mM) (data not shown).
FIG. 4.
Dose dependence of the increase in HIV-1 infectivity mediated by chloroquine. Infectivity of HIV-1SF2 in the presence of increasing concentrations of chloroquine (0 to 50 μM). Each point represents the average of three independent determinations. For purposes of comparison, the infectivity of the virus in the absence of chloroquine was arbitrarily designated as 1.
Increase in infectivity is not due to contaminating plasmid DNA.
All viral stocks used in the previous experiments were generated by transfecting 293T cells with plasmids encoding replication-competent HIV proviruses. This raised the possibility that the observed increased infectivity is simply an artifact caused by plasmid contamination of the viral stocks. We treated viral stocks with DNase I to remove contaminating plasmid DNA to examine this possibility. DNase I-treated virus was then added to cells incubated in the presence or absence of either BFLA-1 or chloroquine. DNase I treatment did not diminish the increase in infectivity observed when cells were preincubated with either BFLA-1 or chloroquine (Fig. 5). The increase in infectivity observed for DNase I-treated HIV-1SF2 was similar to that of observed in cultures infected with untreated virus in the presence of either chloroquine or BFLA-1. In order to confirm this result, we also examined the infectivity of HIV-1SF2 in the presence of both BFLA-1 and zidovudine (AZT). AZT blocks the activity of reverse transcriptase and prevents virus particles from productively infecting target cells. However, AZT will not block expression of Tat from proviral DNA. The presence of AZT blocked infection of target cells, regardless of whether they were pretreated with BFLA-1 (data not shown). These data suggest that the observed increase in infectivity is due to intact virus particles and not to contaminating plasmid DNA.
The level of HIV-1 LTR expression is not increased in the presence of inhibitors of lysosomal acidification.
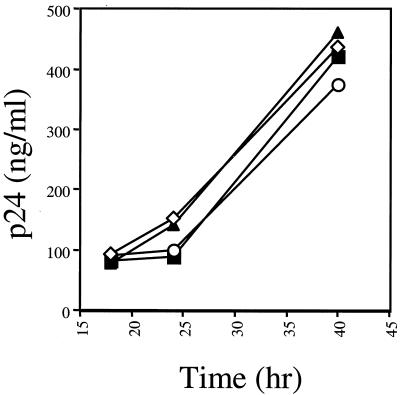
An alternative explanation of our observations is that the agent used increased the expression of the HIV-1 LTR (24). To address this question, we transfected 293T cells with plasmids encoding the HIV-1SF2 provirus, and at 18 h posttransfection, the medium was replaced with medium containing amantadine, chloroquine, or BFLA-1. The amount of virus produced at various times posttransfection was then determined by p24 ELISA. If amantadine, chloroquine, or BFLA-1 affects expression from the HIV-1 LTR either positively or negatively, then the level of viral protein production from transfected cells incubated in the presence of these drugs would be altered compared to that of control cells. At 18 h posttransfection and prior to the addition of the different drugs, all samples contained similar levels of p24, suggesting that the transfection efficiencies were similar in all samples (Fig. 6). In the presence of either amantadine or BFLA-1, there was a slight reduction in p24 levels 6 and 18 h after addition of the drugs. Chloroquine treatment had no effect on the levels of p24 produced at any of the time points evaluated. These data suggest that these drugs do not enhance proviral expression.
FIG. 6.
Lysosomotropic agents do not alter HIV expression. Virus production from transfected 293T cells was evaluated in the presence of amantadine (1 mM [▪]), chloroquine (50 μM [◊]), or BFLA-1 (0.1 μM [○]). Cells were maintained in culture in the presence of each drug (added 18 h posttransfection) for the duration of the time course. Virus production was determined as a function of p24 in the culture supernatant. Individual points represent the average of three determinations. ▴, control, no drug.
Effect of lysosomal inhibitors on surface expression of CD4 and CXCR4.
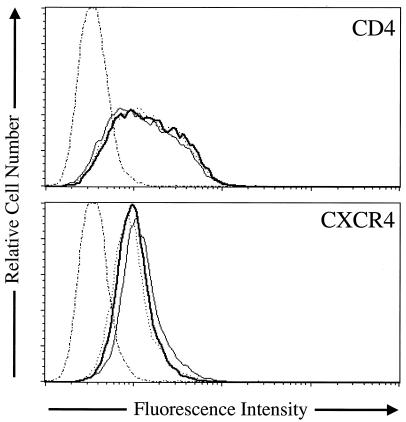
Previous studies have demonstrated that changes in the level of surface expression of CD4 significantly alter the ability of HIV to enter host cells (7, 26, 42). This raises the possibility that the increase in infectivity observed when cells are pretreated with inhibitors of lysosomal acidification is due to an increase in CD4 surface expression. We therefore examined the effects of concanamycin A, amantadine, chloroquine, and BFLA-1 on CD4 surface expression. CD4 surface expression was not altered by the presence of these drugs (Fig. 7) (data not shown), indicating that the increase in infectivity was not simply due to an increase in CD4 cell surface expression. We also examined the level of CXCR4 surface expression in the presence of concanamycin A, amantadine, chloroquine, and BFLA-1. The presence of these drugs did not increase the surface expression of CXCR4, but rather slightly decreased the cell surface expression of CXCR4 (Fig. 7) (data not shown). These results demonstrate that the increase infectivity is not due to an increase of receptor or coreceptor cell surface expression.
FIG. 7.
Effect of lysosomotropic agents on CD4 and CXCR4 surface levels. The effect of concanamycin A (dotted line) and BFLA-1 (dark solid line) on CD4 (top) and CXCR4 (bottom) expression was determined by flow cytometry with PE-labeled anti-CD4 or PE-labeled anti-CXCR4. Cells were incubated in the presence of the respective inhibitor for 3 h at 37°C, dislodged from the tissue culture plate with Versene, and stained at 4°C. For controls, cells treated with drugs were stained with an isotype-matched nonspecific immunoglobulin G1 PE-labeled antibody (thin dotted and dashed line), or cells were not treated with drugs, but stained with anti-CD4 or anti-CXCR4-PE labeled antibodies to determine the normal levels of surface expression (thin solid line).
DISCUSSION
The host cell's plasma membrane represents a considerable barrier that all viruses must cross in order to establish a productive infection. In the case of enveloped viruses, they gain entrance into the host cell by fusing with host cell membranes. This fusion event occurs either at the plasma membrane via a pH-independent fusion process or with endocytic vesicles through a fusion event triggered by the exposure of the virion to low pH (2, 17, 23, 46). Lysosomotropic agents, such as chloroquine, have traditionally been used as reagents for distinguishing the route of entry of a virus. Such agents are nonspecific weak bases that diffuse across membranes in a concentration-dependent manner and thereby neutralize the pH of endocytic vesicles (16, 43). Viruses unaffected by lysosomotropic agents are said to enter by fusing in a pH-independent manner with the plasma membrane, and viruses whose infectivity was decreased or blocked by these agents are said to enter the host cell by fusing with endocytic vesicles in a pH-dependent manner (25, 38). However, these agents are nonspecific and have other side effects on the cell. The isolation of reagents such as BFLA-1, concanamycin B, and concanamycin A, which specifically block vascular ATPases, has allowed a more conclusive determination of the role played by endosomal pH in the process of viral entry (14).
In this article, we demonstrate that HIV-1 infection can be modulated by treating cells with agents capable of elevating endosomal pH. We show that BFLA-1 pretreatment can dramatically increase the overall infectivity of HIV-1 isolates such as HIV-1SF2. We focused our investigation on BFLA-1 because previous reports have demonstrated the effectiveness of this reagent in blocking pH-dependent viral entry (41). In order to confirm that the infectivity enhancement noted for HIV-1SF2 was not a nonspecific side effect of BFLA-1, we also examined the effect of chloroquine, amantadine, concanamycin A, and ammonium chloride, all of which are drugs known to increase endosomal pH. In all cases, we observed an increase in infectivity in the presence of these drugs. One important conclusion of our results is that HIV-1SF2 does not require an acid environment in order to effectively enter the target cell; rather, our data suggest that the normal acidic environment of the endosome is actually detrimental to entry by this virus. These results are in contrast with observations made by Fackler and Peterlin (11) with ammonium chloride and concanamycin A. They also observed a reproducible increase in the infectivity of HIV-1NL4-3 in the presence of either of these drugs. However, they detected a decrease in infectivity by HIV-1SF2. It is possible that the discrepancies between these two studies are due to differences in the experimental approaches employed. Fackler and Peterlin initiated their infections at the same time the lysosomotropic agents were added (11). In our case, we incubated our target cells with the lysosomotropic agents prior to addition of the virus stocks. By pretreating the cells with the inhibitors, we ensured maximal inhibition of lysosomal acidification prior to virus infection and therefore minimized the likelihood of lysosomal degradation of infectious viruses. Alternatively, these differences could be due to differences in the amino acid sequences of the envelope proteins of the HIV-1 strains used. Single-amino-acid changes in the fusion protein of influenza virus (HA) been shown to alter the pH at which these proteins undergo the conformational changes necessary to mediate fusion (4, 7). However, this is unlikely the case, since for both studies, provirus clones were used to generate virus stocks.
Although the most likely explanation for our results is that inhibition of lysosomal acidification prevents the degradation of infectious particles, other possibilities were also evaluated. First, we confirmed that the increase in infection observed in the presence of these drugs was not an artifact caused by the presence of contaminating proviral DNA in our virus stocks. Second, we determined that elevation of endosomal pH did not increase p24 expression from the provirus, suggesting that the effect of these drugs is not due to an increase in the level of transcription from the proviral LTR. Finally, we determined that the observed increase in infectivity is not due to increased surface expression of either CD4 and/or CXCR4. These results further support our conclusion that it is the inhibition of endosomal or lysosomal acidification that is responsible for the increase in infectivity observed.
To determine if elevated endosomal pH affects the infectivity of other retroviruses, we evaluated the effect of BFLA-1 on an amphotropic pseudotyped MLV. Our results indicate that the infectivity of amphotropic MLV is increased approximately sevenfold in the presence of BFLA-1. This increase is similar in magnitude to those observed with both HIV-1NL4-3 and HIV-1LAI. That the infectivity of amphotropic MLV can be increased if target cells are pretreated with BFLA-1 suggests that this may be a general mode of entry for retroviruses. More importantly, it suggests that inhibitors of lysosomal and endosomal acidification might serve to enhance the infectivity of retrovirus vectors commonly used for gene therapy and gene transfer experiments.
It has been previously established that the majority of HIV particles that bind to the cell surface are endocytosed into the host cell (33, 44). Under standard infection conditions, these particles do not result in a productive infection (11, 33, 44). In all probability, these particles fail to fuse with endosomal membranes and are simply degraded in the lysosome (11, 44). Therefore, the increase in infectivity observed with these drugs is due in part to the fact that they inhibit activation of degradative enzymes in the endocytic pathway. However, the effects of these agents on vesicular transport may also contribute to the observed alteration in infectivity (5). Blocking or slowing degradation may allow sufficient time for some of the endocytosed virus particles to fuse with cellular membranes and deposit their viral genomes into the cytoplasm of the host cell. In the case of HIV-1SF2, the increase in infectivity caused by drugs such as BFLA-1 is significant, resulting in infectivity beyond that of more infectious clones, such as HIV-1NL4-3 or HIV-1LAI. Our results suggest that the lower infectivity observed with HIV-1SF2 may be due to an inability of this virus to fuse with the cell membrane and enter the host cells in an appropriate time frame. Therefore, under normal culture conditions, these particles are endocytosed and ultimately degraded. Our results are in agreement with the conclusion reached by Fackler and Peterlin that endocytosis of HIV-1 can result in productive infection provided the proper conditions are present (11). We also agree with their assertion that endocytic entry may potentially result in an expanded cellular range for HIV-1 and that, by this mechanism, HIV might create additional latent reservoirs that could complicate the eradication of the virus.
An important point to address is why the uptake of virions increases infectivity when the acidification of the endosomes is inhibited. HIV interacts with its coreceptors in lipid rafts, where these molecules form clusters (32, 45). These structures are intimately involved in the endocytic process. Thus, in lipid rafts, HIV could be endocytosed before it can fuse with the plasma membrane. Consistent with these tenets is the fact that the majority of HIV-1 virions that enter the cell do so mainly via endocytosis (33). Once in the endosomes, HIV would be normally degraded, but in the neutralized endosomes, the coreceptor-rich environment could allow for a more efficient rate of fusion with the overall result of significantly higher levels of infection. One point that should be made clear is that regardless of how HIV enters the cell, infection is dependent on membrane fusion events that take place at the plasma membrane or in the cytoplasm.
Chloroquine is one of the drugs commonly used to treat malaria in parts of sub-Saharan Africa, where HIV infection is endemic (3, 27, 31, 47). Our initial observation that chloroquine increased the infectivity of HIV suggested that administration of chloroquine to HIV-infected patients could potentially exacerbate the course of HIV infection. However, studies describing the administration of chloroquine to HIV-infected patients have not reported complications regarding the HIV status of patients under these regiments (21, 39). A more thorough examination of the effects of chloroquine on the infectivity of HIV demonstrated that the concentration of chloroquine necessary to detect an increase in infectivity in our in vitro assay is above that reported in the blood of patients under treatment (∼1 μM) (10, 47). However, it should be noted that chloroquine and hydroxychloroquine overdoses (although not common) do occur with a certain frequency and can result in significantly higher levels of drug (∼30 μM) in the blood of these patients (19, 22). Amantadine has been used as a therapy for influenza virus A infection (18, 34). However, its use has diminished due to the fact that resistant strains emerge both in the laboratory and in patients. In addition, amantadine has been used extensively to treat a variety of neurological disorders, including Parkinson's disease (29, 37). Experience with amantadine in the context of HIV infection has not been extensively documented, but the levels of drug in the plasma of patients under treatment (300 ng/ml) do not reach those needed to affect the infectivity of HIV as described herein (1). Based on these observations, there is no apparent reason to suggest discontinuation of the use of these currently available drugs in the setting of HIV infection. However, this might not be the case with future, more effective inhibitors. Our observations emphasize the fact that clinically useful drugs that alter cellular processes can greatly modulate the infectivity of HIV. In addition, these drugs might have applications for increasing the infectivity of retrovirus-based gene transfer vectors.
Acknowledgments
We thank A. Varley and Y. Lin for critical reading of the manuscript; H. Ploegh and M. Emerman for the generous gift of concanamycin B and HeLa-Magi cells, respectively; and R. Munford, R. Gaynor, and D. Foster for continued support of this work.
This work was supported by National Institutes of Health grant AI-33331 (J.V.G.).
REFERENCES
- 1.Aoki, F. Y., and D. S. Sitar. 1988. Clinical pharmacokinetics of amantadine hydrochloride. Clin. Pharmacokinet. 14:35-51. [DOI] [PubMed] [Google Scholar]
- 2.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 3.Bloland, P. B., M. Ettling, and S. Meek. 2000. Combination therapy for malaria in Africa: hype or hope? Bull. W. H. O. 78:1378-1388. [PMC free article] [PubMed] [Google Scholar]
- 4.Daniels, R. S., J. C. Downie, A. J. Hay, M. Knossow, J. J. Skehel, M. L. Wang, and D. C. Wiley. 1985. Fusion mutants of the influenza virus hemagglutinin glycoprotein. Cell 40:431-439. [DOI] [PubMed] [Google Scholar]
- 5.de Duve, C., T. de Barsy, B. Poole, A. Trouet, P. Tulkens, and F. Van Hoof. 1974. Lysosomotropic agents. Biochem. Pharmacol. 23:2495-2531. [DOI] [PubMed] [Google Scholar]
- 6.Demicheli, V., T. Jefferson, D. Rivetti, and J. Deeks. 2000. Prevention and early treatment of influenza in healthy adults. Vaccine 18:957-1030. [DOI] [PubMed] [Google Scholar]
- 7.Doms, R. W. 2000. Beyond receptor expression: the influence of receptor conformation, density, and affinity in HIV-1 infection. Virology 276:229-237. [DOI] [PubMed] [Google Scholar]
- 8.Drose, S., and K. Altendorf. 1997. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J. Exp. Biol. 200:1-8. [DOI] [PubMed] [Google Scholar]
- 9.Drose, S., K. U. Bindseil, E. J. Bowman, A. Siebers, A. Zeeck, and K. Altendorf. 1993. Inhibitory effect of modified bafilomycins and concanamycins on P- and V-type adenosinetriphosphatases. Biochemistry 32:3902-3906. [DOI] [PubMed] [Google Scholar]
- 10.Ducharme, J., and R. Farinotti. 1996. Clinical pharmacokinetics and metabolism of chloroquine. Focus on recent advancements. Clin. Pharmacokinet. 31:257-274. [DOI] [PubMed] [Google Scholar]
- 11.Fackler, O. T., and B. M. Peterlin. 2000. Endocytic entry of HIV-1. Curr. Biol. 10:1005-1008. [DOI] [PubMed] [Google Scholar]
- 12.Fleming, D. M. 2001. Managing influenza: amantadine, rimantadine and beyond. Int. J. Clin. Pract. 55:189-195. [PubMed] [Google Scholar]
- 13.Frazier, A. L., and J. V. Garcia. 1994. Retrovirus-mediated transfer and long-term expression of HIV type 1 tat gene in murine hematopoietic tissues. AIDS Res. Hum. Retrovir. 10:1517-1519. [DOI] [PubMed] [Google Scholar]
- 14.Gagliardi, S., M. Rees, and C. Farina. 1999. Chemistry and structure activity relationships of bafilomycin A1, a potent and selective inhibitor of the vacuolar H+-ATPase. Curr. Med. Chem. 6:1197-1212. [PubMed] [Google Scholar]
- 15.Garcia, J. V., and A. D. Miller. 1994. Retrovirus vector-mediated transfer of functional HIV-1 regulatory genes. AIDS Res. Hum. Retrovir. 10:47-52. [DOI] [PubMed] [Google Scholar]
- 16.Grabe, M., and G. Oster. 2001. Regulation of organelle acidity. J. Gen. Physiol. 117:329-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez, L. D., L. R. Hoffman, T. G. Wolfsberg, and J. M. White. 1996. Virus-cell and cell-cell fusion. Annu. Rev. Cell Dev. Biol. 12:627-661. [DOI] [PubMed] [Google Scholar]
- 18.Iwahashi, J., K. Tsuji, T. Ishibashi, J. Kajiwara, Y. Imamura, R. Mori, K. Hara, T. Kashiwagi, Y. Ohtsu, N. Hamada, H. Maeda, M. Toyoda, and T. Toyoda. 2001. Isolation of amantadine-resistant influenza A viruses (H3N2) from patients following administration of amantadine in Japan. J. Clin. Microbiol. 39:1652-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan, P., J. G. Brookes, G. Nikolic, and D. G. Le Couteur. 1999. Hydroxychloroquine overdose: toxicokinetics and management. J. Toxicol. Clin. Toxicol. 37:861-864. [DOI] [PubMed] [Google Scholar]
- 20.Kain, K. C., G. D. Shanks, and J. S. Keystone. 2001. Malaria chemoprophylaxis in the age of drug resistance. I. Currently recommended drug regimens. Clin. Infect. Dis. 33:226-234. [DOI] [PubMed] [Google Scholar]
- 21.Kalyesubula, I., P. Musoke-Mudido, L. Marum, D. Bagenda, E. Aceng, C. Ndugwa, and K. Olness. 1997. Effects of malaria infection in human immunodeficiency virus type 1-infected Ugandan children. Pediatr. Infect. Dis. J. 16:876-881. [DOI] [PubMed] [Google Scholar]
- 22.Kelly, J. C., G. S. Wasserman, W. D. Bernard, C. Schultz, and J. Knapp. 1990. Chloroquine poisoning in a child. Ann. Emerg. Med. 19:47-50. [DOI] [PubMed] [Google Scholar]
- 23.Kielian, M., and S. Jungerwirth. 1990. Mechanisms of enveloped virus entry into cells. Mol. Biol. Med. 7:17-31. [PubMed] [Google Scholar]
- 24.Kimpton, J., and M. Emerman. 1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J. Virol. 66:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kooi, C., M. Cervin, and R. Anderson. 1991. Differentiation of acid-pH-dependent and -nondependent entry pathways for mouse hepatitis virus. Virology 180:108-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozak, S. L., E. J. Platt, N. Madani, F. E. Ferro, Jr., K. Peden, and D. Kabat. 1997. CD4, CXCR-4, and CCR-5 dependencies for infections by primary patient and laboratory-adapted isolates of human immunodeficiency virus type 1. J. Virol. 71:873-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kramer, M. H., and H. O. Lobel. 2001. Antimalarial chemoprophylaxis in infants and children. Paediatr. Drugs 3:113-121. [DOI] [PubMed] [Google Scholar]
- 28.Long, J. K., S. B. Mossad, and M. P. Goldman. 2000. Antiviral agents for treating influenza. Clevel. Clin. J. Med. 67:92-95. [DOI] [PubMed] [Google Scholar]
- 29.Luginger, E., G. K. Wenning, S. Bosch, and W. Poewe. 2000. Beneficial effects of amantadine on l-dopa-induced dyskinesias in Parkinson's disease. Movement Disorders 15:873-878. [DOI] [PubMed] [Google Scholar]
- 30.Luo, T., J. L. Douglas, R. L. Livingston, and J. V. Garcia. 1998. Infectivity enhancement by HIV-1 Nef is dependent on the pathway of virus entry: implications for HIV-based gene transfer systems. Virology 241:224-233. [DOI] [PubMed] [Google Scholar]
- 31.Makono, R., and S. Sibanda. 1999. Review of the prevalence of malaria in Zimbabwe with specific reference to parasite drug resistance (1984-96). Trans. R. Soc. Trop. Med. Hyg. 93:449-452. [DOI] [PubMed] [Google Scholar]
- 32.Manes, S., G. del Real, R. A. Lacalle, P. Lucas, C. Gomez-Mouton, S. Sanchez-Palomino, R. Delgado, J. Alcami, E. Mira, and A. Martinez. 2000. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 1:190-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maréchal, V., F. Clavel, J. M. Heard, and O. Schwartz. 1998. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J. Virol. 72:2208-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masuda, H., H. Suzuki, H. Oshitani, R. Saito, S. Kawasaki, M. Nishikawa, and H. Satoh. 2000. Incidence of amantadine-resistant influenza A viruses in sentinel surveillance sites and nursing homes in Niigata, Japan. Microbiol. Immunol. 44:833-839. [DOI] [PubMed] [Google Scholar]
- 35.Mellman, I., R. Fuchs, and A. Helenius. 1986. Acidification of the endocytic and exocytic pathways. Annu. Rev. Biochem. 55:663-700. [DOI] [PubMed] [Google Scholar]
- 36.Montalto, N. J., K. D. Gum, and J. V. Ashley. 2000. Updated treatment for influenza A and B. Am. Family Physician 62:2467-2476. [PubMed] [Google Scholar]
- 37.Munchau, A., and K. P. Bhatia. 2000. Pharmacological treatment of Parkinson's disease. Postgrad. Med. J. 76:602-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natale, V. A., and K. C. McCullough. 1998. Macrophage cytoplasmic vesicle pH gradients and vacuolar H+-ATPase activities relative to virus infection. J. Leukoc. Biol. 64:302-310. [DOI] [PubMed] [Google Scholar]
- 39.Okereke, C. S. 1999. Management of HIV-infected pregnant patients in malaria-endemic areas: therapeutic and safety considerations in concomitant use of antiretroviral and antimalarial agents. Clin. Ther. 21:1456-1496. [DOI] [PubMed] [Google Scholar]
- 40.Peden, K., M. Emerman, and L. Montagnier. 1991. Changes in growth properties on passage in tissue culture of viruses derived from infectious molecular clones of HIV-1LAI, HIV-1MAL, and HIV-1ELI. Virology 185:661-672. [DOI] [PubMed] [Google Scholar]
- 41.Perez, L., and L. Carrasco. 1994. Involvement of the vacuolar H(+)-ATPase in animal virus entry. J. Gen. Virol. 75:2595-2606. [DOI] [PubMed] [Google Scholar]
- 42.Platt, E. J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabat. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pless, D. D., and R. B. Wellner. 1996. In vitro fusion of endocytic vesicles: effects of reagents that alter endosomal pH. J. Cell. Biochem. 62:27-39. [DOI] [PubMed] [Google Scholar]
- 44.Schaeffer, E., R. Geleziunas, and W. C. Greene. 2001. Human immunodeficiency virus type 1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J. Virol. 75:2993-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singer, I. I., S. Scott, D. W. Kawka, J. Chin, B. L. Daugherty, J. A. DeMartino, J. DiSalvo, S. L. Gould, J. E. Lineberger, L. Malkowitz, M. D. Miller, L. Mitnaul, S. J. Siciliano, M. J. Staruch, H. R. Williams, H. J. Zweerink, and M. S. Springer. 2001. CCR5, CXCR4, and CD4 are clustered and closely apposed on microvilli of human macrophages and T cells. J. Virol. 75:3779-3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69:531-569. [DOI] [PubMed] [Google Scholar]
- 47.Wetsteyn, J. C., P. J. De Vries, B. Oosterhuis, and C. J. Van Boxtel. 1995. The pharmacokinetics of three multiple dose regimens of chloroquine: implications for malaria chemoprophylaxis. Br. J. Clin. Pharmacol. 39:696-699. [DOI] [PMC free article] [PubMed] [Google Scholar]