Abstract
Hepatitis C virus (HCV) is the major causative agent of blood-borne non-A, non-B hepatitis. Although a strong humoral response is detectable within a few weeks of primary infection and during viral persistence, the role played by antibodies against HCV envelope glycoproteins in controlling viral replication is still unclear. We describe how human monoclonal anti-HCV E2 antibody fragments isolated from a chronically HCV-infected patient differ sharply in their abilities to neutralize infection of HepG2 cells by a vesicular stomatitis virus pseudotype bearing HCV envelope glycoproteins. Two clones were able to neutralize the pseudotype virus at a concentration of 10 μg/ml, while three other clones completely lacked this activity. These data can explain the lack of protection and the possibility of reinfection that occur even in the presence of a strong antiviral antibody response.
Hepatitis C virus (HCV) is the major causative agent of blood-borne non-A, non-B hepatitis (10), infecting more than 200 million people worldwide (3). The tendency of HCV infection toward chronicity (8), with persistent viral replication (3), suggests that the host immune response is unable to tackle and eradicate the infection in the majority of cases. The role of the humoral response against viral envelope glycoproteins (E1 and E2) in the control of viral replication is still unclear (8). The study and demonstration of neutralizing antibodies have been hampered by the lack of a robust neutralization system, by the paucity of animal models, and by the differences between human and animal antibody responses to HCV antigens (1, 4). To investigate the effects of human antibodies on key viral steps such as viral entry into target cells, we used a model that reproduces HCV infection by means of vesicular stomatitis virus (VSV) pseudotypes bearing HCV envelope glycoproteins (VSV/HCV). A recombinant VSV, in which the glycoprotein (G) gene had been replaced with a reporter gene (VSVΔG*) encoding green fluorescent protein (GFP), was used to produce VSV/HCV pseudotypes possessing the HCV E1 and E2 envelope glycoproteins (13). The infectivity of pseudotypes can be determined by quantifying the number of cells expressing the GFP reporter gene using fluorescence microscopy. Briefly, the VSVΔG*/HCVE1-E2 pseudotype (VSV/HCV) consisted of VSV in which the G envelope protein was replaced with chimeric HCV E1 and E2 envelope glycoproteins consisting of the ectodomains of E1 and E2 proteins of a type 1b HCV cDNA clone (NIH-J1) (2) fused to the N-terminal signal sequences, transmembrane, and cytoplasmic domain of VSV G protein (13, 15, 19). VSV/HCV pseudotypes were prepared by infecting CHO cells with a VSV in which the G protein-coding region had been replaced with GFP (18). The VSVΔG*/G pseudotype (VSV/G), used as a control (as well as to produce the VSV/HCV pseudotype), was produced by infecting a cell line transiently expressing G protein with VSVΔG*. To determine whether the chimeric proteins could be incorporated into VSV particles, the CHO cell line expressing E1 and E2 on its surface was infected with VSVΔG*/G. After 16 h, the infected cell supernatant was collected and the pseudotyped viruses were purified by centrifugation through sucrose density gradients and analyzed by immunoblotting (13). VSVΔG*/G and VSVΔG* were produced as controls by infecting CHO cells transiently expressing VSV G protein or the parental CHO cells with VSVΔG*/G. The VSV structural proteins N, P, and M (matrix protein) were present in all of the purified virions. VSV G protein was detected in VSVΔG*/G but not in VSVΔG*-negative controls or in the HCV pseudotype virus (13). Virions produced in cells expressing the chimeric HCV envelope proteins contained both E1 and E2, indicating that HCV glycoproteins were correctly assembled into VSV particles. The relative infectivities of VSVΔG*/G and VSVΔG*/HCVE1-E2 for HepG2 cells were comparable in experiments repeated with 3 different batches of viruses (13).
To evaluate the efficiency of the humoral anti-HCV immune response in inhibiting viral entry into cells, we examined whether the pseudotypes could be neutralized by a panel of human monoclonal antibody Fab fragments derived from a phage display repertoire library containing the immunoglobulin G1 kappa (IgG1κ) repertoire of a patient chronically infected with HCV of type 1b (7). The antibody fragments, selected with purified recombinant HCV/E2 of type 1a (strain H) (12) expressed in CHO cells, have been fully characterized and have been demonstrated to correspond to clones actually present in the serum of chronically infected patients (4). All Fabs demonstrated similar affinity for the E2 antigen, with monomer Fab antigen binding constants on the order of 107 to 108 mol/liter−1 (7). Each of the five antibodies used in this study represents one of the five families in which the whole anti-E2 antibody repertoire of this patient was grouped. Fabs belonging to the same family share similar biological activities and have strong homologies of DNA sequences (7). Extensive competition studies have demonstrated that these antibody fragments recognize five different epitopes on the surface of the E2 glycoprotein (4). Epitope mapping of these Fabs was carried out by means of a peptide scan using 15-amino-acid long overlapping peptides, covering the whole sequence of the E2 protein, for selection and screening. Fabs did not show any reaction (7), indicating their recognition of the conformational epitopes that are not reproduced in the peptide scan approach. Immunoprecipitation experiments were carried out to demonstrate specific binding of human recombinant Fabs to VSV/HCV pseudoviruses. Briefly, 10 μl of the purified pseudovirus preparation described above was incubated for 4 h at 37°C with FLAG-tagged purified human recombinant Fabs (5) at a final concentration of 20 ng/μl; after this step, 40 μl of anti-FLAG M2 affinity gel (Sigma, Saint Louis, Mo.), consisting of a purified murine IgG1 monoclonal antibody specifically recognizing the FLAG epitope covalently attached to agarose, was added and the mixture was incubated overnight at 4°C with gentle shaking. After this step, the resin was washed five times at 4°C with phosphate-buffered saline (PBS) with 0.5% fetal bovine serum, followed by 15 washes with PBS alone. Viral RNA that might have been present in the pellet was extracted and demonstrated by reverse transcription-PCR with a modification of established methods (14). Briefly, VSV RNA in the pellet was retrotranscribed with the primer IS (5′-GGTGTTGCAGACTATGTTGGAC-3′), and the cDNA thus obtained was amplified by PCR (denaturation at 93°C for 1 min, annealing at 37°C for 1 min, and extension at 72°C for 2 min, repeated for 35 cycles) with the IS primer and the IA primer (5′-GGTGTTGCAGACTATGTTGAC-3′). The expected 395-bp DNA derived from correct amplification of the VSV genome was demonstrated in a 2% agarose gel. All human anti-HCV E2 Fabs precipitated the VSV/HCV pseudotypes but not the VSV/G controls. No precipitation was demonstrated with a control Fab (6) or in control tubes where no FLAG-tagged Fab was added. In enzyme-linked immunosorbent assay inhibition studies with sera derived from HCV-positive patients (4), the human recombinant monoclonal Fabs used in this study were demonstrated to be not artifacts but representative of clones existing in the human anti-HCV immunoresponse.
Divergences of variable-region DNA sequences from the relative germ line sequences are typical of antigen-driven affinity maturation (Table 1), suggesting a prolonged exposure to the antigen.
TABLE 1.
Germ lines and V gene mutations in variable regions of anti-HCV E2 human monoclonal antibodiesa
| Polypeptide chain | Antibody | V gene | % of mutated nucleotides in:
|
% of mutated amino acids in:
|
||
|---|---|---|---|---|---|---|
| FRs | CDRs | FRs | CDRs | |||
| Heavy | e8 | VH1-18 | 9.5 | 22.2 | 14.9 | 33.3 |
| e20 | VH1-69 | 9.4 | 16.9 | 19 | 38 | |
| e137 | VH1-69 | 11.5 | 15.3 | 14 | 41.7 | |
| e301 | VH1-69 | 8.9 | 19.4 | 15.6 | 45.8 | |
| e509 | VH1-69 | 5.2 | 15.9 | 10.9 | 33.3 | |
| Light | e8 | KV3-20 | 2.7 | 16 | 2.6 | 33.3 |
| e20 | KV1-9 | 4.3 | 7.7 | 9.7 | 22.2 | |
| e137 | KV1-8 | 2.2 | 9 | 3.2 | 15.4 | |
| e301 | KV3-15 | 3.8 | 14.3 | 9.7 | 23 | |
| e509 | KV3-15 | 3.2 | 1.3 | 6.5 | 0 | |
Sequences were determined as described previously (7) and aligned with germ line sequences in the IMGT database (11). The percentages of nucleotide and amino acid mutations were calculated according to the alignment method of Kabat et al. (9), taking into account framework regions (FRs) 1, 2, and 3 for heavy and light chains and complementarity-determining regions (CDRs) 1 and 2 for heavy chains and 1, 2, and 3 for light chains.
The neutralization of binding (NOB) activity of each Fab was also determined as described previously (7, 17). Briefly, 20 μl of E2 glycoprotein (0.5 μg/ml in PBS) was mixed with progressive dilutions of purified Fab preparations in 96-well U-bottom microplates. After incubation at 4°C for 1 h, pellets of MOLT-4 cells (105/well) were added and incubated for 1 h at 4°C. Unbound HCV proteins and antibodies were removed by two centrifugations in PBS at 200 ×g for 5 min at 4°C. Cells were subsequently incubated for 30 min at 4°C with human polyclonal anti-HCV E2 serum at a 1/100 dilution. Cells were washed twice in PBS and incubated for 30 min at 4°C with fluorescein isothiocyanate-labeled goat anti-human IgG at a 1/100 dilution. Cells were washed as described above and resuspended in 100 μl of PBS; cell-bound fluorescence was analyzed with a FACScan flow cytometer (Becton Dickinson, Sparks, Md.) and evaluated as described above. The concentrations at which NOB and 50% NOB were achieved were calculated as described previously (7, 17). The results clearly indicated that some clones (e137 and e8) were unable to inhibit HCV E2 binding to cells and that others inhibited binding even at very low concentrations (Table 2). Evaluation of the NOB of pseudotyped viruses to CD81 was not possible due to the very low binding of VSV/HCV to this molecule compared to the background (R. Burioni and Y. Matsuura, unpublished observation).
TABLE 2.
Neutralization of E2 binding to target cell and of VSV/HCV entry into hepatoma cells by the human recombinant anti-E2 Fabs used in this study
| Fab clone | 50% NOB concentration (μg/ml) (relative level of NOB activity)a | Effect on VSV/HCV infection |
|---|---|---|
| e8 | >40 (none) | None |
| e20 | 3 (high) | None |
| e137 | 40 (low) | Inhibition |
| e301 | 3 (high) | Strong inhibition |
| e509 | <0.035 (highest) | Enhancement |
NOB activity data are derived from data previously published (7).
The pseudotype neutralization assay performed was a modification of a previously described method (13). Briefly, dilutions of purified human recombinant Fabs were incubated with 2.4 × 103 infectious units of the VSV/HCV or VSV/G pseudotype for 30 min at 37°C and inoculated into HepG2 cells (4 × 104 cells) prepared in a 96-well plate. After adsorption for 60 min at 37°C, the cells were washed three times with Dulbecco's modified Eagle medium containing 10% fetal bovine serum and incubated at 37°C for 16 h; then, the number of infectious units of virus was determined by counting the number of GFP-expressing cells by fluorescence microscopy. The data are presented as the percentages of inhibition compared with the results from control wells to which no antibody was added and represent the average of three experiments performed in duplicate.
Two of the Fabs, e8 and e20, recognizing different epitopes on the surface of HCV E2 (4), did not neutralize VSV/HCV pseudotype infection (data not shown) even at the highest concentration used (80 μg/ml). Remarkably, one of these two Fabs, e20, was demonstrated to have strong NOB activity (7), confirming the notion that even antibodies inhibiting E2 binding may fail to prevent viral infection.
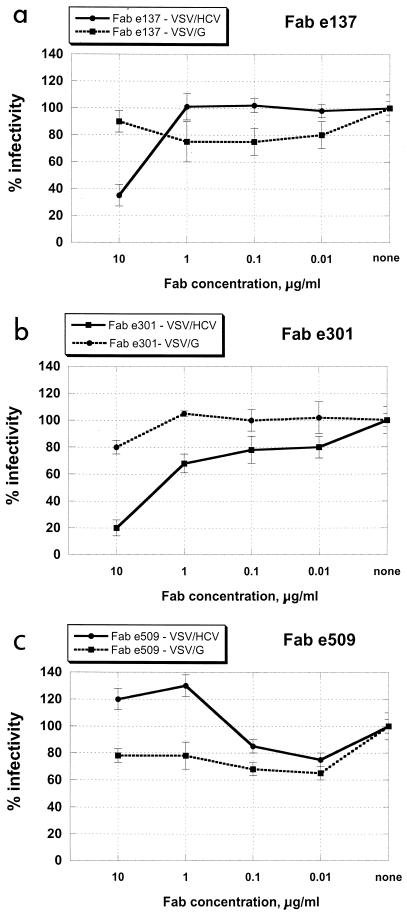
Two other Fabs, e137 and e301, efficiently neutralized VSV/HCV at a concentration of 10 μg/ml, while VSV pseudotypes bearing the VSV G envelope protein (VSV/G pseudotypes) were not affected (Fig. 1a and b). These data are congruent with previous findings (4) indicating that these two clones compete for the same E2 region, probably recognized by human antibodies endowed with neutralizing activity, as shown in a two-dimensional surface map of the human epitopes on HCV E2 (Fig. 2).
FIG. 1.
Inhibition of infection of VSV/HCV and VSV/G pseudotypes by purified anti-HCV E2 human recombinant Fabs e137 (a), e301 (b), and e509 (c) at different concentrations. HepG2 cells infected with Fab-treated pseudotypes were incubated for 16 h, and the number of GFP-expressing cells was determined by fluorescence microscopy. Data are presented as percentages of the infection detected in control wells (no Fabs added). The results shown are the average of three independent assays performed in duplicate, with error bars representing standard deviations.
FIG. 2.
Two-dimensional surface-like map of the human B-cell epitopes present on the surface of HCV E2, as recognized by the monoclonal antibodies used in this study. Overlapping circles indicate reciprocal inhibition. Fabs endowed with VSV/HCV pseudotype-neutralizing activity are underlined. The putative region mediating the interaction between HCV E2 and the cellular target is indicated by the dotted-line square. The putative region recognized by neutralizing antibodies is indicated by a thick black circle. Due to modifications that can be induced by antigen-antibody interactions, this diagram does not correspond to the actual physical map. The map without neutralization data is derived from previously published work (4).
Fab e509 is currently the strongest available antibody in terms of NOB activity, inhibiting binding between E2 and the cellular target at a very low concentration (Table 2). Incubation of VSV/HCV pseudotypes with this Fab at concentrations as low as 1 μg/ml apparently enhanced virus entry into hepatoma cells. No increase in infectivity was demonstrated when VSV/G pseudotypes were used, thus ruling out the possibility that a nonspecific interaction between this Fab and the cellular membrane promoted viral entry into the cell (Fig. 1c).
A control antibody (6) exerted no effect on the pseudotype system, as it failed to neutralize both VSV/HCV and VSV/G pseudotypes (data not shown). The VSV/G pseudotype was duly neutralized by a polyclonal anti-VSV antiserum at dilutions of up to 1:1,000 used as a neutralization control in these experiments (13), which had no effect on VSV/HCV (data not shown). Since a neutralizing effect was first demonstrated in the present work, a neutralizing control for VSV/HCV pseudotypes was not available. Polyclonal and monoclonal anti-E1 and anti-E2 antibodies raised in several hosts showed no neutralizing effect on VSV/HCV pseudotypes (Y. Matsuura, unpublished data)
Several aspects of the data presented here deserve special attention. First, the neutralizing activity of monovalent Fabs showed that HCV entry can be inhibited without the need for virion aggregation or cross-linking; furthermore, it is unlikely that the blocking of the interaction between the virus and its cellular target is a key factor in HCV neutralization, reinforcing the growing skepticism about CD81 playing a crucial role in HCV entry in susceptible cells. These data can explain at the molecular level the lack of correlation between NOB activity in the serum and protection from disease. Since the antibodies were elicited by epitopes exposed during natural infection, they are unlikely to be artifacts created by epitopes present only on recombinant proteins, as demonstrated by the inhibition of their binding to HCV E2 with sera derived from HCV-positive patients (4). Finally, we demonstrate that some degree of cross-protection is provided by anti-HCV antibodies. Although formal proof of the cross-reactivity of our Fabs with E2 of a type different from 1a was not provided due to the difficulty of obtaining purified E2 of other types, antibodies selected with E2 of type 1a were able to neutralize and immunoprecipitate a pseudotype bearing E2 of type 1b.
Another finding described in this paper is that Fab e509 was able to enhance the infectivity of the VSV/HCV pseudotype virus, although no effect on the VSV/G construct was apparent. Even though further studies are needed to conclusively demonstrate this activity, Fab e509 seems to be an important tool to use in studying the interactions between HCV and the cell surface.
The observation that, out of a panel of human Fabs derived from an infected patient, two Fabs were able to inhibit viral entry, two were not influential, and one promoted infection can help us to understand how HCV escapes the control of the immune system. These data strongly suggest that a part of the effort of the immune system may be directed toward the production of antibodies that are not necessarily beneficial to the host. Given that antibodies able to inhibit the antigen binding of these Fabs have been isolated in the sera of patients, and that an excess of these Fabs can inhibit serum binding to E2 by more than 70% (4), the artifactual nature of the Fabs used in this study can be ruled out. Assays able to quantify the amount of antibodies directed against the different epitopes in the sera of patients (R. Burioni, submitted for publication) will make it possible to demonstrate whether the amount of antibodies directed against each epitope correlates with a different outcome of the viral infection.
Finally, the results shown here will help in the design of possible vaccine strategies and their in vitro evaluation (16). This would be a considerable advance in the case of an infection lacking an animal model. Molecules demonstrated in vitro to selectively stimulate the production of neutralizing antibodies but not to elicit the production of immunoglobulins that are not beneficial to the host will be the best candidates for further in vivo studies.
Acknowledgments
R.B. and Y.M. contributed equally to this paper.
We thank A. Grieco, G. Malcangi, S. Bighi, M. Perotti, S. Bellagamba, S. Carletti, and M. Vecchi for their valuable assistance. R.B. is deeply grateful to Giovanni Gasbarrini for critical help and continuous support.
This work was supported by grants from Istituto Superiore di Sanità to R.B. and M.C.
REFERENCES
- 1.Ahmed, M., T. Shikata, and M. Esumi. 1996. Murine humoral immune response against recombinant structural proteins of hepatitis C virus distinct from those of patients. Microbiol. Immunol. 40:169-176. [DOI] [PubMed] [Google Scholar]
- 2.Aizaki, H., Y. Aoki, T. Harada, K. Ishii, T. Suzuki, S. Nagamori, G. Toda, Y. Matsuura, and T. Miyamura. 1998. Full-length complementary DNA of hepatitis C virus genome from an infectious blood sample. Hepatology 27:621-627. [DOI] [PubMed] [Google Scholar]
- 3.Alter, M. J., H. S. Margolis, K. Krawczynski, F. N. Judson, A. Mares, W. J. Alexander, P. Y. Hu, J. K. Miller, M. A. Gerber, R. E. Sampliner, et al. 1992. The natural history of community-acquired hepatitis C in the United States. N. Engl. J. Med. 327:1899-1905. [DOI] [PubMed] [Google Scholar]
- 4.Bugli, F., N. Mancini, C. -Y. Kang, C. Di Campli, A. Grieco, A. Manzin, A. Gabrielli, A. Gasbarrini, G. Fadda, P. E. Varaldo, M. Clementi, and R. Burioni. 2001. Mapping B-cell epitopes of hepatitis C virus E2 glycoprotein using human monoclonal antibodies from phage display libraries. J. Virol. 75:9986-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burioni, R., F. Bugli, N. Mancini, and G. Fadda. 2001. A novel expression vector for production of epitope-tagged recombinant Fab fragments in bacteria. Hum. Antibodies 10:149-154. [PubMed] [Google Scholar]
- 6.Burioni, R., P. Plaisant, F. Bugli, L. Solforosi, V. D. Carri, P. E. Varaldo, and G. Fadda. 1998. A new subtraction technique for molecular cloning of rare antiviral antibody specificities from phage display libraries. Res. Virol. 149:327-330. [DOI] [PubMed] [Google Scholar]
- 7.Burioni, R., P. Plaisant, A. Manzin, D. Rosa, V. Delli Carri, F. Bugli, L. Solforosi, S. Abrignani, P. E. Varaldo, G. Fadda, and M. Clementi. 1998. Dissection of human humoral immune response against hepatitis C virus E2 glycoprotein by repertoire cloning and generation of recombinant Fab fragments. Hepatology 28:810-814. [DOI] [PubMed] [Google Scholar]
- 8.Cerny, A., and F. V. Chisari. 1999. Pathogenesis of chronic hepatitis C: immunological features of hepatic injury and viral persistence. Hepatology 30:595-601. [DOI] [PubMed] [Google Scholar]
- 9.Kabat, E. A., T. T. Wu, H. M. Perry, K. S. Gottesman, and C. Foeller. 1991. Sequences of proteins of immunological interest, 5th ed. U.S. Department of Health and Human Services, Bethesda, Md.
- 10.Kuo, G., Q. L. Choo, H. J. Alter, G. L. Gitnick, A. G. Redeker, R. H. Purcell, T. Miyamura, J. L. Dienstag, M. J. Alter, C. E. Stevens, et al. 1989. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science 244:362-364. [DOI] [PubMed] [Google Scholar]
- 11.Lefranc, M. P., V. Giudicelli, C. Ginestoux, J. Bodmer, W. Muller, R. Bontrop, M. Lemaitre, A. Malik, V. Barbie, and D. Chaume. 1999. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 27:209-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lesniewski, R., G. Okasinski, R. Carrick, C. Van Sant, S. Desai, R. Johnson, J. Scheffel, B. Moore, and I. Mushahwar. 1995. Antibody to hepatitis C virus second envelope (HCV-E2) glycoprotein: a new marker of HCV infection closely associated with viremia. J. Med. Virol. 45:415-422. [DOI] [PubMed] [Google Scholar]
- 13.Matsuura, Y., H. Tani, K. Suzuki, T. Someya, R. Suzuki, H. Aizaki, K. Ishii, K. Morishii, C. S. Robison, M. A. Whitt, and T. Miyamura. 2001. Characterization of pseudotype VSV possessing HCV envelope proteins. Virology 286:263-275. [DOI] [PubMed] [Google Scholar]
- 14.Nunez, J. I., E. Blanco, T. Hernandez, C. Gomez-Tejedor, M. J. Martin, J. Dopazo, and F. Sobrino. 1998. A RT-PCR assay for the differential diagnosis of vesicular viral diseases of swine. J. Virol. Methods 72:227-235. [DOI] [PubMed] [Google Scholar]
- 15.Ohashi, H., K. Maruyama, Y. C. Liu, and A. Yoshimura. 1994. Ligand-induced activation of chimeric receptors between the erythropoietin receptor and receptor tyrosine kinases. Proc. Natl. Acad. Sci. USA 91:158-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parren, P. W., P. Fisicaro, A. F. Labrijn, J. M. Binley, W. P. Yang, H. J. Ditzel, C. F. Barbas, and D. R. Burton. 1996. In vitro antigen challenge of human antibody libraries for vaccine evaluation: the human immunodeficiency virus type 1 envelope. J. Virol. 70:9046-9050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosa, D., S. Campagnoli, C. Moretto, E. Guenzi, L. Cousens, M. Chin, C. Dong, A. J. Weiner, J. Y. Lau, Q. L. Choo, D. Chien, P. Pileri, M. Houghton, and S. Abrignani. 1996. A quantitative test to estimate neutralizing antibodies to the hepatitis C virus: cytofluorimetric assessment of envelope glycoprotein 2 binding to target cells. Proc. Natl. Acad. Sci. USA 93:1759-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takada, A., C. Robison, H. Goto, A. Sanchez, K. G. Murti, M. A. Whitt, and Y. Kawaoka. 1997. A system for functional analysis of Ebola virus glycoprotein. Proc. Natl. Acad. Sci. USA 94:14764-14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takikawa, S., K. Ishii, H. Aizaki, T. Suzuki, H. Asakura, Y. Matsuura, and T. Miyamura. 2000. Cell fusion activity of hepatitis C virus envelope proteins. J. Virol. 74:5066-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]