Abstract
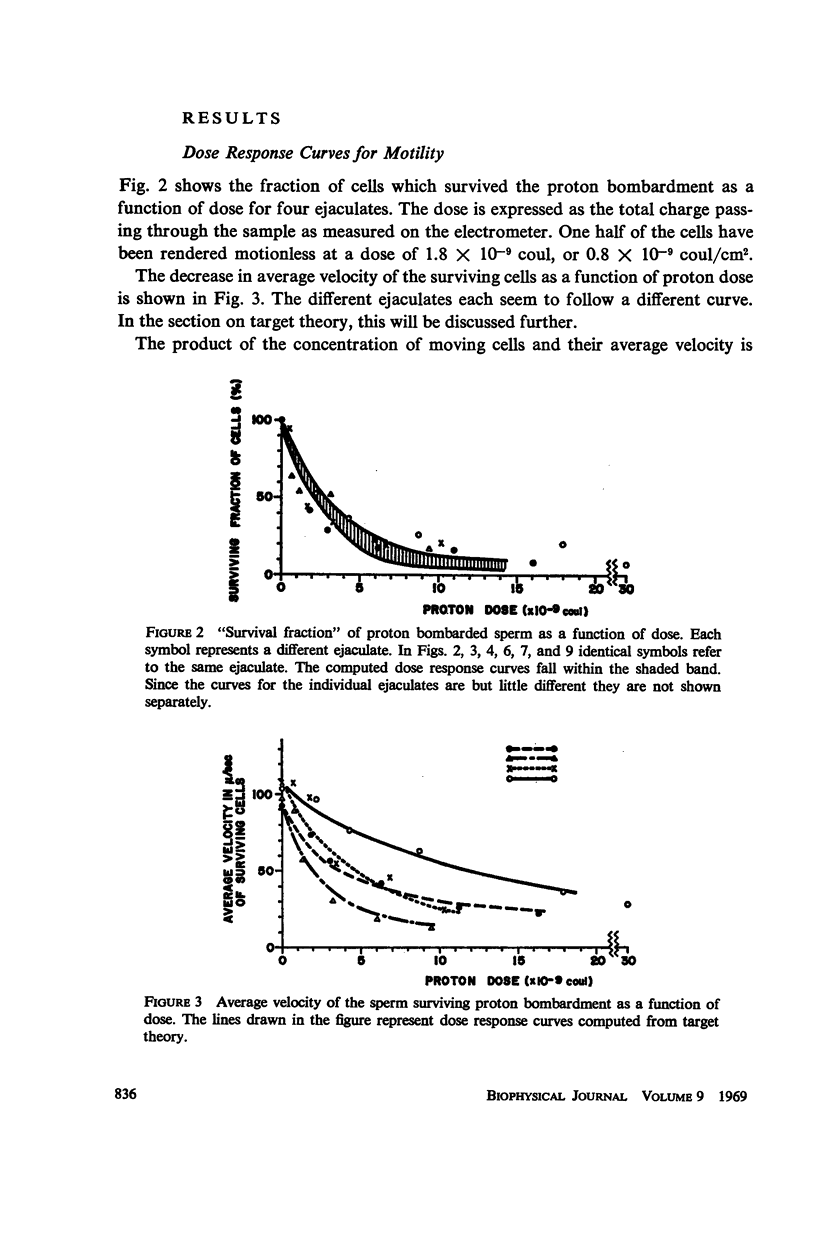
Diluted bull semen samples were bombarded with a 24 Mev proton beam. Dose response curves for the fraction of cells which survived the bombardment and for the average velocity of the surviving cells were measured. Target theory indicated a cross section of the sensitive volume of 2.1 × 10-10 cm2. Respiration measurements showed that the oxidative phosphorylation in the sperm remained coupled after the bombardments. The efficiency with which free energy from ATP hydrolysis was converted into mechanical work by the sperm was found to decrease after proton bombardment. The half-value dose for this effect was two and a half times higher than the half-value dose for motility damage. These respiration measurements indicate that the damage due to the bombardment is not to the metabolic system or to the contractile system in the sperm flagellum, but to a control system for the motility. The results of the target theory shows that this control system is localized in a small element of approximately 1600 A diameter. The centriole is tentatively proposed as being this control element.
Full text
PDF


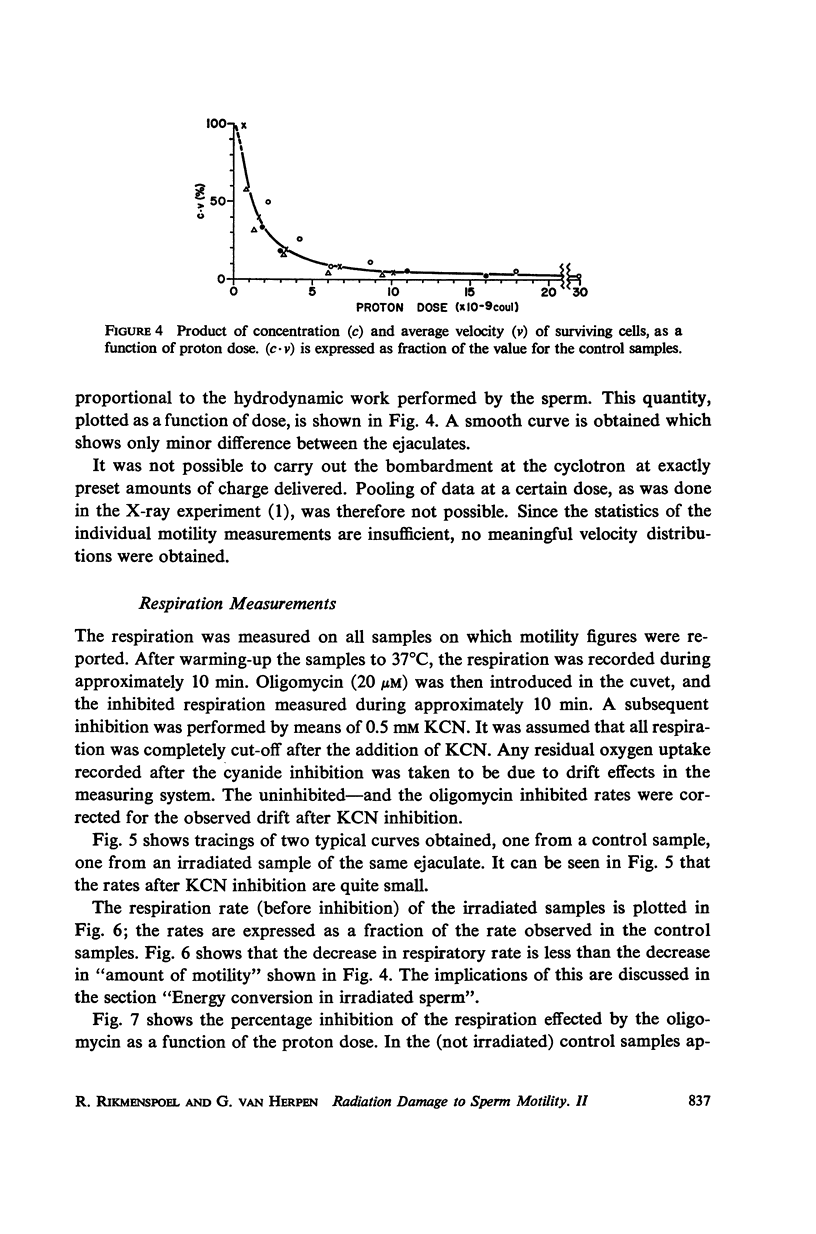




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brokaw C. J., Benedict B. Mechanochemical coupling in flagella. II. Effects of viscosity and thiourea on metabolism and motility of Ciona spermatozoa. J Gen Physiol. 1968 Aug;52(2):283–299. doi: 10.1085/jgp.52.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARDY H. A., JOHNSON D., McMURRAY W. C. Antibiotics as tools for metabolic studies. I. A survey of toxic antibiotics in respiratory, phosphorylative and glycolytic systems. Arch Biochem Biophys. 1958 Dec;78(2):587–597. doi: 10.1016/0003-9861(58)90383-7. [DOI] [PubMed] [Google Scholar]
- MOHRI H., MOHRI T., ERNSTER L. ISOLATI N AND ENZYMIC PROPERTIES OF THE MIDPIECE OF BULL SPERMATOZOA. Exp Cell Res. 1965 May;38:217–246. doi: 10.1016/0014-4827(65)90400-3. [DOI] [PubMed] [Google Scholar]
- RIKMENSPOEL R. THE INHIBITION BY AMYTAL OF RESPIRATION AND MOTILITY OF BULL SPERMATOZOA. Exp Cell Res. 1965 Feb;37:312–326. doi: 10.1016/0014-4827(65)90180-1. [DOI] [PubMed] [Google Scholar]
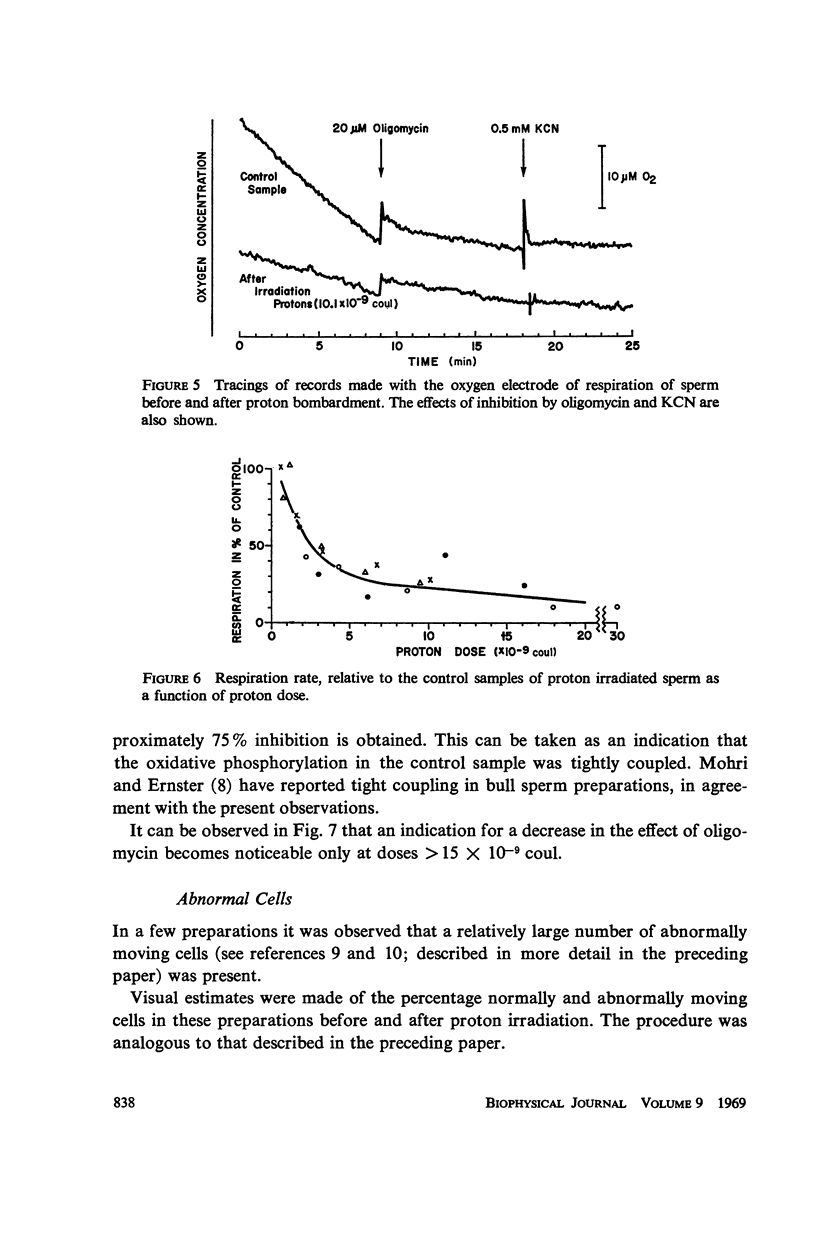
- RIKMENSPOEL R., van HERPEN, EIJKHOUT P. Cinematographic observations of the movements of bull sperm cells. Phys Med Biol. 1960 Oct;5:167–181. doi: 10.1088/0031-9155/5/2/306. [DOI] [PubMed] [Google Scholar]
- Rikmenspoel R. The tail movement of bull spermatozoa. Observations and model calculations. Biophys J. 1965 Jul;5(4):365–392. doi: 10.1016/S0006-3495(65)86723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAACKE R. G., ALMQUIST J. O. ULTRASTRUCTURE OF BOVINE SPERMATOZOA. II. THE NECK AND TAIL OF NORMAL, EJACULATED SPERM 1,2,3. Am J Anat. 1964 Jul;115:163–183. doi: 10.1002/aja.1001150110. [DOI] [PubMed] [Google Scholar]


