Abstract
Homozygous human immunodeficiency virus type 1 (HIV-1)-transgenic mice (Tg26) appear normal at birth but die within 3 to 4 weeks. The skin of these animals shows diffuse scaling and high-level expression of both HIV-1 mRNA and gp120. Previous experiments showed that treatment with human chorionic gonadatropin (hCG) prevented death and the expression of HIV-1 mRNA and gp120. The present experiments were initiated to study the role of tumor necrosis factor alpha (TNF-α) in HIV-1-induced pathology. Examination of the sera of Tg26 mice revealed a 50-fold increase in TNF-α levels compared to those in nontransgenic mice. Treatment with antibody to TNF-α prevented death, resulted in near normal growth, and produced a marked decrease in skin lesions and a profound reduction in the expression of HIV-1 mRNA and gp120. Both TNF-α antibody and hCG reduced TNF-α levels in sera by approximately 75%. We conclude that TNF-α contributes in a major way to HIV-1-induced pathology in transgenic mice and that both hCG and antibody to TNF-α prevent the development of pathology by suppressing the level of TNF-α.
Infants vertically infected with human immunodeficiency virus type 1 (HIV-1) show growth retardation and severe weight loss that can lead to death (15, 30). A variety of factors, including the overproduction of certain cytokines, have been implicated as possible causes (3, 26, 31). The HIV-1-transgenic mouse line Tg26, which carries a 7.4-kb HIV-1 construct lacking a 3.0-kb sequence encompassing the gag-pol (Δgag/pol) region of the provirus pNL4-3 (8), has been used to study HIV-1-induced pathology in mice (17). Heterozygous Tg26 mice exhibit normal appearance and near normal growth but develop nephropathy and hyperproliferative skin lesions (e.g., papillomas) in adult life. Homozygous Tg26 mice are normal in appearance and weight at birth but develop debilitating cachexia and diffuse scaling of the skin and die within 3 to 4 weeks after birth (10, 17-19). Previously, it was shown that treatment of newborn homozygous Tg26 mice with human chorionic gonadotropin (hCG) prevented death, reduced skin lesions, and resulted in near normal growth (7). At the molecular level, treatment with hCG reduced the expression of HIV-1 mRNA and gp120 protein. The exact mechanism by which hCG or still unidentified peptides within hCG preparations (21) mediate these effects is not known, however.
In vitro and in vivo studies have shown that HIV-1 infection can induce the secretion and elevation of proinflammatory cytokines such as interleukin 1β (IL-1β), IL-6, and tumor necrosis factor alpha (TNF-α) (6, 16, 27). In some cases, a direct correlation between the level of proinflammatory molecules and viral load has been observed (2, 14, 29). One of these molecules, TNF-α, is known to act upstream to many proinflammatory molecules and can contribute to inflammation and tissue damage (36). What role TNF-α actually plays in HIV-1-induced pathology in Tg26 mice is not clear, however. The present study was initiated to examine the role of TNF-α in HIV-1-induced pathology in homozygous Tg26 mice.
Quantitation of inflammatory cytokines in sera of Tg26 mice.
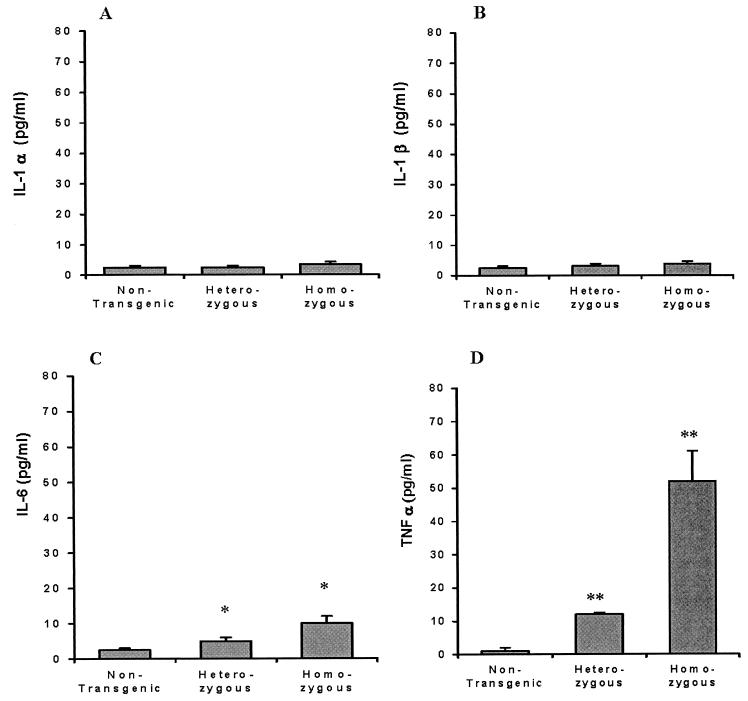
The inflammatory cytokines IL-1α, IL-1β, IL-6, and TNF-α were measured by enzyme-linked immunosorbent assay in the sera of 3- to 4-week-old Tg26 homozygous, Tg26 heterozygous, and nontransgenic mice. IL-1α and IL-1β remained in the normal range in the three groups of mice (Fig. 1A and B). IL-6 levels were elevated about twofold in the heterozygous mice and nearly fourfold in the homozygous mice compared to those found in the nontransgenic mice (Fig. 1C). In contrast, TNF-α was elevated 6- to 12-fold in the heterozygous mice and nearly 50-fold in the homozygous mice (Fig. 1D).
FIG. 1.
Cytokine levels in sera of nontransgenic, Tg26 heterozygous, and Tg26 homozygous mice. (A) IL-1α; (B) IL-1β; (C) IL-6; (D) TNF-α. Serum samples from eight animals were collected and analyzed in triplicate. Bars denote the standard error of the mean. *P < 0.05; **P < 0.005.
Effect of TNF-α and anti-TNF-α antibody on growth of homozygous Tg26 mice.
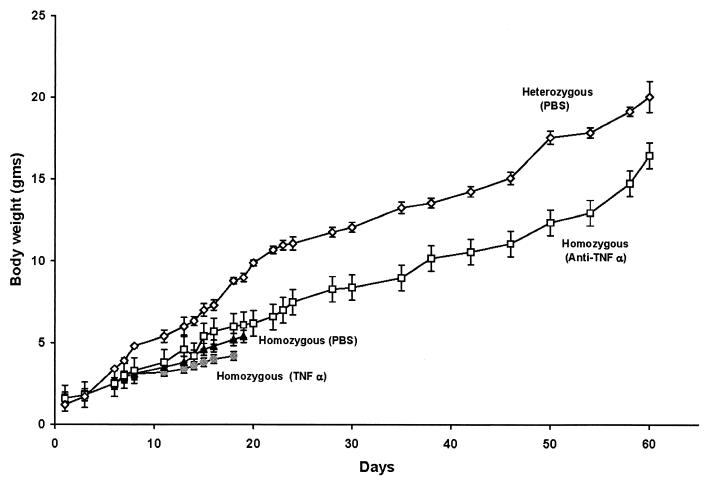
To see if the elevated levels of TNF-α contributed to cachexia and death, homozygous Tg26 mice were treated with anti-TNF-α or TNF-α. One day after the pups were born, mothers were given subcutaneously 2 μg of anti-mouse TNF-α polyclonal antibody (R & D Systems, Minneapolis, Minn.) twice a week. When the pups were 5 days old, they were given subcutaneously 1 μg of anti-mouse TNF-α antibody twice a week. At 6 weeks of age, the dosage was increased to 2 μg twice a week. In other experiments, mice were treated subcutaneously with 200 ng of recombinant mouse TNF-α (R & D Systems) twice a week. As shown in Fig. 2, TNF-α-treated homozygous mice grew at a somewhat slower rate than phosphate-buffered saline (PBS)-treated homozygous mice and mice in both groups died within 20 days. In contrast, homozygous Tg26 animals that had received anti-TNF-α did not die and exhibited progressive weight gain, although at a pace somewhat slower than that for untreated heterozygous transgenic mice (Fig. 2) or untreated nontransgenic mice (7).
FIG. 2.
Body weight of Tg26 homozygous mice treated with PBS (8 animals), TNF-α (4 animals), or anti-TNF-α (10 animals) and body weight of Tg26 heterozygous mice treated with PBS (7 animals). Bars denote the standard error of the mean.
Expression of gp120 in homozygous Tg26 mice.
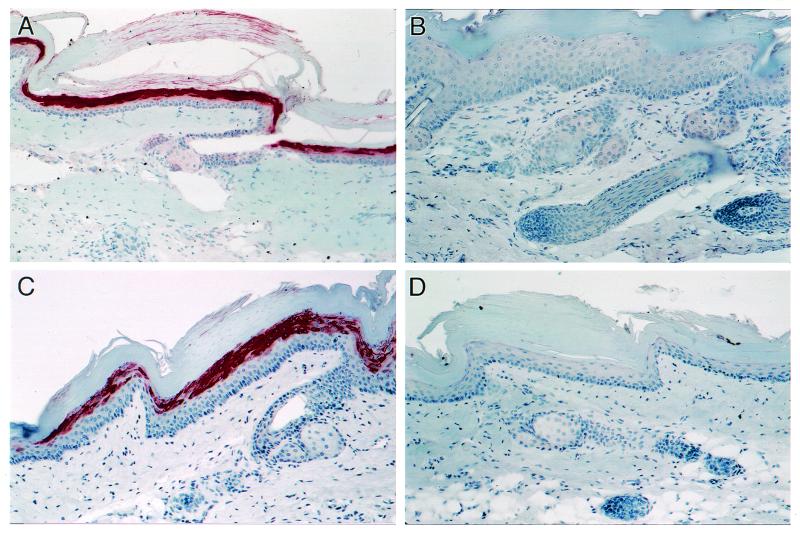
Homozygous Tg26 mice develop proliferative lesions characterized by diffuse epidermal hyperplasia and keratosis (19). To study the effect of TNF-α and anti-TNF-α on these lesions, skin samples from TNF-α- and anti-TNF-α-treated mice were stained for HIV-1 gp120 expression as described previously (7). Figure 3A shows that the expression of gp120 was high in PBS-treated homozygous mice. The pattern was similar to that observed previously (7). Treatment with anti-TNF-α inhibited almost completely the expression of gp120 (Fig. 3B), whereas treatment with TNF-α (Fig. 3C) enhanced the expression of gp120 compared to that for the PBS-treated mice (Fig. 3A). These findings are similar to those observed when homozygous Tg26 mice were treated with hCG (7).
FIG. 3.
Effects of TNF-α and anti-TNF-α on gp120 expression in the skin of Tg26 homozygous mice. Sections of skin from Tg26 mice treated with PBS (A), anti-TNF-α (B), or TNF-α (C) and incubated with polyclonal sheep anti-gp120 immunoglobulin G (1:2,000 dilution); sections of skin from Tg26 mice incubated with normal sheep serum (D). Immunoperoxidase staining was performed by the streptavidin-biotin complex technique using a Histostain-SP kit (Zymed, Burlingame, Calif.). All sections were counterstained with hematoxylin.
Expression of HIV-1 mRNA in homozygous Tg26 mice.
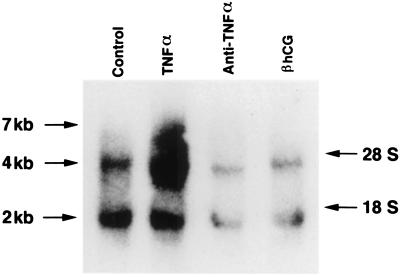
Previous studies showed that the muscle and skin from Tg26 homozygous mice expressed high levels of HIV-1 mRNA, with the 2- and 4-kb messages being more abundant than the 7-kb message (7). Treatment with hCG markedly reduced the expression of HIV-1 mRNA (7). Since TNF-α is known to up regulate HIV-1 mRNA expression (14), we wanted to see whether treatment with TNF-α would enhance, whereas treatment with anti-TNF-α would suppress, HIV-1 mRNA expression in Tg26 mice. Figure 4 shows the expected 2- and 4-kb bands from the skin of PBS-treated homozygous Tg26 mice. Treatment with TNF-α substantially increased the expression of the 4-kb band as well as that of the 7-kb band. In contrast, treatment with anti-TNF-α or hCG (Sigma Chemical Co., St. Louis, Mo.) greatly reduced the expression of both the 2- and 4-kb bands.
FIG. 4.
Effect of TNF-α, anti-TNF-α, and hCG on HIV-1 mRNA expression in the skin of Tg26 homozygous mice as determined by Northern blot analysis. Hybridization was carried out with an HIV-1-specific Nef cDNA probe (1). RNA samples in each lane were equalized prior to loading on the gel and confirmed by ethidium bromide staining of the gel (not shown).
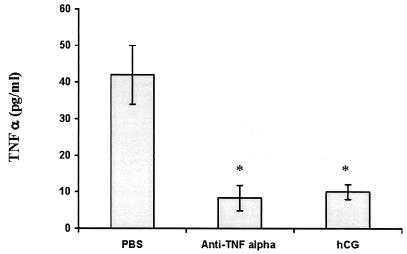
TNF-α levels in sera of anti-TNF-α- and hCG-treated mice.
The level of TNF-α in the sera of Tg26 homozygous mice that had been treated with anti-TNF-α or hCG was determined by enzyme-linked immunosorbent assay. As shown in Fig. 5, both anti-TNF-α and hCG reduced the level of TNF-α by 75 to 80% compared to that in samples taken from PBS-treated mice.
FIG. 5.
TNF-α concentration in sera of 20- to 30-day-old Tg26 homozygous mice that had been treated with PBS, anti-TNF-α, or hCG. Each sample was assayed in triplicate, and the bars denote the standard error of the mean. *P < 0.005.
Large amounts of TNF-α are secreted into the serum in response to a wide variety of inflammatory stimuli and infections including HIV-1 (40). High-level expression of TNF-α induces the biosynthesis of molecules that cause tissue necrosis (e.g., collagenases, proteases, and reactive oxygen species). TNF-α also can cause cell death by apoptosis (20). Elevated levels of circulating TNF-α have been linked to a wide variety of diseases, including arthritis, diabetes, Crohn's disease, and cachexia associated with terminal cancer and AIDS (23). Studies over the last several years have shown that the down regulation of TNF-α by TNF-α blockers can reverse some of the clinical effects of these disorders in both animals and humans (4, 13, 28, 32, 36, 37). Our experiments with anti-TNF-α show this to be the case in homozygous Tg26 mice.
It is known that up regulated TNF-α can, in turn, up regulate the transcription factors NF-κB and AP-1, which mediate the expression of a number of downstream TNF-α-responsive genes (5, 34, 35). NF-κB and AP-1 are known to affect the actual replication of HIV-1 (41). NF-κB and AP-1 bind to specific DNA sites in the long terminal repeats of HIV-1, and this enhances HIV-1 replication (9, 11, 12, 25). Since the TNF-α promoter also contains NF-κB and AP-1 binding sites, TNF-α can positively up regulate its own synthesis, leading to the persistence of inflammation and tissue damage (24, 38, 39). Thus, the down regulation of TNF-α by anti-TNF-α is the most likely explanation for the suppression of HIV-1-induced disease in Tg26 homozygous mice.
Previously, it was shown that treatment of Tg26 mice with hCG prevented cachexia and death (7). In the present study, we demonstrated that treatment of Tg26 mice with hCG reduced TNF-α levels in serum. This suggests that hCG may also exert its protective effect on Tg26 mice by suppressing TNF-α-induced activation pathways. Similarly, the suppression of streptococcus cell wall-induced arthritis by hCG may be mediated through the reduction of TNF-α levels (33). Evidence that hCG actually can suppress TNF-α-induced pathways, particularly NF-κB and AP-1, was recently provided by Manna and colleagues (22).
In conclusion, our studies show that TNF-α is elevated in HIV-1-Tg26 mice and that reduction of TNF-α levels by either anti-TNF-α or hCG inhibits the expression of HIV-1 proteins and prevents cachexia and death. Anti-TNF-α acts by neutralizing extracellular TNF-α, whereas hCG acts intracellularly by blocking steps in the TNF-α-induced NF-κB gene activation cascade. The combination of these two molecules may prove to be of therapeutic value not only in AIDS but also in a variety of other TNF-α-associated disorders.
REFERENCES
- 1.Ahmad, N., and S. Venkatesan. 1988. Nef protein of HIV-1 is a transcriptional repressor of HIV-1 LTR. Science 241:1481-1485. [DOI] [PubMed] [Google Scholar]
- 2.Arany, I., M. Muldrow, and S. K. Tyring. 2001. Correlation between mRNA levels of IL-6 and TNF alpha and progression rate in anal squamous epithelial lesions from HIV-positive men. Anticancer Res. 21:425-428. [PubMed] [Google Scholar]
- 3.Arditi, M., W. Kabat, and R. Yogev. 1991. Serum tumor necrosis factor alpha, interleukin 1-beta, p24 antigen concentrations and CD4+ cells at various stages of human immunodeficiency virus 1 infection in children. Pediatr. Infect. Dis. J. 10:450-455. [DOI] [PubMed] [Google Scholar]
- 4.Barrera, P., L. A. Joosten, A. A. den Broeder, L. B. van de Putte, P. L. van Riel, and W. B. van den Berg. 2001. Effects of treatment with a fully human anti-tumour necrosis factor alpha monoclonal antibody on the local and systemic homeostasis of interleukin 1 and TNF alpha in patients with rheumatoid arthritis. Ann. Rheum. Dis. 60:660-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baud, V., and M. Karin. 2001. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 11:372-377. [DOI] [PubMed] [Google Scholar]
- 6.Bergamini, A., E. Faggioli, F. Bolacchi, S. Gessani, L. Cappannoli, I. Uccella, F. Demin, M. Capozzi, R. Cicconi, R. Placido, S. Vendetti, G. M. Colizzi, and G. Rocchi. 1999. Enhanced production of tumor necrosis factor-alpha and interleukin-6 due to prolonged response to lipopolysaccharide in human macrophages infected in vitro with human immunodeficiency virus type 1. J. Infect. Dis. 179:832-842. [DOI] [PubMed] [Google Scholar]
- 7.De, S. K., C. R. Wohlenberg, N. J. Marinos, D. Doodnauth, J. L. Bryant, and A. L. Notkins. 1997. Human chorionic gonadotropin hormone prevents wasting syndrome and death in HIV-1 transgenic mice. J. Clin. Investig. 99:1484-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickie, P., J. Felser, M. Eckhaus, J. Bryant, J. Silver, N. Marinos, and A. L. Notkins. 1991. HIV-associated nephropathy in transgenic mice expressing HIV-1 genes. Virology 185:109-119. [DOI] [PubMed] [Google Scholar]
- 9.Duh, E. J., W. J. Maury, T. M. Folks, A. S. Fauci, and A. B. Rabson. 1989. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc. Natl. Acad. Sci. USA 86:5974-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franks, R. R., P. E. Ray, C. C. Babbott, J. L. Bryant, A. L. Notkins, T. J. Santoro, and P. E. Klotman. 1995. Maternal-fetal interactions affect growth of human immunodeficiency virus type 1 transgenic mice. Pediatr. Res. 37:56-63. [DOI] [PubMed] [Google Scholar]
- 11.Griffin, G. E., K. Leung, T. M. Folks, S. Kunkel, and G. J. Nabel. 1989. Activation of HIV gene expression during monocyte differentiation by induction of NF-kappa B. Nature 339:70-73. [DOI] [PubMed] [Google Scholar]
- 12.Gualberto, A., M. L. Hixon, T. S. Finco, N. D. Perkins, G. J. Nabel, and A. S. Baldwin, Jr. 1995. A proliferative p53-responsive element mediates tumor necrosis factor alpha induction of the human immunodeficiency virus type 1 long terminal repeat. Mol. Cell. Biol. 15:3450-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassard, P. V., S. W. Binder, V. Nelson, and E. A. Vasiliauskas. 2001. Anti-tumor necrosis factor monoclonal antibody therapy for gastrointestinal Behcet's disease: a case report. Gastroenterology 120:995-999. [DOI] [PubMed] [Google Scholar]
- 14.Hittinger, G., C. Poggi, E. Delbeke, N. Profizi, and A. Lafeuillade. 1998. Correlation between plasma levels of cytokines and HIV-1 RNA copy number in HIV-infected patients. Infection 26:100-103. [DOI] [PubMed] [Google Scholar]
- 15.Jason, J., E. Gomperts, D. N. Lawrence, R. C. Holman, J. D. Bouhasin, R. Miller, and B. L. Evatt. 1989. HIV and hemophilic children's growth. J. Acquir. Immune Defic. Syndr. 2:277-282. [PubMed] [Google Scholar]
- 16.Khanna, K. V., X. F. Yu, D. H. Ford, L. Ratner, J. K. Hildreth, and R. B. Markham. 2000. Differences among HIV-1 variants in their ability to elicit secretion of TNF-alpha. J. Immunol. 164:1408-1415. [DOI] [PubMed] [Google Scholar]
- 17.Klotman, P. E., J. Rappaport, P. Ray, J. B. Kopp, R. Franks, L. A. Bruggeman, and A. L. Notkins. 1995. Transgenic models of HIV-1. AIDS 9:313-324. [PubMed] [Google Scholar]
- 18.Kopp, J. B., M. E. Klotman, S. H. Adler, L. A. Bruggeman, P. Dickie, N. J. Marinos, M. Eckhaus, J. L. Bryant, A. L. Notkins, and P. E. Klotman. 1992. Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc. Natl. Acad. Sci. USA 89:1577-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopp, J. B., J. F. Rooney, C. Wohlenberg, N. Dorfman, N. J. Marinos, J. L. Bryant, S. I. Katz, A. L. Notkins, and P. E. Klotman. 1993. Cutaneous disorders and viral gene expression in HIV-1 transgenic mice. AIDS Res. Hum. Retrovir. 9:267-275. [DOI] [PubMed] [Google Scholar]
- 20.Lee, E. G., D. L. Boone, S. Chai, S. L. Libby, M. Chien, J. P. Lodolce, and A. Ma. 2000. Failure to regulate TNF-induced NF-kappa B and cell death responses in A20-deficient mice. Science 289:2350-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lunardi-Iskandar, Y., J. L. Bryant, W. A. Blattner, C. L. Hung, L. Flamand, P. Gill, P. Hermans, S. Birken, and R. C. Gallo. 1998. Effects of a urinary factor from women in early pregnancy on HIV-1, SIV and associated disease. Nat. Med. 4:428-434. [DOI] [PubMed] [Google Scholar]
- 22.Manna, S. K., A. Mukhopadhyay, and B. B. Aggarwal. 2000. Human chorionic gonadotropin suppresses activation of nuclear transcription factor-kappa B and activator protein-1 induced by tumor necrosis factor. J. Biol. Chem. 275:13307-13314. [DOI] [PubMed] [Google Scholar]
- 23.Matthys, P., and A. Billiau. 1997. Cytokines and cachexia. Nutrition 13:763-770. [DOI] [PubMed] [Google Scholar]
- 24.Newell, C. L., A. B. Deisseroth, and G. Lopez-Berestein. 1994. Interaction of nuclear proteins with an AP-1/CRE-like promoter sequence in the human TNF-alpha gene. J. Leukoc. Biol. 56:27-35. [DOI] [PubMed] [Google Scholar]
- 25.Rabbi, M. F., L. Al-Harthi, and K. A. Roebuck. 1997. TNF alpha cooperates with the protein kinase A pathway to synergistically increase HIV-1 LTR transcription via downstream TRE-like cAMP response elements. Virology 237:422-429. [DOI] [PubMed] [Google Scholar]
- 26.Rautonen, J., N. Rautonen, N. L. Martin, R. Philip, and D. W. Wara. 1991. Serum interleukin-6 concentrations are elevated and associated with elevated tumor necrosis factor-alpha and immunoglobulin G and A concentrations in children with HIV infection. AIDS 5:1319-1325. [DOI] [PubMed] [Google Scholar]
- 27.Reuben, J. M., J. A. Turpin, B. N. Lee, M. Doyle, B. Gonik, R. Jacobson, and W. T. Shearer. 1996. Induction of inflammatory cytokines in placental monocytes of gravidae infected with the human immunodeficiency virus type 1. J. Interferon Cytokine Res. 16:963-971. [DOI] [PubMed] [Google Scholar]
- 28.Richard-Miceli, C., and M. Dougados. 2001. Tumour necrosis factor-alpha blockers in rheumatoid arthritis: review of the clinical experience. BioDrugs 15:251-259. [DOI] [PubMed] [Google Scholar]
- 29.Rizzardi, G. P., W. Barcellini, G. Tambussi, F. Lillo, M. Malnati, L. Perrin, and A. Lazzarin. 1996. Plasma levels of soluble CD30, tumour necrosis factor (TNF)-alpha and TNF receptors during primary HIV-1 infection: correlation with HIV-1 RNA and the clinical outcome. AIDS 10:F45-F50. [DOI] [PubMed] [Google Scholar]
- 30.Rubinstein, A. 1986. Pediatric AIDS. Curr. Probl. Pediatr. 16:361-409. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz, L. J., Y. St. Louis, R. Wu, A. Wiznia, A. Rubinstein, and P. Saenger. 1991. Endocrine function in children with human immunodeficiency virus infection. Am. J. Dis. Child. 145:330-333. [DOI] [PubMed] [Google Scholar]
- 32.Siegel, S. A., D. J. Shealy, M. T. Nakada, J. Le, D. S. Woulfe, L. Probert, G. Kollias, J. Ghrayeb, J. Vilcek, and P. E. Daddona. 1995. The mouse/human chimeric monoclonal antibody cA2 neutralizes TNF in vitro and protects transgenic mice from cachexia and TNF lethality in vivo. Cytokine 7:15-25. [DOI] [PubMed] [Google Scholar]
- 33.Song, X. Y., L. Zeng, W. Jin, C. M. Pilo, M. E. Frank, and S. M. Wahl. 2000. Suppression of streptococcal cell wall-induced arthritis by human chorionic gonadotropin. Arthritis Rheum. 43:2064-2072. [DOI] [PubMed] [Google Scholar]
- 34.Stancovski, I., and D. Baltimore. 1997. NF-κB activation: the I κB kinase revealed? Cell 91:299-302. [DOI] [PubMed] [Google Scholar]
- 35.Tang, G., Y. Minemoto, B. Dibling, N. H. Purcell, Z. Li, M. Karin, and A. Lin. 2001. Inhibition of JNK activation through NF-kappa B target genes. Nature 414:313-317. [DOI] [PubMed] [Google Scholar]
- 36.Taylor, G. A., E. Carballo, D. M. Lee, W. S. Lai, M. J. Thompson, D. D. Patel, D. I. Schenkman, G. S. Gilkeson, H. E. Broxmeyer, B. F. Haynes, and P. J. Blackshear. 1996. A pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity 4:445-454. [DOI] [PubMed] [Google Scholar]
- 37.Taylor, P. C., R. O. Williams, and R. N. Maini. 2001. Immunotherapy for rheumatoid arthritis. Curr. Opin. Immunol. 13:611-616. [DOI] [PubMed] [Google Scholar]
- 38.Trede, N. S., A. V. Tsytsykova, T. Chatila, A. E. Goldfeld, and R. S. Geha. 1995. Transcriptional activation of the human TNF-alpha promoter by superantigen in human monocytic cells: role of NF-kappa B. J. Immunol. 155:902-908. [PubMed] [Google Scholar]
- 39.Udalova, I. A., J. C. Knight, V. Vidal, S. A. Nedospasov, and D. Kwiatkowski. 1998. Complex NF-kappa B interactions at the distal tumor necrosis factor promoter region in human monocytes. J. Biol. Chem. 273:21178-21186. [DOI] [PubMed] [Google Scholar]
- 40.Van der Meide, P. H., and H. Schellekens. 1996. Cytokines and the immune response. Biotherapy 8:243-249. [DOI] [PubMed] [Google Scholar]
- 41.Yang, X., Y. Chen, and D. Gabuzda. 1999. ERK MAP kinase links cytokine signals to activation of latent HIV-1 infection by stimulating a cooperative interaction of AP-1 and NF-kappa B. J. Biol. Chem. 274:27981-27988. [DOI] [PubMed] [Google Scholar]