Abstract
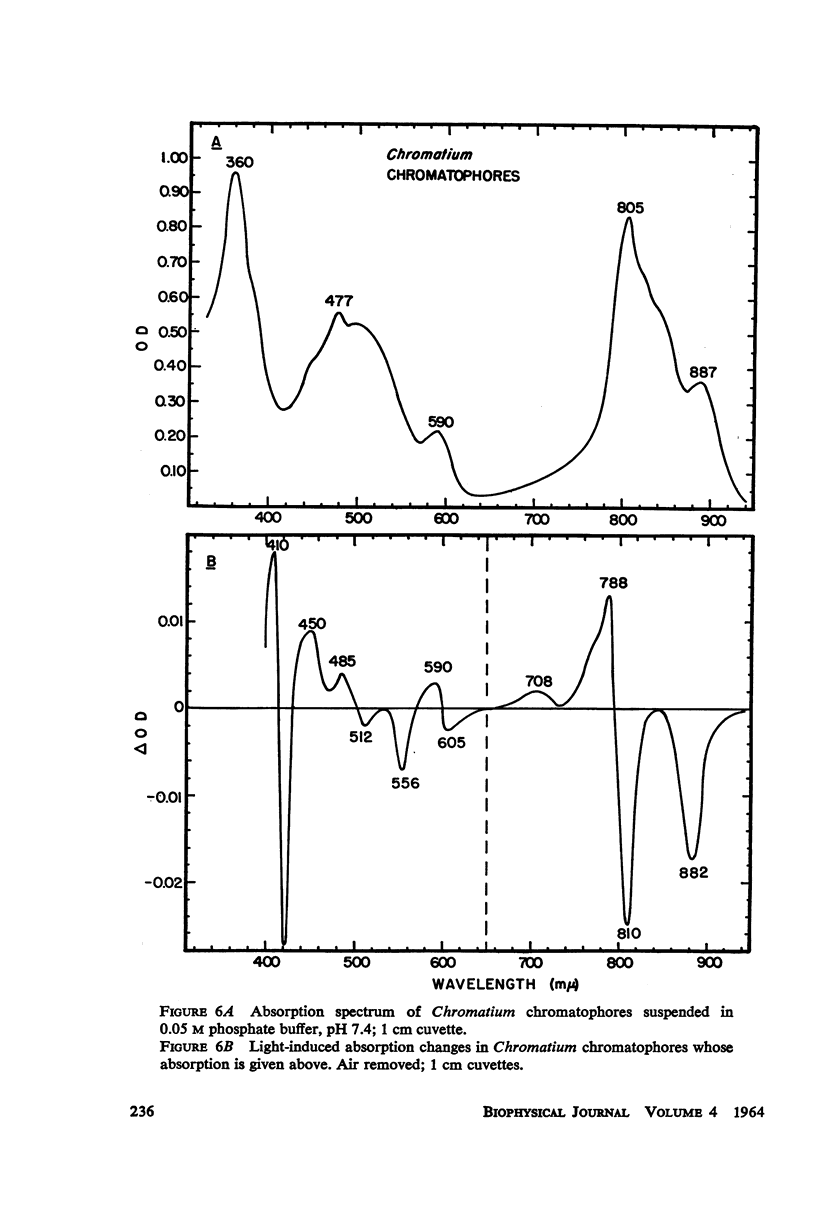
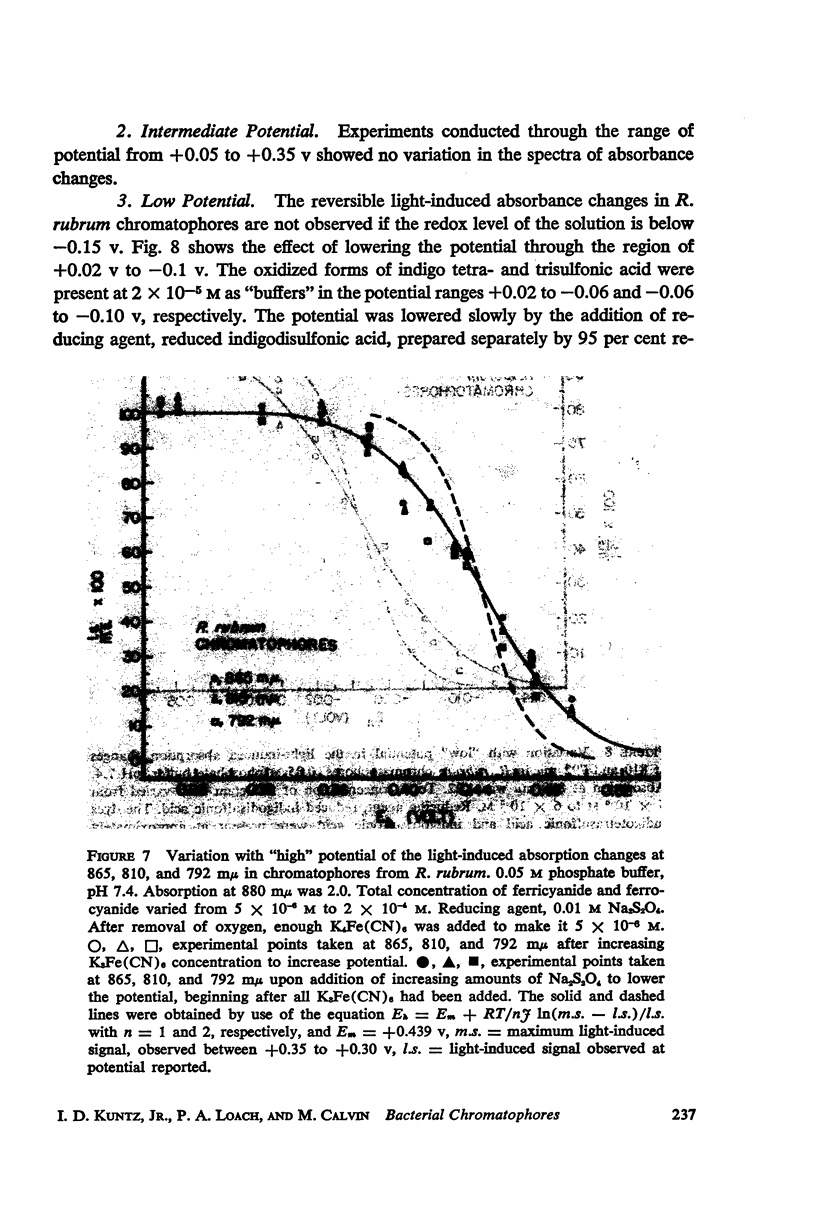
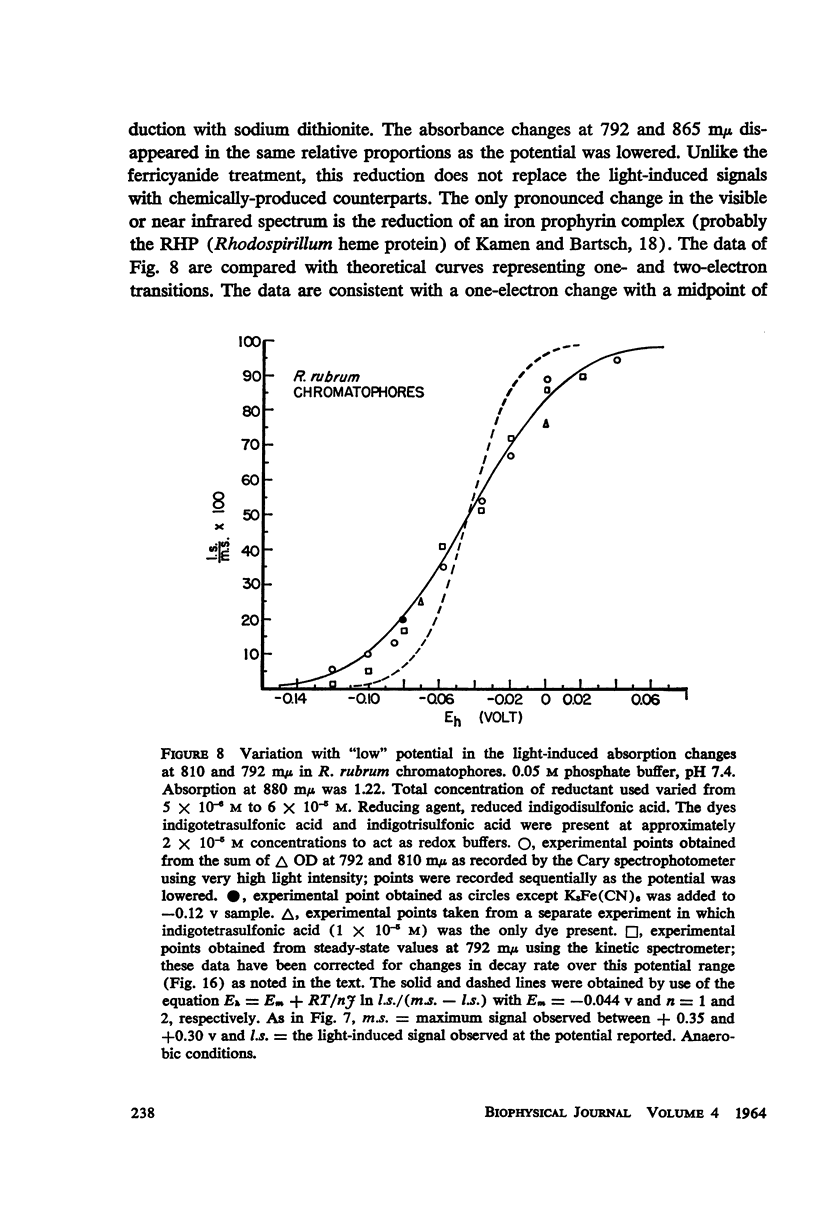
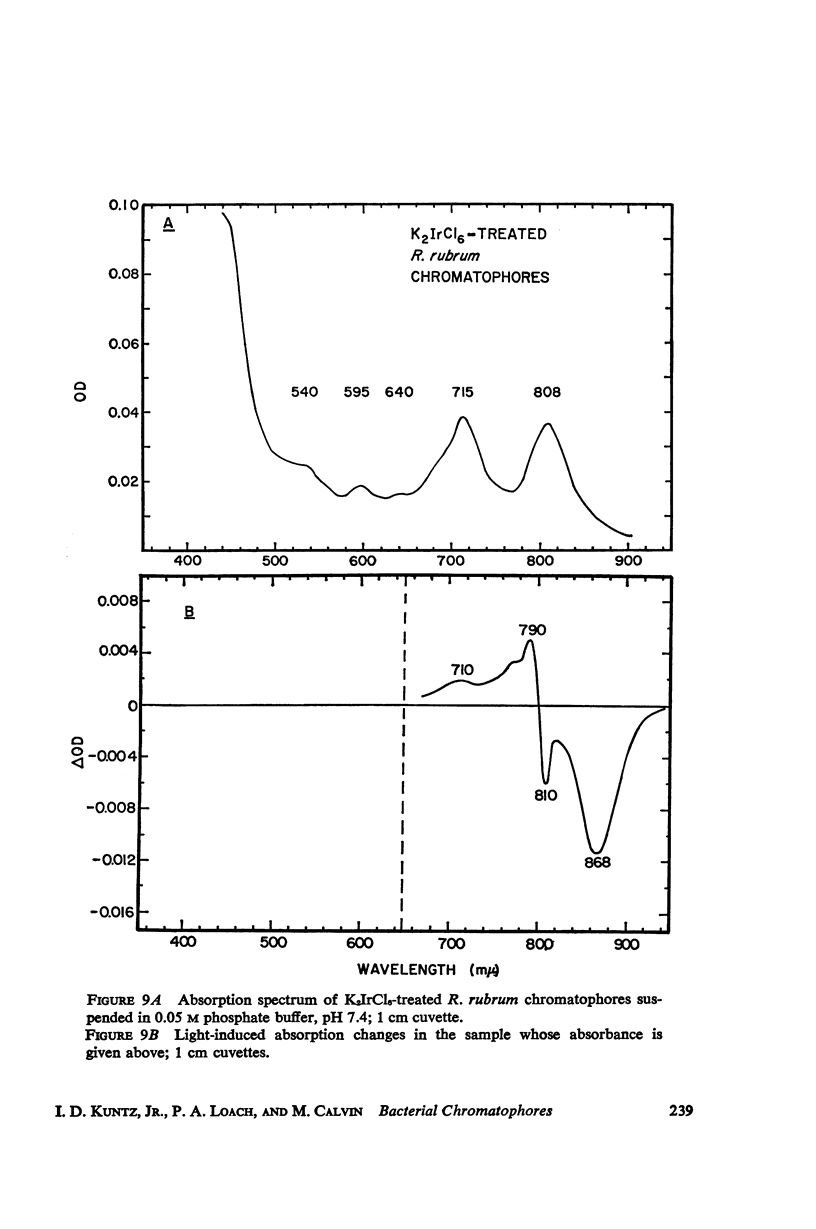
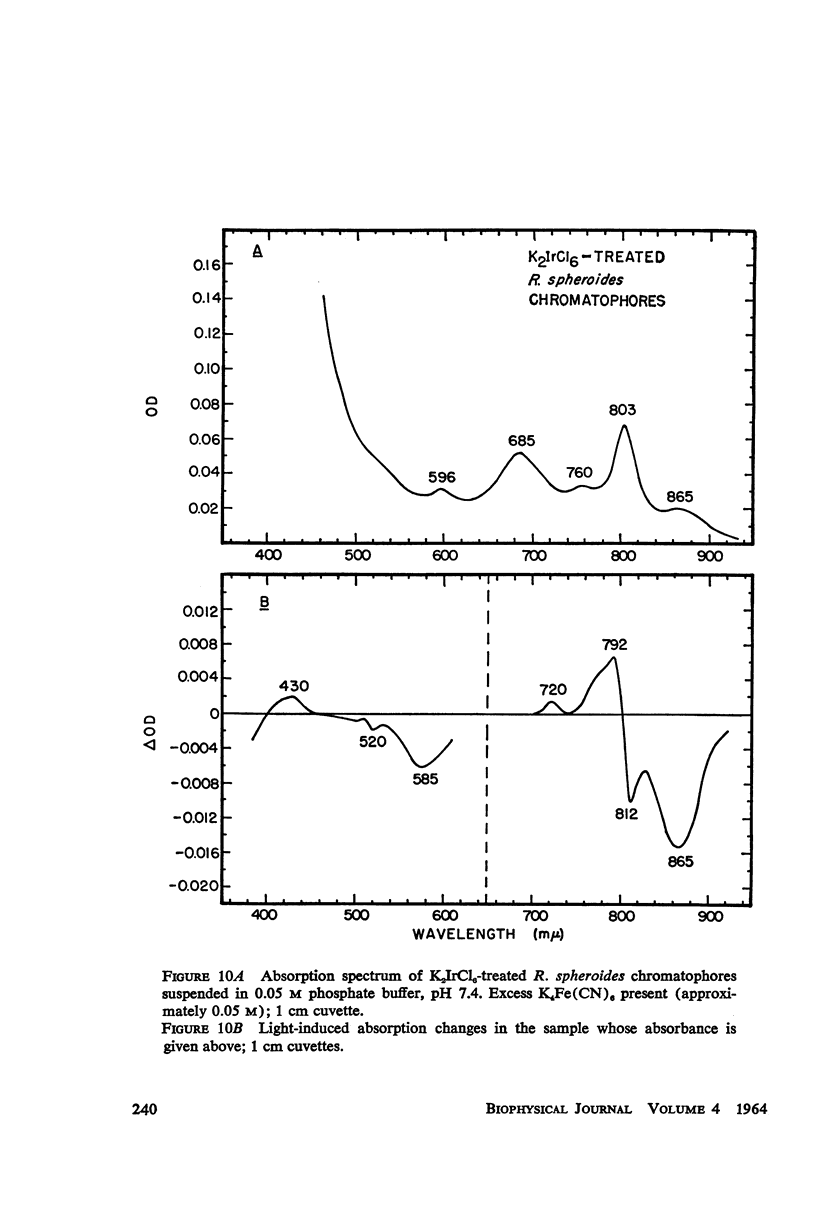
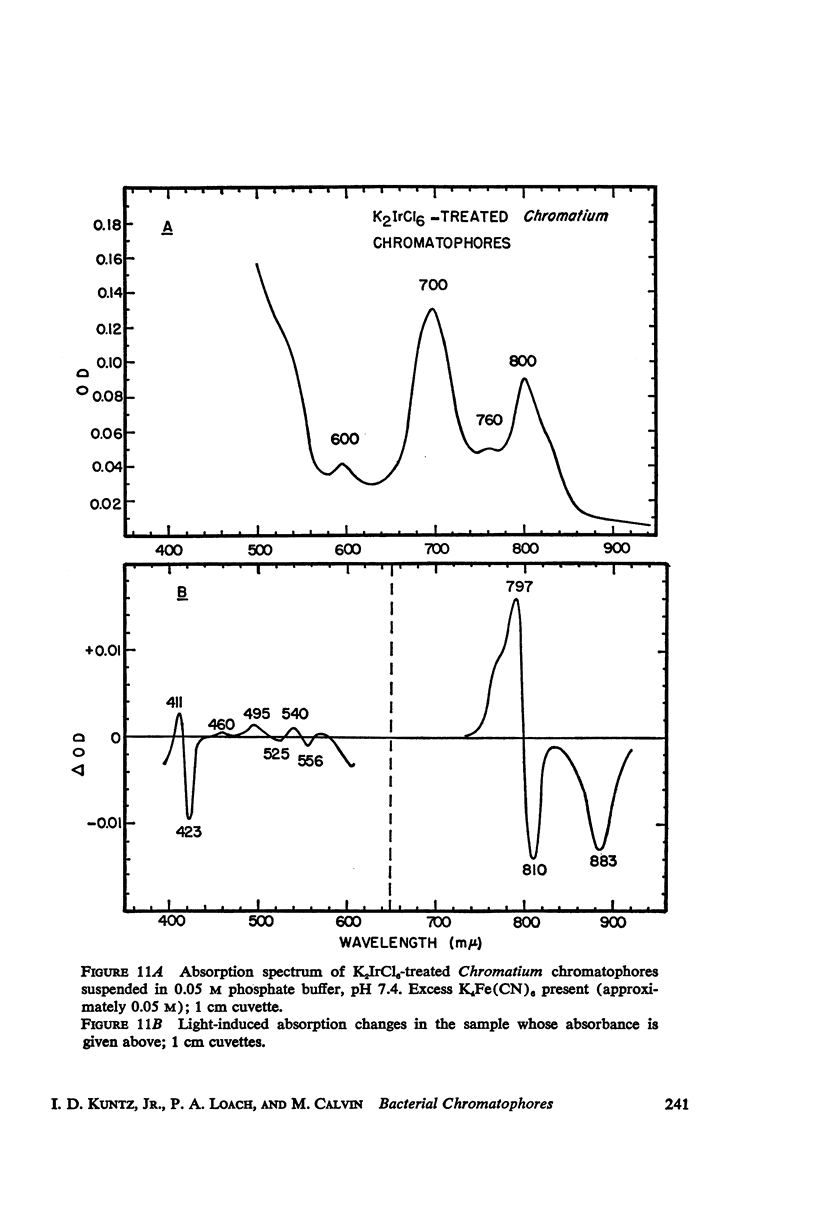
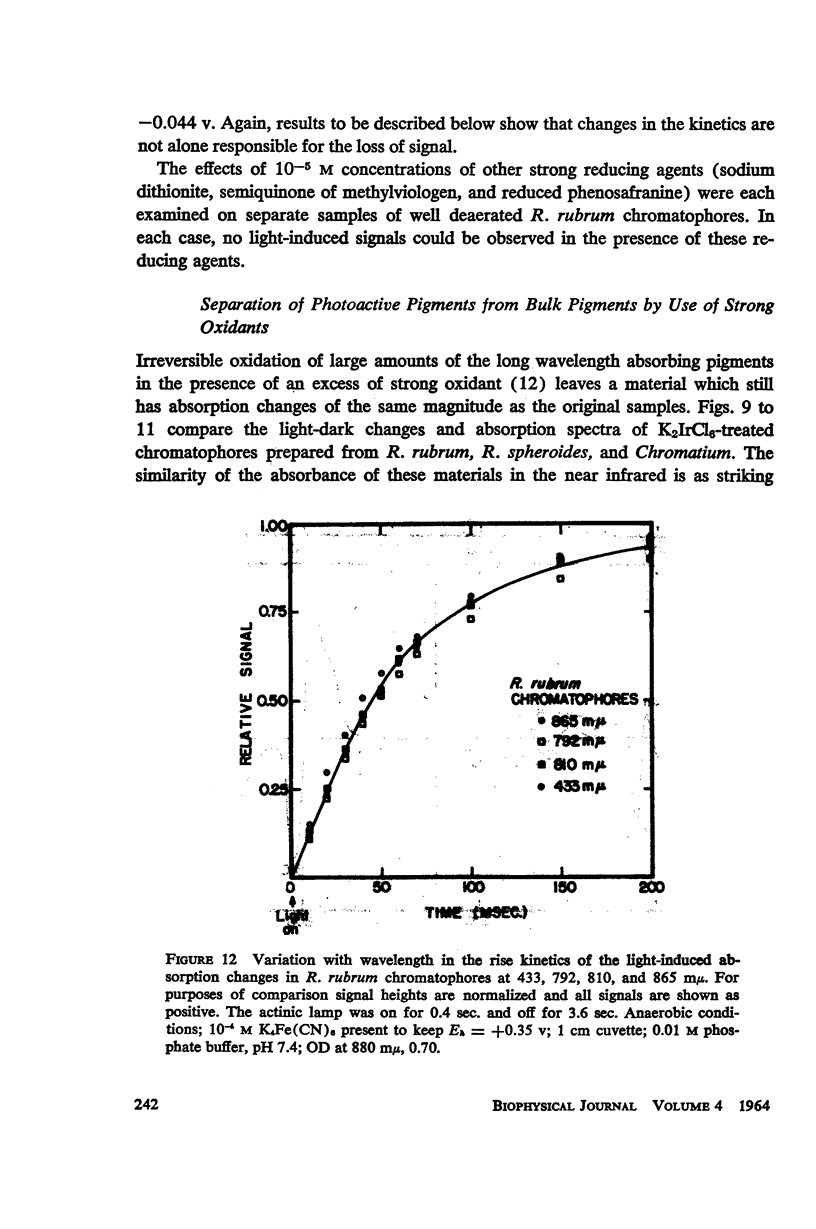
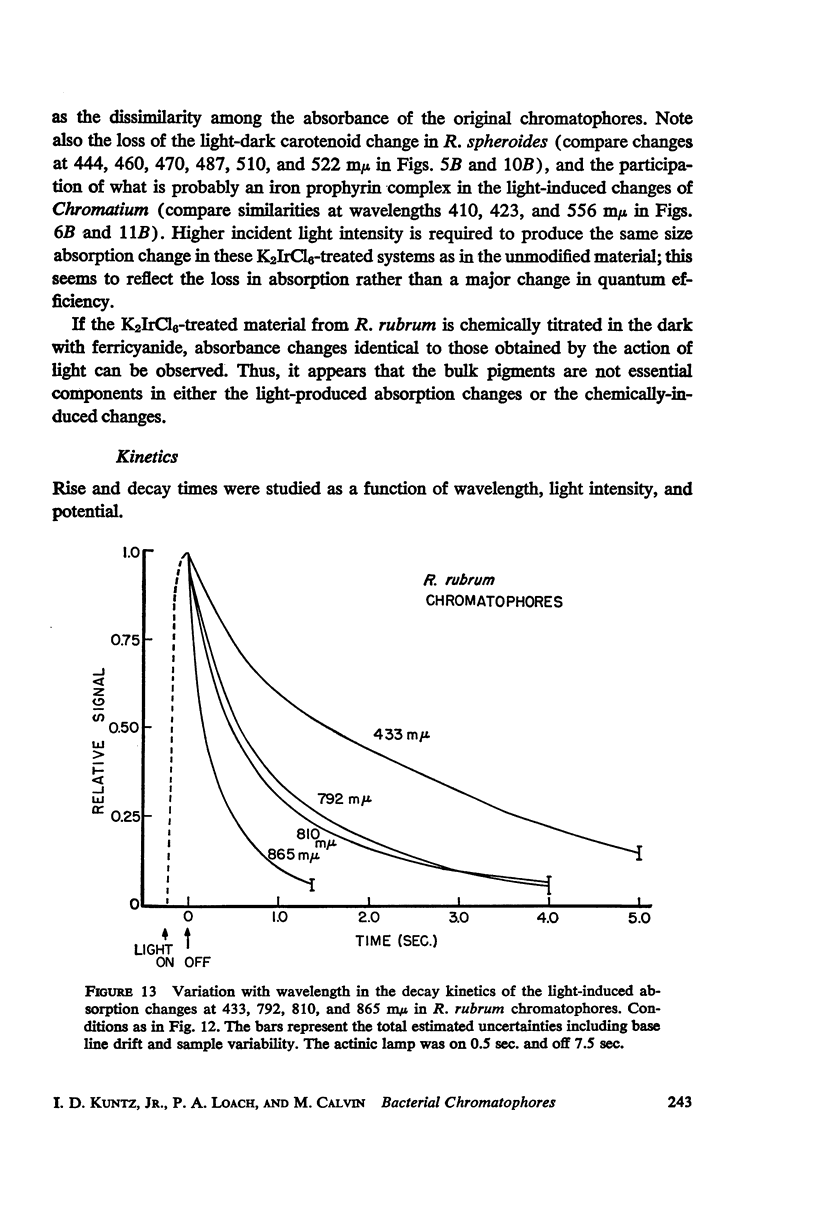
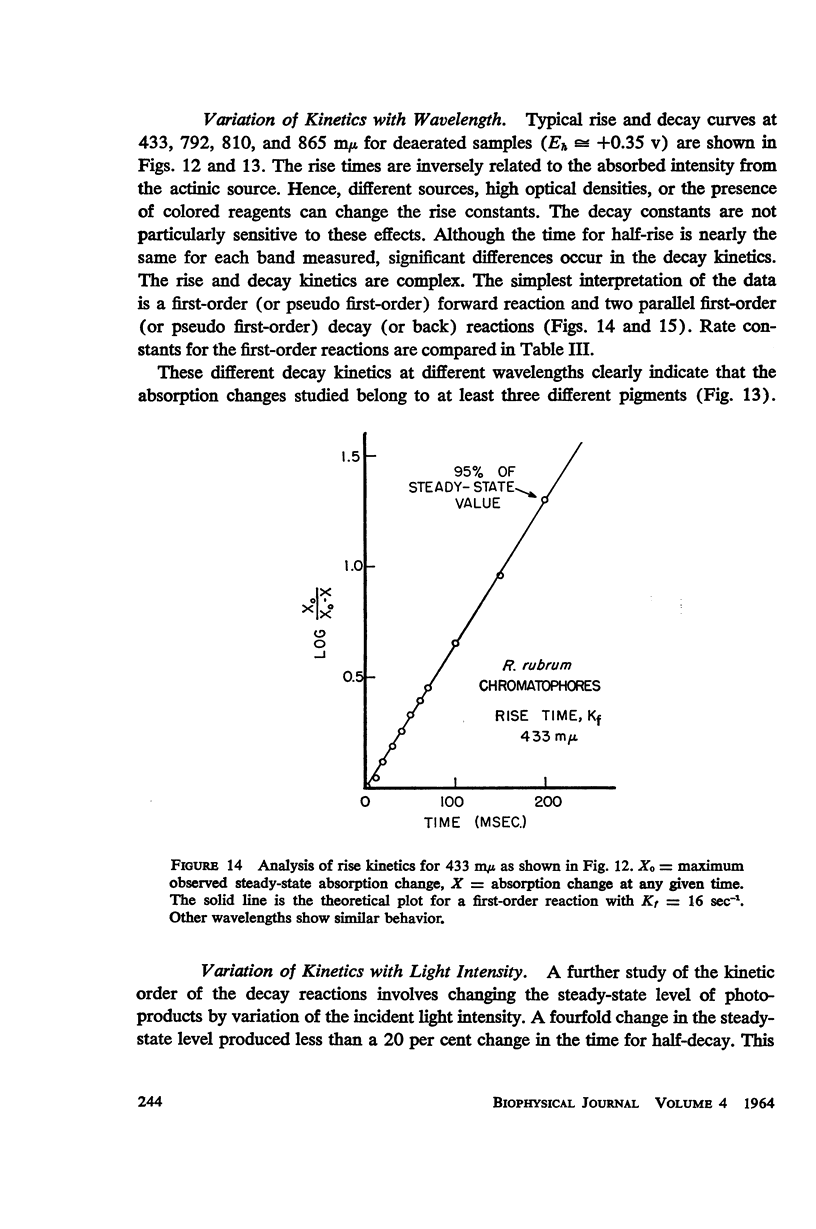
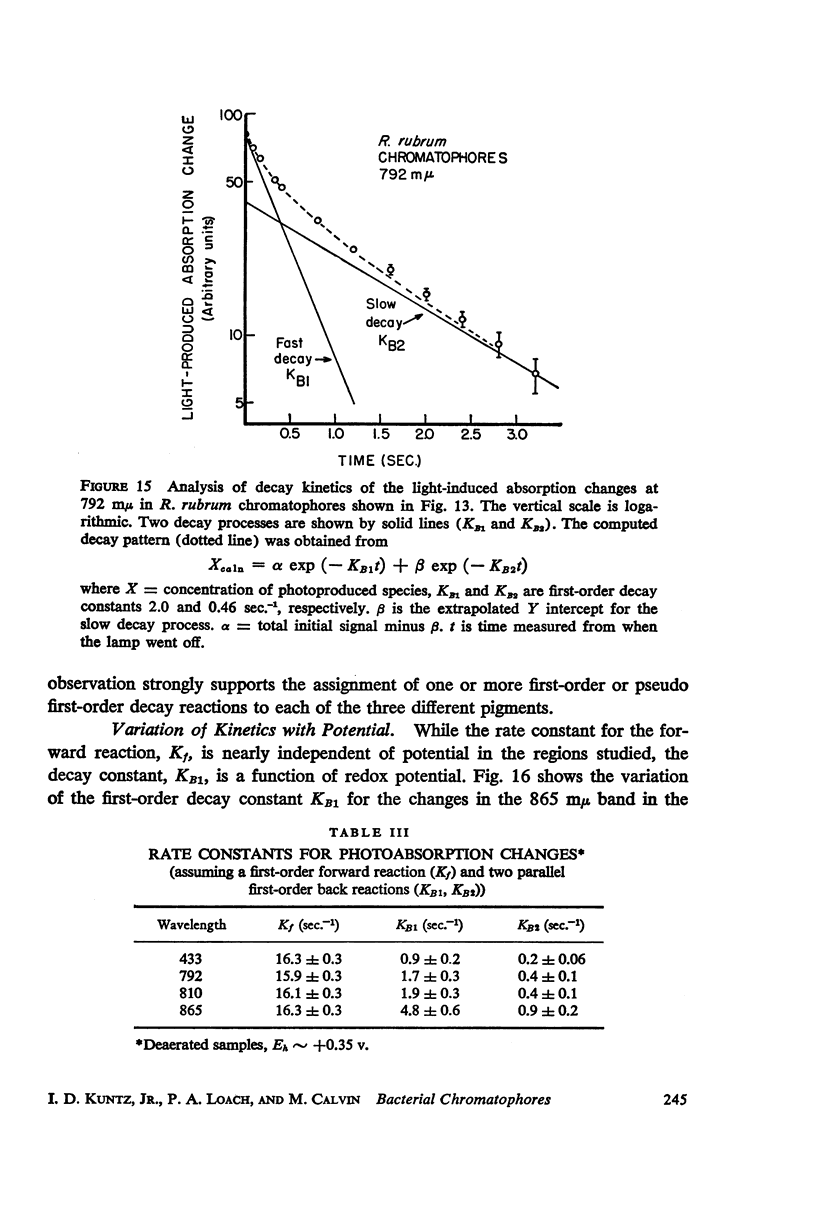
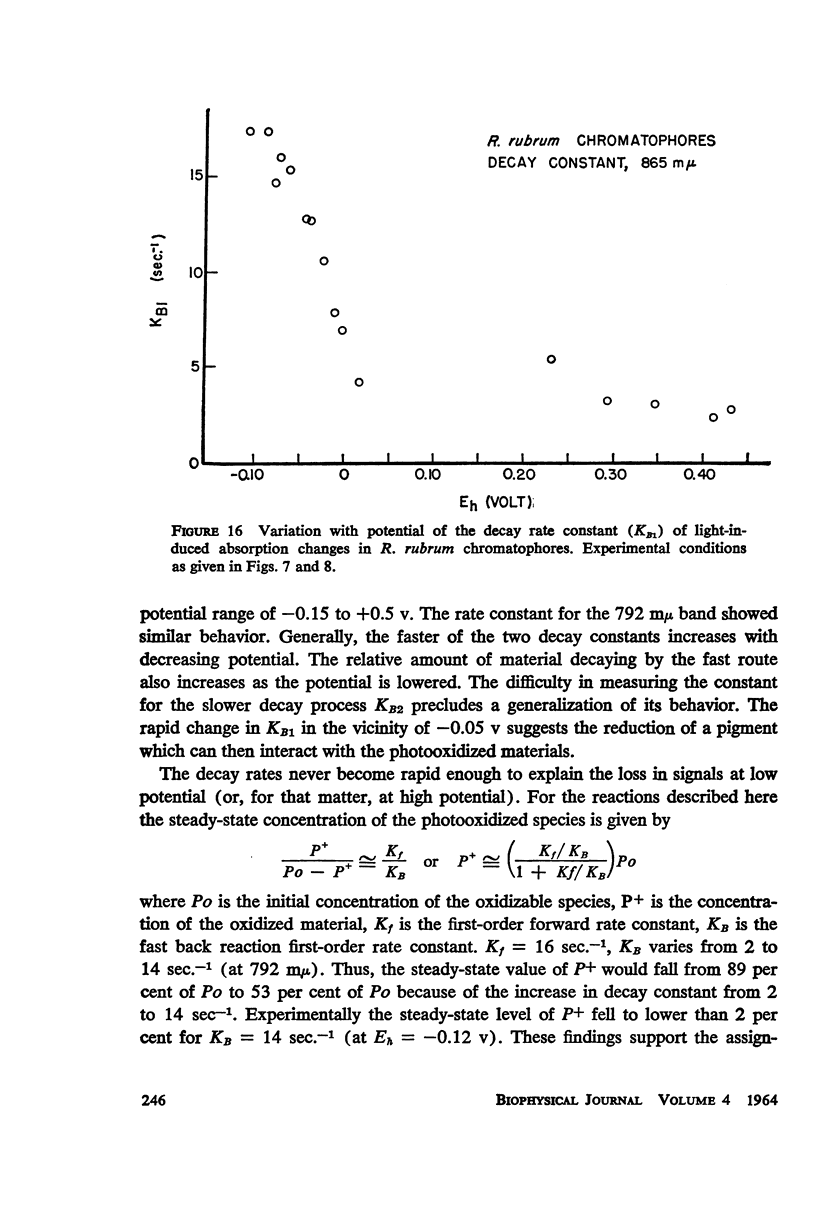
The magnitude and kinetics of photo-induced absorption changes in bacterial chromatophores (R. rubrum, R. spheroides and Chromatium) have been studied as a function of potential, established by added redox couples. No photochanges can be observed above +0.55 v or below -0.15 v. The loss of signal at the higher potential is centered at +0.439 v and follows a one-electron change. The loss of signal at the lower potential is centered at -0.044 v and is also consistent with a one-electron change. Both losses are reversible. A quantitative relationship exists between light-minus-dark and oxidized-minus-reduced spectra in the near infrared from +0.30 to +0.55 v. Selective treatment of the chromatophores with strong oxidants irreversibly bleaches the bulk pigments but appears to leave intact those pigments responsible for the photo- and chemically-induced absorption changes. Kinetic studies of the photochanges in deaerated samples of R. rubrum chromatophores revealed the same rise time for bands at 433, 792, and 865 mμ (t½ = 50 msec.). However, these bands had different decay rates (t½ = 1.5, 0.5, 0.15 sec., respectively), indicating that they belong to different pigments. Analysis of the data indicates, as the simplest interpretation, a first-order (or pseudo first-order) forward reaction and two parallel first-order (or pseudo first-order) decay reactions at each wavelength. These results imply that all pigments whose kinetics are given are photooxidized and the decay processes are dark reductions. These experiments are viewed as supporting and extending the concept of a bacterial photosynthetic unit, with energy migration within it to specific sites of electron transfer.
Full text
PDF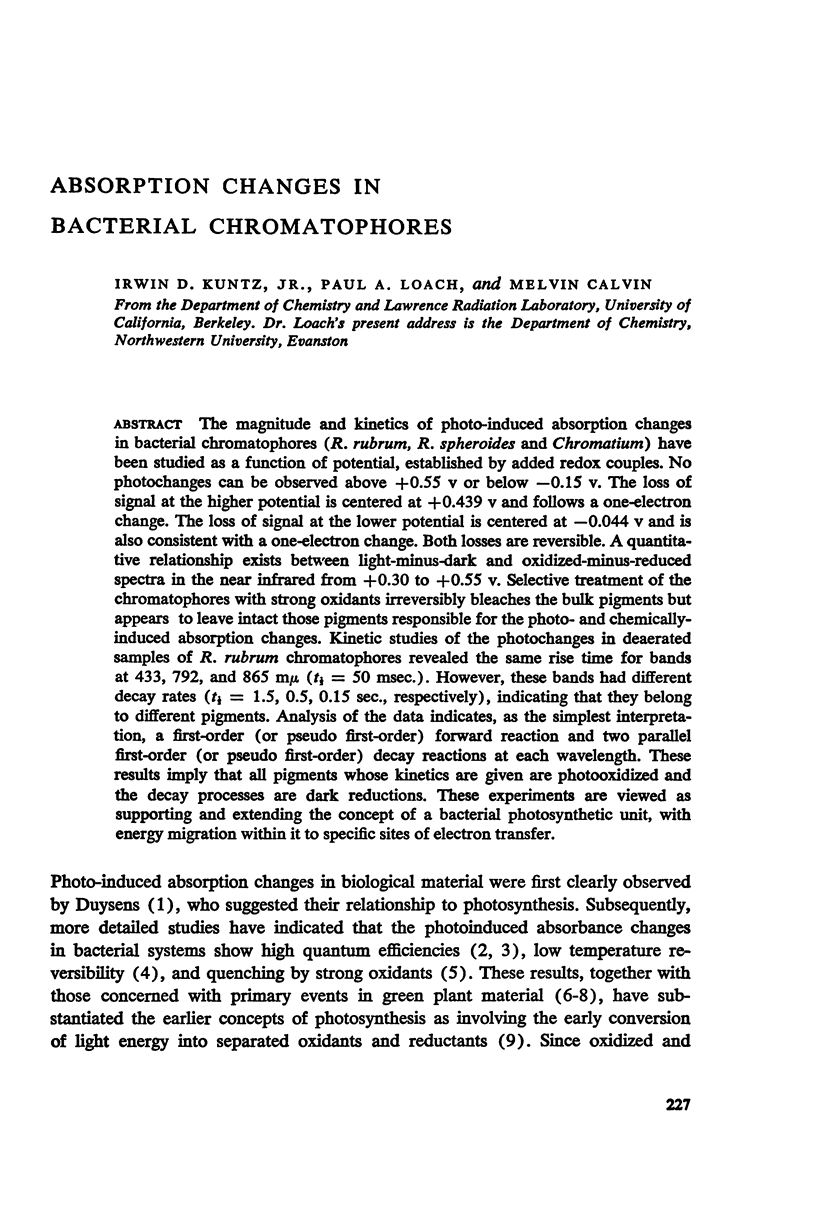

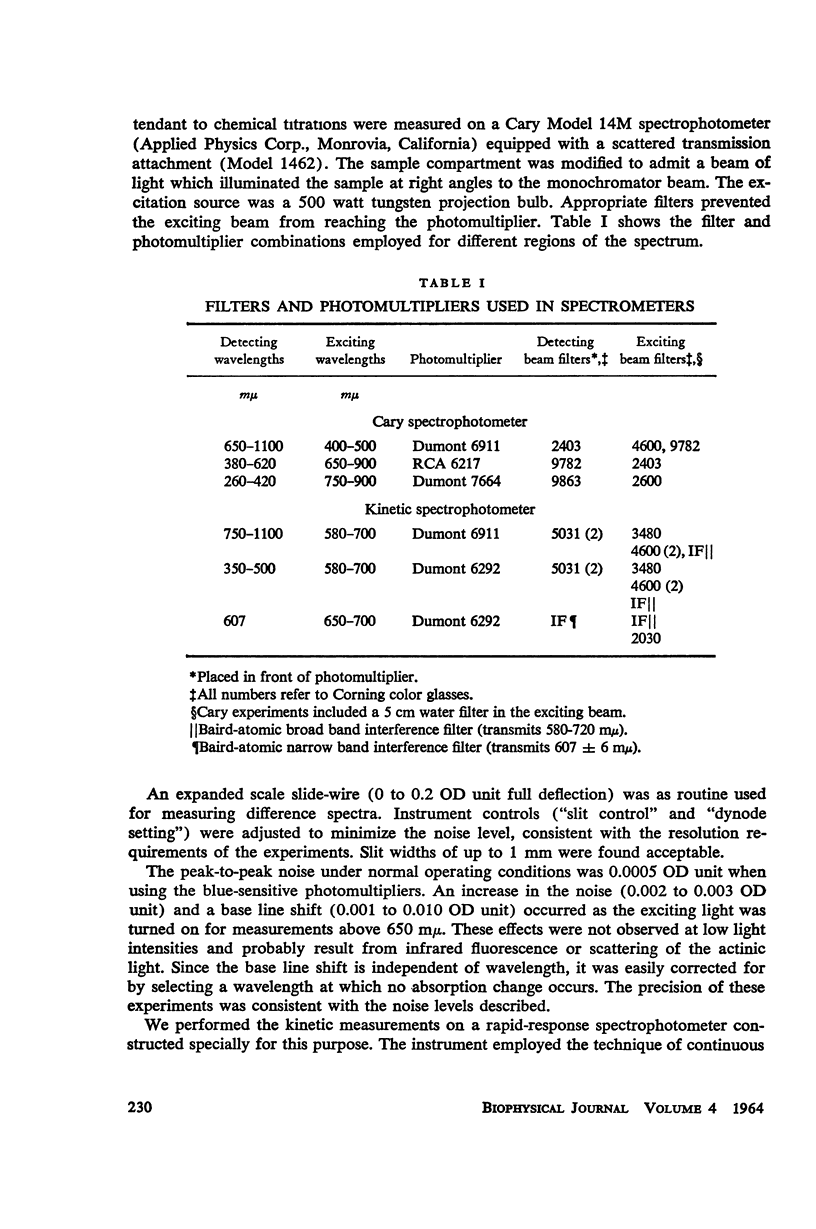
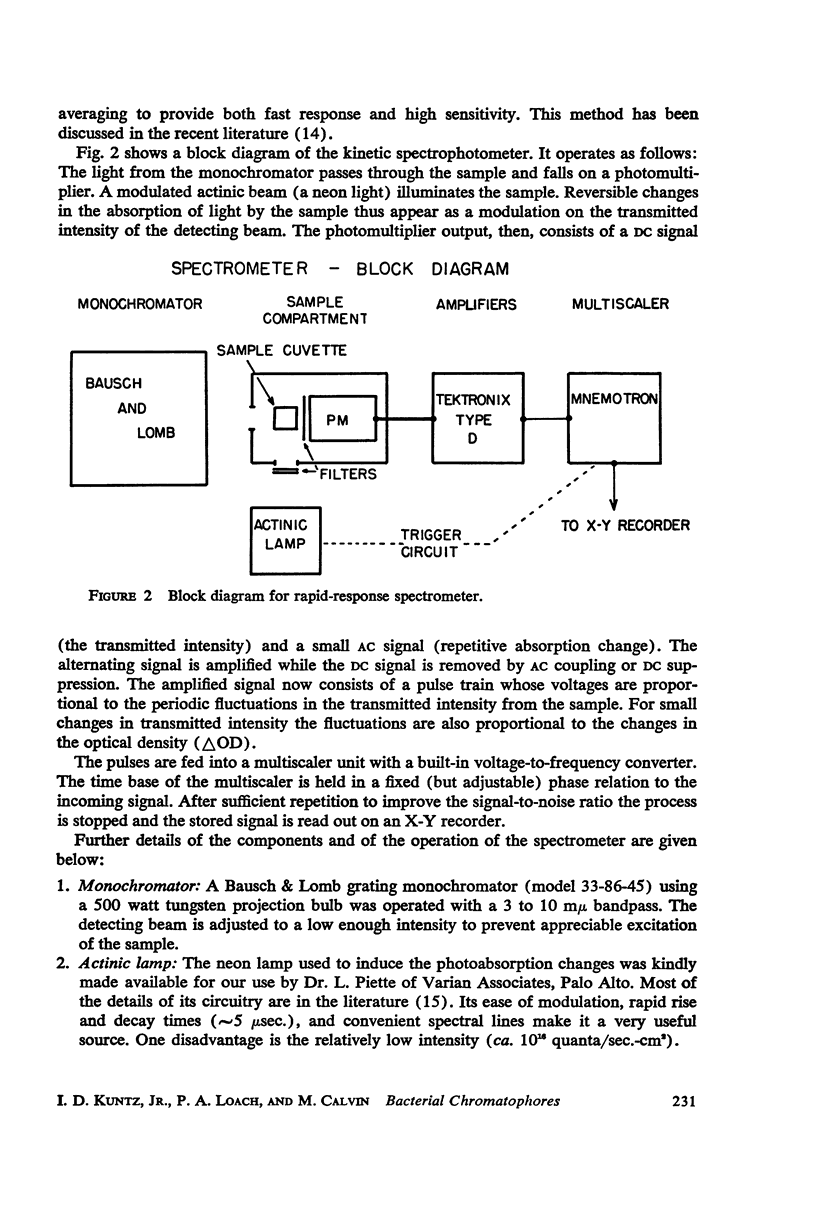



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W., Clayton R. K. THE FIRST STEP IN PHOTOSYNTHESIS: EVIDENCE FOR ITS ELECTRONIC NATURE. Proc Natl Acad Sci U S A. 1960 Jun;46(6):769–776. doi: 10.1073/pnas.46.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin M., Androes G. M. Primary Quantum Conversion in Photosynthesis: Low-temperature photoparamagnetism bespeaks electron transfer and migration as the earliest event. Science. 1962 Nov 23;138(3543):867–873. doi: 10.1126/science.138.3543.867. [DOI] [PubMed] [Google Scholar]
- HARBURY H. A. Oxidation-reduction potentials of horseradish peroxidase. J Biol Chem. 1957 Apr;225(2):1009–1024. [PubMed] [Google Scholar]
- KOK B. Partial purification and determination of oxidation reduction potential of the photosynthetic chlorophyll complex absorbing at 700 millimicrons. Biochim Biophys Acta. 1961 Apr 15;48:527–533. doi: 10.1016/0006-3002(61)90050-6. [DOI] [PubMed] [Google Scholar]
- OLSON J. M., CHANCE B. Oxidation-reduction reactions in the photosynthetic bacterium Chromatium. II. Dependence of light reactions on intensity of irradiation and quantum efficiency of cytochrome oxidation. Arch Biochem Biophys. 1960;88:40–53. doi: 10.1016/0003-9861(60)90194-6. [DOI] [PubMed] [Google Scholar]
- Sogo P. B., Pon N. G., Calvin M. PHOTO SPIN RESONANCE IN CHLOROPHYLL-CONTAINING PLANT MATERIAL. Proc Natl Acad Sci U S A. 1957 May 15;43(5):387–393. doi: 10.1073/pnas.43.5.387. [DOI] [PMC free article] [PubMed] [Google Scholar]



