Abstract
Synthetic oligonucleotides containing CpG motifs in specific sequence contexts have been shown to induce potent immune responses. We have evaluated mucosal administration of two immunostimulatory sequence (ISS)-containing phosphorothioate-stabilized oligonucleotides for antiherpetic efficacy in animal models. The ISS oligonucleotides, suspended in phosphate-buffered saline, were tested in mouse and guinea pig vaginal models of herpes simplex virus type 2 (HSV-2) infection. For comparison, groups of untreated, non-ISS oligonucleotide-treated, and acyclovir-treated animals also were monitored. The results indicated that vaginal epithelial application of ISS (up to 6 h after viral inoculation) with mice lethally challenged with HSV-2 delayed disease onset and reduced the number of animals that developed signs of disease (P = 0.003). ISS application significantly increased survival rates over those of controls (P = 0.0014). The ISS also impacted an established infection in the guinea pig model of HSV-2 disease. A single administration of ISS (21 days after viral inoculation) significantly reduced the frequency and severity of HSV-2 lesions compared to results with non-ISS oligonucleotide-treated and untreated guinea pigs (P < 0.01). HSV-2 is shed from the vaginal cavity of the guinea pig in the absence of lesions, similar to the case with humans. As an additional indication of ISS efficacy, the magnitude of viral shedding also was significantly reduced in ISS-treated animals (P < 0.001). These effects appeared to be immunologically mediated, since ISS had no direct effect on HSV-2 replication in vitro using standard plaque assays. These data suggest that ISS may be useful in the treatment and control of genital herpes in humans.
Treatment of herpes simplex virus type 2 (HSV-2) infection is limited to the use of topical, oral, or intravenous antiviral nucleoside analogs (11, 27). Evidence of an increasing incidence and prevalence of HSV-2 infections (12, 26, 58) suggests that more efficacious control strategies are needed. Clinical infection occurs in 20 to 30% of adults (7, 43), while up to 85% of females develop HSV-2 antibodies in their lifetime (20). Recent preclinical studies have suggested that direct application of vaccines or microbicides to the vaginal mucosa may offer an alternative to traditional chemotherapy (5, 8, 13, 30, 43). Immunotherapy of recurrent HSV-2 infections through vaccination and immunomodulation has been shown to be effective in preliminary clinical trials (26, 47, 49, 50).
Studies have indicated that cell-mediated immunity is necessary for protection against HSV-2 infection and disease (17, 37-40, 46, 52). The cytokine milieu associated with a Th1 immune response (e.g., gamma interferon [IFN-γ], interleukin 12 [IL-12], etc.) has been shown to be important for controlling herpetic disease (15, 28, 35, 38, 57). For these reasons, we have tested DNA-based immunotherapy known to enhance Th1-type immune responses for the control of experimental genital herpes.
Immunostimulatory CpG DNA sequences (ISS), originally described for mycobacterial DNA (reviewed in references 21 and 23), have been shown to be active in a variety of contexts including bacterial, plasmid, and synthetic oligonucleotide DNA (21, 23). ISS, depending in part on the sequence context, have a variety of immunomodulating properties that can stimulate humoral and cellular immune responses (23). ISS have been reported to induce B-cell proliferation and immunoglobulin production, natural killer cell activation, and secretion of IFN-α, -β, and -γ, IL-6, IL-12, and IL-18 (2, 22-24, 44, 54). Recently an indication of the mechanism of action of ISS has been reported and involves the toll-like receptor 9 (3, 25, 51). Bauer and colleagues (3) examined a series of CpG motifs with various activities in mouse and human. Their results indicated that murine and human TLR9 respond to different CpG motifs helping to explain the different stimulation afforded by certain ISS. To date, ISS have been tested as adjuvants designed to elicit stronger and more appropriate immune responses to vaccines (13, 32) and to redirect Th2 immune responses to allergens toward nonallergic Th1 type responses (33, 41, 53).
To our knowledge, no work has been done to evaluate the direct efficacy of ISS alone against primary or recurrent HSV disease. Based on the knowledge that selected ISS stimulated Th1 immune responses and that Th1 responses were important in the control of HSV infections, we hypothesized that ISS treatment would afford some protection against HSV infections. We further speculated that induction of local responses, those at the site of the infection, likely would provide the greatest effect. To test this hypothesis, we evaluated the antiherpesvirus activity of two ISS-containing, phosphorothioate-stabilized oligonucleotides and tested their efficacy in mouse and guinea pig models of genital HSV-2 infection. These ISS are potent immunostimulators in a broad range of animal species including mice, rabbits, and primates (53) and in cultured human cells (33), making them good candidates for controlling HSV infection both in animal models and in humans. One of the ISS (1018 ISS) recently was shown to have immunostimulatory activity in human clinical trials with ragweed allergy sufferers when linked to ragweed allergen (10) and when used as an immunopotentiater for hepatitis B virus vaccine (32; S. A. Halperin, G. Van Nest, B. Halperin, B. Smith, S. Abtahi, H. Whiley, and J. Eiden, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. G-1578, 2001).
We observed that a single topical vaginal application of an ISS-containing oligonucleotide in phosphate-buffered saline provided significant protection in the mouse model against vaginal HSV-2 challenge when applied after viral inoculation. To establish if ISS therapy could affect an existing infection, in the guinea pig model of recurrent genital HSV-2 infection we showed that administration of a single dose of ISS significantly reduced the frequency of recurrent lesions and the magnitude of viral shedding. These results indicate the potential efficacy of mucosal application of ISS-containing oligonucleotides in the prevention and/or treatment of genital herpes and other mucosal pathogens.
MATERIALS AND METHODS
ISS production.
The active ISS-containing phosphorothioate-stabilized oligonucleotides used in these studies were 1018 ISS (sequence: TGA CTG TGA ACG TTC GAG ATG A) and C106 ISS (sequence: TCG TCG AAC GTT CGT TAA CGT TCG). 1019 non-ISS (sequence: TGA CTG TGA AGG TTA GAG ATG A) and 1040 non-ISS (sequence: TGA CTG TGA ACC TTA GAG ATG A) were control, inactive sequences. 1018 ISS is 22 bases in length and contains two CpG motifs. C106 ISS is 24 bases in length and contains 6 CpG motifs. The 22-base 1040 and 1019 non-ISS oligonucleotides both contain two base changes from 1018 ISS that eliminate both CpG motifs. Oligonucleotides were purchased from Avecia (Boston, Mass.).
Cells and viruses.
Vero cells (ATCC CCL81) were cultured in Dulbecco's modified Eagle's medium containing heat-inactivated 10% newborn calf serum (Life Technologies, Gaithersburg, Md.) plus 50 μg of penicillin/ml, 50 μg of streptomycin/ml, and 0.15 μg of Fungizone (Life Technologies)/ml at 37°C and 5% CO2. Clarified stocks of HSV-2 strains 186 (37) and MS (ATCC VR-540) were prepared from infected Vero monolayers and stored at −80°C until used. Titers of virus were determined by standard plaque assays as described below.
Animals.
Female outbred Swiss Webster mice (Harlan, Indianapolis, Ind.) weighing 20 to 22 g and Hartley guinea pigs (Charles River [breeding] Laboratories, Wilmington, Mass.) weighing 350 to 450 g were housed in American Association for Laboratory Animal Care-approved quarters and provided with unlimited access to food and water. Animals were acclimated for at least 7 days after arrival and prior to any procedures. For each study, groups of animals (n = 12 to 18) were generated randomly prior to initiating treatment.
Mouse vaginal model of HSV-2 infection.
Mice were treated with Depo-Provera (UpJohn, Kalamazoo, Mich.) hormone injections subcutaneously (150 mg/kg of body weight/dose) 7 and 1 day prior to viral inoculation (38). A lethal dose of HSV-2 strain 186 (104 PFU) was instilled in the vaginal cavity following wet and then dry vaginal swabbing with a calcium alginate swab (Fisher Scientific, Pittsburgh, Pa.). Animals were assessed daily for symptomatic disease through 14 days postinoculation (p.i.). Survival was followed through 21 days p.i. As an additional indicator of infection, vaginal swabs were collected and then tested for viral content on Vero cells.
Treatments.
In the initial study, groups of mice (n = 15) received 100 μg of 1018 ISS oligonucleotide in phosphate-buffered saline (PBS) (5 mg/ml) intravaginally by instillation (10 μl) and topically (10 μl) in the anogenital area at 2 or 6 h after viral inoculation. Additional groups of mice served as controls and were vehicle treated or untreated. In a second study, groups of mice (n = 16) were treated with one of two ISS oligonucleotides (1018 ISS or C106 ISS), one of two non-ISS control oligonucleotides (1019 non-ISS or 1040 non-ISS), PBS, or acyclovir (Zovirax ointment; GlaxoSmithKline, Research Triangle Park, N.C. (3 times per day for 7 days) or were not treated.
Guinea pig model of genital herpes.
On the day of inoculation, the vaginal closure membrane was ruptured with a premoistened calcium alginate swab. The vaginal vault was swabbed subsequently with a dry calcium alginate swab, and 105.7 PFU of the MS strain of HSV-2 was instilled into the vaginal vault with a syringe and a 20-gauge plastic catheter (48). This dose is sublethal while providing infection of nearly every inoculated animal. During the acute genital infection, animals were evaluated daily through 14 days p.i. for genital skin disease and urinary retention. Disease was quantified by a skin lesion scoring system ranging from 0, representing no disease, to 4, representing severe disease characterized by large ulcers with maceration (48). Following acute disease, animals were distributed to produce statistically similar groups based upon disease severity. Daily scoring of each animal proceeded from day 21 through 56 p.i. to establish the frequency of external recurrent herpetic lesions.
Treatments.
Guinea pigs were distributed to one of four groups (n = 12). Animals in two of the groups received 1 mg of oligonucleotide in PBS (5 mg/ml) via vaginal instillation (100 μl) and topical anogenital application (100 μl) 21 days after inoculation, a time after animals have recovered from the primary disease and have established latent infections from which virus can reactivate spontaneously, leading to disease-free shedding or recurrent lesion formation (42). The oligonucleotides delivered were the 1018 ISS or the 1019 non-ISS. A third group received a course of acyclovir (Zovirax; GlaxoSmithKline) beginning 6 h p.i. and continuing three times a day for the next 7 days. The animals in the final group were not treated and served as a baseline for disease. All animals were scored daily for genital lesions through day 56 p.i.
Viral shedding detection.
Guinea pigs spontaneously shed HSV-2 from the vaginal cavity even in the absence of signs of disease (42). Unfortunately, infectious virus is recovered from no more than 1% of the collected swabs, limiting the usefulness of such assays, since they would require the use of a prohibitively large number of animals to produce statistically meaningful data. Viral DNA, however, can be detected in 10 to 20% of the vaginal swabs from latently infected guinea pigs (Pyles and Stanberry, unpublished findings) allowing for the study of viral shedding frequencies and comparisons of the magnitudes. Therefore, to assess the impact of the oligonucleotides on shedding, the vaginal cavity of each guinea pig was swabbed daily with a calcium alginate-tipped swab from 22 through 43 days p.i. DNA was extracted from each swab sample and subjected to quantitative PCR for HSV-2 DNA. Template preparation was completed using the QIAmp DNA extraction system (Qiagen, Inc., Chatsworth, Calif.) and included mock swab blanks as monitors for sample contamination. Each PCR mixture contained 1× reaction buffer including 15 mM MgCl2 (PCR core kit; Boehringer Mannheim Biochemicals, Indianapolis, Ind.), 50 uM deoxynucleoside triphosphate mix, 50 pM of each primer (Life Technologies), and 3 U of Taq DNA polymerase (Boehringer Mannheim Biochemicals). The primers for HSV-2 detection targeted the DNA polymerase gene (HSVDP5, 5′-ATGGTGAACATCGACATGTACGG-3′; and HSVDP3-2, 5′-CCTCCTTGTCGAGGCCCCGAAAC-3′) and generated a single amplimer of 391 bp (19). A separate reaction was performed for each of the specimens to address template quality and quantity. For this control, the single-copy guinea pig albumin gene served as a target for amplification using a second set of primers (lacabf, 5′-AGTCCATTTCTTGTCTGTCTCTCT-3′; and lacabr, 5′-CTGGGGAACAAAGTAAGAGTCAAC-3′). The resulting 498-bp amplimer allowed for normalization of DNA concentration and a more quantitative estimate of the HSV-2 burden in each specimen. Positive specimens were compared to amplification of a series of 10-fold serial dilutions of established genomic equivalents using MS HSV-2 stocks. The reactions were run in a GeneAmp PCR System 9600 machine (Perkin-Elmer Corp., Norwalk, Conn.) beginning with an initial “hot start” at 95°C for 2 min and then 35 cycles consisting of denaturation at 95°C for 1 min, annealing for 1 min at 65°C, 72°C extension for 1 min 30 s, and a final 7-min extension at 72°C.
The amplification products of each sample, positive and negative controls, and the series of known standards were separated on 1.5% agarose gels and then Southern blotted on nitrocellulose membranes that were prehybridized at 59°C for 4 to 6 h in 10 to 20 ml of 2× SSC (0.3 M NaCl, 0.03 M Na citrate), 6× Denhardt's solution (0.12% polyvinylpyrrolidone, 0.12% Ficoll, 0.12% bovine serum albumin), 200 μg of salmon sperm DNA/ml, and 0.1% sodium dodecyl sulfate. Hybridization was performed for 12 to 14 h at 59°C with 2 pmol of the appropriate probe end-labeled with digoxigenin-11-ddUTP (Boehringer Mannheim Biochemicals) (GGCGTAGTAGGCGGGGATGTCGCG, HG52, bp 64939 to 64962; or CTTCAGATCATCAAGATAGTC, albumin, bp 1550 to 1570)/ml. After hybridization, the blots were washed in 2× SSC and 0.1% sodium dodecyl sulfate for 15 min at 49°C. Chemiluminescence detection was performed as recommended by the manufacturer (Roche Biochemicals; Indianapolis, Ind.). Blots then were exposed to BioMax MR X-ray film (Eastman Kodak; Rochester, N.Y.). Densitometry was completed with a ChemImager 4400 and AlphaEase software (Alpha-Innotech; San Leandro, Calif.).
In vitro antiviral analysis of ISS-containing oligonucleotides.
Direct plaque reduction assays were performed for strains of HSV-2. Vero cells were seeded into 24-well culture dishes (Fisher Scientific) at 90 to 100% confluence. Twenty-four hours, 10 min, or 30 s prior to viral inoculation, the culture medium was aspirated and replaced with 1.0 or 10 μg of each oligonucleotide/10 μl in 1 ml of culture medium. A set of wells received an equivalent volume of PBS. At time 0, the medium was aspirated and replaced with 0.2 ml of culture medium containing 50 PFU of HSV-2 strain 186. After an hour's adsorption, 1 ml of blocking medium (growth medium supplemented with 0.3% human gamma globulin) was added to each well. In a separate set of wells, the viral inocula (50 PFU of HSV-2 strain 186) were generated by mixing the virus with 1 or 10 μg of oligonucleotide or PBS for 10 min prior to addition to the cells. Three days later, when well-defined plaques were visualized, the cells were fixed in methanol and stained in 0.8% crystal violet (Sigma, St. Louis, Mo.), rinsed, and then air dried. Plaque counts were recorded in triplicate, and then averages were calculated.
Statistical analysis.
Data were analyzed by log rank analyses or in some cases by one-way analysis of variance (ANOVA) using Prism software (GraphPad, San Diego, Calif.). A P value of <0.05 was considered significant with 95% confidence. Mean values in the text are presented ± standard errors of the means [SEM].
RESULTS
Topical administration of ISS after HSV-2 exposure.
In this initial study, the efficacy of topical application of 1018 ISS was evaluated using four groups of 15 mice each. Single 100-μg vaginal treatments of the 1018 ISS in PBS were administered either 2 or 6 h after the mice were challenged with a lethal dose of HSV-2. Mice in a control group were treated similarly with the PBS vehicle alone. In every case, the treatments were well tolerated with no adverse signs observed. The three groups were compared with a final group that was untreated.
Mice treated with the 1018 ISS at either 2 or 6 h p.i. had better outcomes for symptomatic disease (as indicated by hair loss and erythema near the vagina) and survival than PBS-treated or untreated controls (Table 1). Mice treated with 1018 ISS, on average, experienced a significantly delayed onset of hair loss and erythema compared to control groups (Fig. 1A) (P < 0.0001 or 0.003 for 2 or 6 h p.i., respectively). The average time to development of hair loss and erythema for animals treated topically 2 h p.i. was 6.6 ± 0.6 days compared to the 4.9 ± 0.2 or 4.7 ± 0.2 days in the PBS or no-treatment control groups, respectively (Table 1). Treatment 6 h p.i. also delayed the onset of hair loss and erythema (mean, 5.7 ± 0.3 days), comparable to treatment at 2 h p.i. All of the PBS-treated and untreated animals developed hair loss and erythema, while 40% of those treated with 1018 ISS were protected from development of these disease signs.
TABLE 1.
Treatment with the 1018 ISS reduced and delayed the incidence and progression of disease and increased the survival rate
| Treatmenta | Incidenceb (%) | Progressionc in animals with disease (%) | Timed to symptoms (days) | Survival timed (days) | Survivale (%) |
|---|---|---|---|---|---|
| 2 h, ISS | 9/15 (60)f | 8/9 (89)f | 6.6 ± 0.6g | 12 ± 1.4g | 6/15 (40)f |
| 6 h, ISS | 12/15 (80)f | 10/12 (83)f | 5.7 ± 0.3h | 10.6 ± 1.1h | 4/15 (27)f |
| 6 h, PBS | 15/15 (100) | 15/15 (100) | 4.9 ± 0.2 | 9.5 ± 0.8 | 0/15 |
| None | 15/15 (100) | 15/15 (100) | 4.7 ± 0.2 | 8.1 ± 0.3 | 0/15 |
Intravaginal topical treatments were delivered 2 or 6 h post-viral inoculation as indicated.
Incidence of disease is measured by the development of hair loss and erythema and is expressed as the number of animals with symptomatic disease/number of animals in the group.
Progression, expressed as number of animals with progression of disease/number of animals with disease, can occur in the animals that develop hair loss and erythema and is indicated similarly by neurologic injury manifested by the chronic release of urine.
Mean for animals with disease signs ± SEM.
Survival is presented as the number of animals alive 21 days p.i./number of animals in the group.
P < 0.01 (ANOVA) versus PBS treatment or no treatment.
P < 0.0001 (log rank analysis) versus PBS treatment or no treatment.
P < 0.001 (log rank) versus PBS treatment or no treatment.
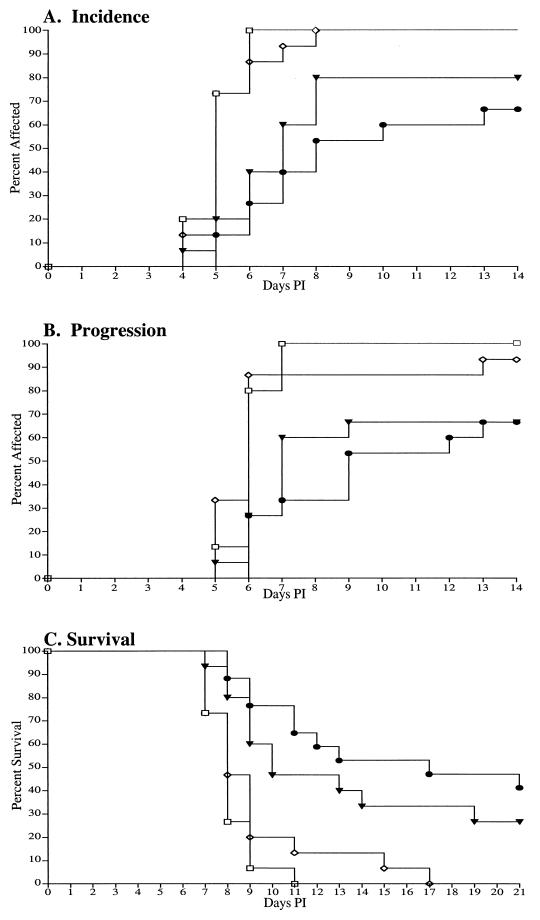
FIG. 1.
Intravaginal and topical perigenital application of 1018 ISS in PBS reduced and delayed the incidence (A) and progression (B) of herpetic disease and increased survival (C) following lethal vaginal HSV-2 challenge. Groups of mice (n = 15 each) were inoculated with HSV-2 and then treated with 1018 ISS 2 h (•) or 6 h (▾) later. As controls, animals were treated similarly with PBS (⋄) or were not treated (□). Daily observations of each animal for initial disease signs of hair loss and erythema (incidence of disease) and for progression (neurologic injury indicated by chronic release of urine) were made for 14 days p.i. Survival was monitored 21 days p.i.
The animals that developed hair loss and erythema following intravaginal 1018 ISS application at 2 or 6 h p.i. showed reduced and delayed disease progression as indicated by chronic release of urine due to neurological injury (Table 1) (83 and 89% of symptomatic animals compared to 100% of PBS-treated or untreated animals). The ISS-treated animals that did experience loss of urinary control developed this complication significantly later than the PBS-treated or untreated control animals (Fig. 1B) (P ⩽ 0.007 for all comparisons).
As expected, 100% mortality was observed with both the untreated and the PBS-treated mice. Application of 1018 ISS 2 h p.i. led to the long-term survival of 40% of the mice, while treatment 6 h p.i. resulted in 27% survival (Table 1) (P < 0.01). Several of the survivors in both groups showed initial signs of disease that resolved over the course of the observation period. The median survival for animals treated with ISS 1018 was 17 and 10 days for the 2- and 6-h p.i. treatment groups compared to 8 days for untreated and PBS-treated mice (P < 0.001). Mean values for each group are presented in Table 1.
An average increased survival time of 2.5 days was observed with animals treated with ISS 1018 2 h p.i. compared to PBS-treated animals, and an average increased survival time of 4 days compared with untreated mice (P < 0.0001) was observed. Mean delays of 1 and 2.5 days were observed for the 6-h p.i. ISS-treated mice compared to PBS-treated and untreated mice, respectively (P < 0.001).
Sequence specificity of ISS efficacy against HSV-2 in the mouse model.
To examine the sequence specificity of the observed efficacy, a second, more extensive mouse study was performed. ISS oligonucleotide administration may have been efficacious by directly inhibiting viral attachment or penetration of the vaginal epithelia or saturation of cellular receptors rather than the hypothesized effects on the immune response. To address the possibility that the extremely large number of DNA strands administered was generating a nonspecific inhibition of viral infection, 1019 and 1040 non-ISS oligonucleotides, identical to 1018 ISS except for two base changes to eliminate the CpG motifs, also were used to treat groups of mice. Oligonucleotide- and vehicle-treated mice received a single treatment delivered 2 h after HSV-2 challenge. An antiviral control group received topical acyclovir ointment three times daily for 7 days beginning 6 h p.i. In addition to the testing of 1018 ISS, another ISS-containing oligonucleotide with characteristics similar to those of 1018 ISS, designated C106 ISS, was tested to establish if the phenomenon was unique for the 1018 ISS. A final group of untreated mice served as baseline controls.
Treatment with either 1018 ISS or C106 ISS significantly protected against the development and progression of skin disease, while no protection was provided by treatment with either 1019 or 1040 non-ISS or the PBS vehicle (P < 0.0001) (Fig. 2 and Table 2). A mean delay of 3 to 4 days was observed for the development of disease in animals treated with either of the ISS oligonucleotides compared to non-ISS treatment or the other controls (P < 0.0001) (Fig. 2A). For animals that showed hair loss and erythema, the progression of herpetic disease was significantly delayed in the animals that received the single ISS oligonucleotide treatment (P < 0.01) (Fig. 2B), and significantly fewer of the animals in these treated groups developed signs of disease (P < 0.01) (Table 2). Nine of the 16 animals treated with 1018 ISS and 6 of 15 animals treated with C106 ISS were protected from disease signs. In comparison, 100% of the control animals developed signs of disease (P < 0.01) (Table 2).
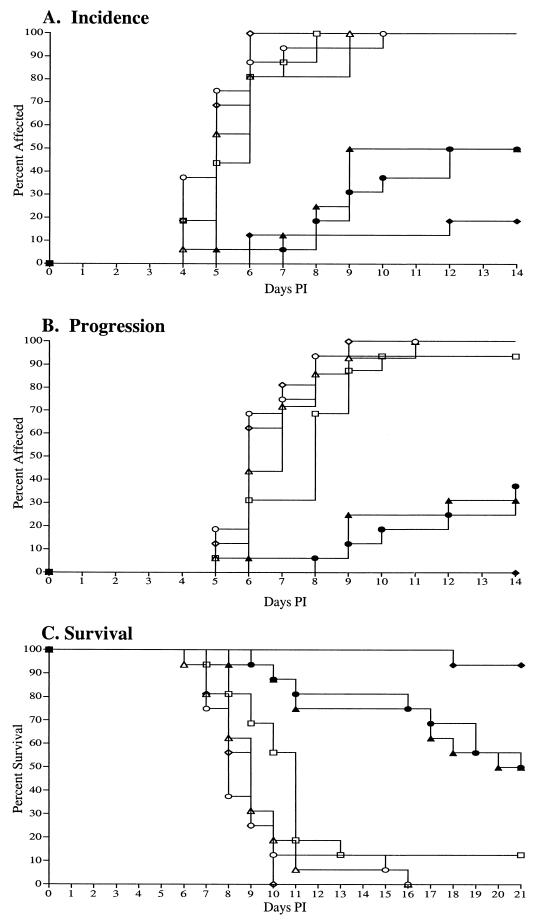
FIG. 2.
Application of either of two ISS-containing oligonucleotides but not non-ISS control oligonucleotides 2 h after lethal HSV-2 challenge significantly reduced and delayed the incidence (A) and progression (B) of herpetic disease and increased survival (C). Mice, treated intravaginally and topically with either 1018 ISS (•) or C106 ISS (▴), were protected against hair loss and erythema (A) and chronic urine release (B) compared to mice treated with the 1019 non-ISS (○) or 1040 non-ISS (▵) control oligonucleotides. As controls, mice were treated with PBS (⋄) or were not treated (□). As a control for intervention, a 21-dose course of acyclovir (♦) was provided to a final group of mice.
TABLE 2.
Treatment with 1018 ISS or C106 ISS but not non-ISS oligonucleotides reduced and delayed the incidence and progression of disease and increased the survival rate
| Treatmenta | Incidenceb (%) | Progressionb in animals with disease (%) | Timec to symptoms (days) | Survival timec (days) | Survivalb (%) |
|---|---|---|---|---|---|
| 1018 ISS | 7/16 (44)d | 5/7 (71)d | 9.4 ± 0.7c | 18.1 ± 1.1f,g | 8/16 (50)d |
| C106 ISS | 9/15 (60)d | 4/9 (44)d | 8.1 ± 0.5e | 17.5 ± 1.2f,g | 8/16 (50)d |
| 1019 non-ISS | 16/16 | 16/16 | 5.2 ± 0.4 | 9.1 ± 0.7 | 0/16 |
| 1040 non-ISS | 15/15 | 15/15 | 5.7 ± 0.3 | 9.2 ± 0.6 | 0/15 |
| PBS | 16/16 | 16/16 | 5.1 ± 0.2 | 8.6 ± 0.3 | 0/16 |
| None | 16/16 | 16/16 | 5.7 ± 0.3 | 10.7 ± 0.8 | 1/16 (6) |
| Acyclovir | 3/16 (19)h | 0/3 | 12 ± 0.7e | 20.8 ± 0.2e | 15/16 (94)h |
Intravaginal and topical treatments delivered 2 h p.i.; acyclovir delivered 6 h p.i., three times per day for 7 days.
Incidence, progression, and survival are scored as indicated in Table 1.
Mean for animals with disease signs ± SEM.
P < 0.01 (ANOVA) versus 1019 non-ISS, 1040 non-ISS, PBS treatments, or no treatment.
P < 0.0001 (log rank analysis) versus 1019 non-ISS, 1040 non-ISS, PBS, or no treatment.
P < 0.0001 (log rank) versus 1019 non-ISS, 1040 non-ISS, or PBS treatments.
P < 0.001 (log rank) versus no treatment.
P < 0.01 (ANOVA) versus 1019 non-ISS, 1040 non-ISS, or PBS treatments.
As in the first study, survival was significantly increased for animals that were treated with the ISS oligonucleotides (P < 0.001) (Fig. 2C). Compared to the complete mortality observed in the non-ISS-treated control animals, 50% of the animals that received the 1018 ISS or C106 ISS oligonucleotides survived the lethal challenge (Table 2 and Fig. 2C). The mean survival time of ISS-treated animals also significantly increased (P < 0.001) compared to that for the non-ISS-treated controls (Table 2). In every case, the oligonucleotide treatments were well tolerated with no adverse signs observed.
ISS efficacy against an existing HSV-2 genital infection in the guinea pig model.
To evaluate the efficacy of ISS against recurrent herpetic disease, we utilized the guinea pig model. This model provides a naturally occurring recurrent disease similar to that seen with human HSV-2 infections (42, 48). Further, latently infected guinea pigs shed virus vaginally (unpublished data) at a frequency similar to that observed with humans, allowing us to establish the impact of ISS therapy on asymptomatic viral shedding.
Latently infected guinea pigs were treated with the 1018 ISS, control 1019 non-ISS oligonucleotide, or acyclovir by vaginal and perigenital application or served as untreated controls. The oligonucleotides were delivered once, 21 days after HSV-2 challenge during the recurrent disease phase. The oligonucleotide-treated and untreated groups were established by distributing animals (n = 10 to 12) based on their primary disease scores collected during the first 14 d p.i. to provide statistically indistinguishable populations. The acyclovir control animals were treated at the beginning of the study with a 21-dose course of acyclovir (3 doses per day for 7 days beginning 6 h p.i.). All of the treatments were well tolerated, with no adverse reactions observed in any of the animals.
An examination of the 7 days following oligonucleotide application indicated that a single administration of 1018 ISS (0.6 ± 0.2 mean cumulative recurrences for 7 days' observation/animal) significantly reduced the frequency of genital lesion development compared to results with either 1019 non-ISS-treated animals (1.9 ± 0.3/animal) or untreated animals (2.1 ± 0.5/animal) (P < 0.05) (Table 3). As expected, the 21-dose acyclovir therapy during primary disease provided significant reduction in lesion frequency (P < 0.05 compared to results with untreated animals) and reduced the number of animals that experienced any recurrences (7 of 12 experienced recurrent disease episodes) (Table 3). Interestingly, for those animals with recurrent disease, a single 1018 ISS application (day 21 p.i.) was as effective as the 7-day, 21-dose acyclovir course delivered beginning 6 h p.i., generating similar lesion frequency in the treated animals (mean number of cumulative recurrences for 1018 ISS treatment, 0.6 ± 0.2/animal versus 0.7 ± 0.3/animal for acyclovir) (Table 3).
TABLE 3.
Treatment with 1018 ISS but not 1019 non-ISS reduced the number of recurrent genital HSV-2 lesions in guinea pigs
| Treatment (n)h | No. with recurrences (days 21-56) | No. of days recurrences observeda between indicated days p.i.
|
|
|---|---|---|---|
| 21-28 | 21-56 | ||
| None (10) | 10 | 2.1 ± 0.5 | 9.1 ± 1.4 |
| 1018 ISSb (11) | 11 | 0.6 ± 0.2e,f | 5.1 ± 1 |
| 1019 non-ISSb (11) | 11 | 1.9 ± 0.3 | 7.6 ± 1.4 |
| Acyclovirc (12) | 7d | 0.7 ± 0.3e,f | 2.9 ± 1e,g |
Mean ± SEM.
Intravaginal and topical oligonucleotide (1 mg) delivered once 21 days p.i.
Intravaginal and topical acyclovir ointment 6 h p.i., t.i.d. for 7 days.
Five of the twelve acyclovir-treated guinea pigs did not experience recurrences.
P < 0.05 (ANOVA) versus 1019 non-ISS treatment.
P < 0.05 (ANOVA) versus no treatment.
P < 0.01 (ANOVA) versus no treatment.
n, no. of subjects in treatment group.
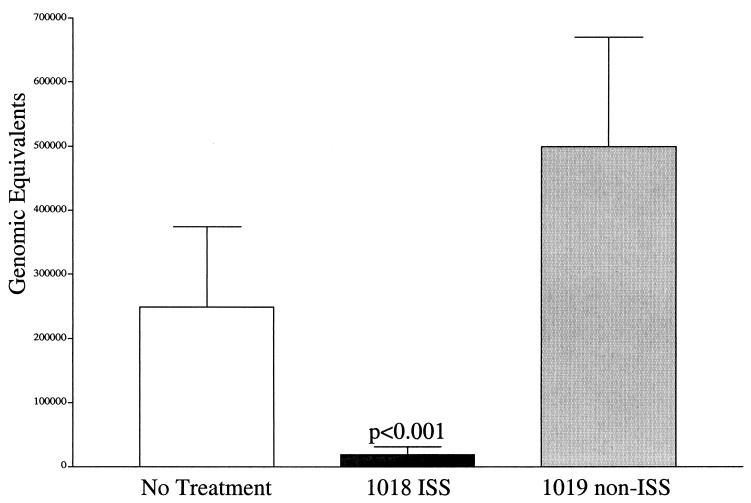
As an additional measure of efficacy, the impact of ISS on the frequency and magnitude of viral shedding was established by using PCR-based detection of HSV-2 DNA. Analysis of daily vaginal swabs collected from 22 to 42 days p.i. established the frequency of vaginal shedding. The results indicated that treatment with 1018 ISS or 1019 non-ISS or no treatment resulted in the same frequency of viral shedding (data not shown). The magnitude of shedding also was examined. Each of the swab samples was amplified for HSV-2 DNA and also for the single-copy guinea pig albumin gene to allow normalization for DNA quality and quantity. HSV-2 DNA burdens were extrapolated from the linear relationship established from band density of a dilution series of known genomic equivalents amplified in parallel to the swab samples. Interestingly, the amount of HSV-2 DNA shed was impacted by treatment with 1018 ISS but not with 1019 non-ISS (Fig. 3). A significant difference in the average amount of viral DNA present in vaginal swabs from 1018 ISS-treated animals (2 × 104 ± 1 × 103 genomic equivalents) was observed compared to either 1019 non-ISS-treated guinea pigs or untreated guinea pigs (5 × 105 ± 1.7 × 105 or 2.5 × 105 ± 1.7 × 105, respectively; P < 0.001). A mean value of 15% of the swabs were positive for each group, allowing, on average, 33 PCR-positive samples for comparison.
FIG. 3.
Vaginal shedding of HSV-2, as detected by quantitative PCR, was significantly reduced by a single application of 1018 ISS but not 1019 non-ISS (P < 0.001). Daily vaginal swabs (22 to 43 days p.i.) were analyzed for HSV-2 DNA content by amplification of a region of the DNA polymerase gene. Resulting amplimers were blotted and then quantified by densitometry and compared to a series of known concentrations of HSV-2 genomic DNA. Genomic equivalents for each positive vaginal swab were extrapolated from the linear relationship between the viral standards and amplimer densities. The average number of genomic equivalents is presented for each group.
ISS do not demonstrate direct antiherpetic activity.
Having shown, in both animal models, that protection against primary and recurrent herpetic disease was specific to oligonucleotides that contained immunostimulatory CpG motifs, we sought to determine if a direct inhibition of viral replication or attachment was the mechanism of action rather than the suspected immunomodulatory effect. For these studies, standard plaque assays were performed using Vero cells pretreated with 1 or 10 μg of ISS/ml for 30 s, 10 min, or 24 h prior to viral inoculation. In separate studies, HSV-2 inocula were treated with the oligonucleotides for 10 min prior to inoculation of the Vero monolayers. As controls, non-ISS oligonucleotides also were tested. Triplicate assays were performed for each condition, and the average was compared to the untreated control value. For each condition, plaque counts were similar to those for the untreated control (Table 4), indicating that the oligonucleotides do not act directly to prevent viral infection. A slight but not significant titer reduction was observed in samples where Vero cells had been pretreated with 10 μg of ISS/ml for 24 h.
TABLE 4.
Oligonucleotide pretreatment of cells or viral inocula did not directly affect infectivity based upon plaque titration assaysa
| Oligonucleotide | Result for cells pretreated with oligonucleotide concn (μg/ml) of:
|
Result for virus pretreated with oligonucleotide concn (μg/ml) of:
|
Mock-treated controls | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1
|
10
|
1
|
10
|
||||||
| 30 s | 10 min | 24 h | 30 s | 10 min | 24 h | 10 min | 10 min | ||
| 1018 ISS | 101 | 98 | 99 | 101 | 101 | 99 | 96 | 102 | 100 |
| C106 ISS | 119 | NT | NT | 106 | NT | NT | 101 | 97 | 106 |
| 1019 non-ISS | 98 | 96 | 103 | 103 | 97 | 99 | 98 | 106 | 101 |
| 1040 non-ISS | 111 | NT | NT | 129 | 100 | 98 | 100 | 97 | 103 |
Each tested parameter was completed in triplicate. Plaque counts from each of three wells were averaged and compared with those for the mock-treated control wells tested in parallel to the 1018 ISS-treated wells. The data are expressed as percentages of plaques present in mock-treated samples. NT, not tested.
DISCUSSION
Immunostimulatory CpG motifs have been tested in a variety of systems for potentiating vaccine activity but have not been tested directly for efficacy against HSV-2 or other mucosal pathogens. The ISS-containing oligonucleotides evaluated in this report have been shown to be active immunostimulators for rodent, rabbit, nonhuman primate, and human systems (10, 33, 53; Halperin et al., 41st ICAAC). In fact, the 1018 ISS oligonucleotide has been used with positive outcomes in clinical trials in allergy and hepatitis B vaccination. 1018 ISS linked to the ragweed allergen Amb a 1 was a potent induced of IgG responses but not IgE responses in ragweed-allergic patients (10). 1018 ISS also provided dramatic stimulation of immunoglobulin G (IgG) responses to hepatitis B vaccine in normal volunteers (Halperin et al., 41st ICAAC). Thus, it is reasonable to anticipate that ISS species such as 1018 ISS would be active in humans and could show anti-HSV-2 efficacy similar to the efficacy observed in the animal models utilized in this study.
In our studies, both of these ISS showed significant efficacy against genital challenge with HSV-2. For the mice that developed infections, ISS treatment at 2 or 6 h after viral inoculation produced significant reductions and delays in disease progression and allowed a portion to recover from the otherwise lethal challenge. The incidence and progression of herpetic disease were significantly delayed in the animals that received the single ISS oligonucleotide treatment or the 21-dose course of acyclovir, and significantly fewer of the animals in these treated groups developed signs of disease. Importantly, up to half the mice survived the HSV-2 infection if treated with 1018 ISS or C106 ISS at 2 h p.i., an infection which was otherwise lethal for all the animals that received the 1019 or 1040 non-ISS control oligonucleotides.
ISS therapy also proved to be effective against an ongoing HSV-2 infection in the guinea pig model. The 21 doses of acyclovir delivered 6 h p.i., three times per day for 7 days protected 5 of 12 animals from recurrent disease and produced an average of 0.7 days of recurrent disease in the remaining 12 animals. The observed efficacy from the single ISS application 21 days after HSV-2 inoculation did not prevent recurrences in any of the tested animals but resulted in a frequency of recurrent disease similar to that with the acyclovir therapy course, indicating that ISS have great potential as antiherpetics. Although not significant, analysis of data collected from days 22 through 56 p.i. indicated a reduction in recurrent lesions in animals treated with 1018 ISS compared to untreated or 1019 non-ISS controls. Animals treated with 1018 ISS, on average, experienced fewer recurrent lesion days than either 1019 non-ISS animals or untreated animals. The fact that the effect of a single treatment significantly reduced clinically observable recurrences (P < 0.05) for 7 days but not for the entire 21-day observation period suggests that more-frequent treatment with ISS (such as once per week) may prove to be even more efficacious.
These findings were extended by viral shedding data that indicated that 1018 ISS treatment did not impact the frequency of reactivation but reduced the amount of viral DNA shed to the vaginal cavity compared to results with 1019-treated or untreated animals. The detection frequency for infectious virus shed from the vaginal cavity is too low to be useful for these studies (42); however, on average 15% of the swabs were positive for HSV-2 DNA for each group, allowing, on average, 33 PCR positive samples for comparison. Together, the unaltered frequency of positives and the reduced magnitude of shedding suggest that the effect of 1018 ISS is to reduce the amount of intact virus that is shed to the vaginal cavity but not the frequency of that event. The reduction in intact virus presumably is due to the immune stimulation provided by 1018 ISS, but the mechanism has not yet been determined.
It is notable that few antiherpetic or antiviral agents have provided significant protection when given as a single dose. A single application of ISS at 2 or 6 h p.i. (for mice) or 21 days p.i. (for guinea pigs) proved to have a significant impact on acute and established HSV-2 infection. The oligonucleotides lacking a CpG motif showed no efficacy, supporting the conclusion that the effects were mediated by the contextual CpG motifs. Further, our in vitro studies indicate that ISS is not directly inhibiting HSV from infecting cells. The mechanism by which ISS control HSV-2 infection is not completely understood. A number of effector arms of both innate and adaptive immunity have been identified as being important in controlling herpes infections (15, 17, 28, 35, 37-39, 57). ISS have been shown to stimulate many of these effector arms, including induction of antigen-nonspecific-B-cell proliferation and immunoglobulin production (34), induction of NK activity (2), secretion of IFN-α (45), IL-12, and IL-18 (44) by macrophages, and IFN-γ secretion from NK cells (9). ISS stimulate the innate immune system to produce IFN-γ and inducers of IFN-γ (IFN-α, IFN-γ, IL-12, and IL-18) and foster a cytokine milieu that drives the adaptive immune response to specific antigens toward the Th1 phenotype. Thus, the effects of ISS on HSV-2 infection could be multifaceted, involving innate immunity, adaptive immunity, or both. Further work will be required to determine which of the many immunostimulating activities of ISS are involved in efficacy against HSV. The positive effects of the ISS alone warrant continued evaluation. We currently are expanding our studies to evaluate alternate formulations and delivery times to optimize the protection afforded by ISS application.
In mice, attenuated strains of HSV-2 applied intravaginally induced humoral (particularly immunization-stimulated IgG) and cellular immunity in both sera and vaginal secretions (30, 43). Vaginally administered mucosal adjuvants, in particular cholera toxin β-subunit, significantly raise IgA and IgG levels in the genital mucosa (18). A recent report has suggested that immunostimulatory oligonucleotides also are effective mucosal adjuvants when used in combination with recombinant HSV-2 gB (13). These studies support the concept that mucosa-associated lymphoid tissue participates in the generation of genital immune-mediated protection.
We also are evaluating the impact of different routes of ISS application on efficacy. In animals, ISS have been shown to be active when applied to the mucosae of the lung and eye as well as other sites (reviewed in reference 29). Studies of oligonucleotides administered to the pulmonary mucosa indicate that these molecules are predominantly retained by lung tissue, with only 7% of the administered dose detected in all others organs combined (1). The oligonucleotides have a reported half-life of 30 h in this tissue, with minimal detectable levels present at 72 h after administration (1). Additionally, in these reports, oligonucleotide doses of as much as 20 mg showed no toxicity (1). To our knowledge, no work has been completed on ISS administration to the genital mucosa. Intraperitoneal administration of ISS have proven to be immunostimulatory (6, 31), but the impact on mucosal viral immunity has not been studied extensively. In guinea pigs, intraperitoneal delivery of ISS on day 21 p.i. reduced the frequency of disease recurrence at levels just reaching significance compared to results with untreated or vehicle-treated animals (data not shown). However, intraperitoneal administration of the ISS in mice did not protect against HSV-2 disease (data not shown). Application of compounds at the site of disease, especially for herpetic infections, likely will be the preferred treatment and should increase usability in the case of genital and oral mucosae.
Recently a family of imidazoquinoline compounds used as topical creams has been tested as immunomodulating therapies for herpetic infections (4, 16, 36). Although not directly antiherpetic, these compounds have produced significant reduction in herpetic disease in animal models (4, 16, 36) and in clinical experiences (14, 47). Mechanistically, imiquimod and resiquimod, members of the imidazoquinoline family, have been shown to induce Th1 cytokine and immune cell profiles (56), stimulating a number of the effector arms involved in the control of herpetic infections. Imiquimod is approved for the treatment of genital warts (55) and has been used in humans for drug-resistant HSV-2 genital infections (14). Work both with animal models and in clinical trials has shown that imidazoquinolines can produce adverse reactions at higher doses (4, 55). The animal and clinical trial data support the continued testing of new immunomodulators as therapies for genital HSV infections. While imidazoquinoline compounds and ISS have many similar properties, ISS does appear to induce more IFN-γ, less TNF-α, and less IL-5 than resiquimod (56), features that could affect efficacy and adverse reactions. The safety profile of 1018 ISS appears very good in toxicological studies using systemic or mucosal delivery and initial clinical trials using systemic delivery (10; Halperin et al., 41st ICAAC).
Local application of ISS could provide an alternative or supplement to standard acyclovir therapy and could be applied in advance of exposure to environmental triggers of reactivation. The PCR data from the vaginal swabs suggest that the ISS effect would also help to reduce the magnitude of virus in shedding events, likely reducing the frequency of transmission. ISS therapy could prove appropriate for HSV-2-seropositive women prior to birth, potentially reducing the risk of neonatal transmission. Studies are planned to establish if ISS therapy will prove effective against primary and recurrent HSV-1 disease.
Acknowledgments
This work was supported by a grant from Dynavax and by grant PO1-AI-37940 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
We thank Tonyia Eaves-Pyles and Jane Strasser for thoughtful review and discussions of the manuscript.
REFERENCES
- 1.Ali, S., S. A. Leonard, C. A. Kukoly, W. J. Metzger, W. R. Wooles, J. F. McGinty, M. Tanaka, A. Sandrasagra, and J. W. Nyce. 2001. Absorption, distribution, metabolism, and excretion of a respirable antisense oligonucleotide for asthma. Am. J. Respir. Crit. Care Med. 163:989-993. [DOI] [PubMed] [Google Scholar]
- 2.Ballas, Z. K., W. L. Rasmussen, and A. M. Krieg. 1996. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J. Immunol. 157:1840-1845. [PubMed] [Google Scholar]
- 3.Bauer, S., C. J. Kirschning, H. Hacker, V. Redecke, S. Hausmann, S. Akira, H. Wagner, and G. B. Lipford. 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA 98:9237-9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernstein, D. I., C. J. Harrison, M. A. Tomai, and R. L. Miller. 2001. Daily or weekly therapy with resiquimod (R-848) reduces genital recurrences in herpes simplex virus-infected guinea pigs during and after treatment. J. Infect. Dis. 183:844-849. [DOI] [PubMed] [Google Scholar]
- 5.Bourne, N., J. Ireland, L. R. Stanberry, and D. I. Bernstein. 1999. Effect of undecylenic acid as a topical microbicide against genital herpes infection in mice and guinea pigs. Antivir. Res. 40:139-144. [DOI] [PubMed] [Google Scholar]
- 6.Broide, D., J. Schwarze, H. Tighe, T. Gifford, M. D. Nguyen, S. Malek, J. Van Uden, E. Martin-Orozco, E. W. Gelfand, and E. Raz. 1998. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J. Immunol. 161:7054-7062. [PubMed] [Google Scholar]
- 7.Burke, R. L., C. Goldbeck, P. Ng, L. Stanberry, G. Ott, and G. Van Nest. 1994. The influence of adjuvant on the therapeutic efficacy of a recombinant genital herpes vaccine. J. Infect. Dis. 170:1110-1119. [DOI] [PubMed] [Google Scholar]
- 8.Clements, J. D. 1997. Surface warfare against pathogens using mucosal vaccines. Nat. Biotechnol. 15:622-623. [DOI] [PubMed] [Google Scholar]
- 9.Cowdery, J. S., J. H. Chace, A. K. Yi, and A. M. Krieg. 1996. Bacterial DNA induces NK cells to produce IFN-gamma in vivo and increases the toxicity of lipopolysaccharides. J. Immunol. 156:4570-4575. [PubMed] [Google Scholar]
- 10.Dieudonne, F., D. Vital Durand, J. Eiden, S. Tuck, G. Van Nest, E. Raz, R. Hamilton, P. S. Creticos, and C. Andre. 2001. ISS linked to Amb a 1 allergen (AIC) stimulates IgG response to Amb a 1 by ragweed-allergic humans. J. Allergy Clin. Immun. 107:S233. [Google Scholar]
- 11.Efstathiou, S., H. J. Field, P. D. Griffiths, E. R. Kern, S. L. Sacks, N. M. Sawtell, and L. R. Stanberry. 1999. Herpes simplex virus latency and nucleoside analogues. Antivir. Res. 41:85-100. [DOI] [PubMed] [Google Scholar]
- 12.Fleming, D. T., G. M. McQuillan, R. E. Johnson, A. J. Nahmias, S. O. Aral, F. K. Lee, and M. E. St Louis. 1997. Herpes simplex virus type 2 in the United States, 1976 to 1994. N. Engl. J. Med. 337:1105-1111. [DOI] [PubMed] [Google Scholar]
- 13.Gallichan, W. S., R. N. Woolstencroft, T. Guarasci, M. J. McCluskie, H. L. Davis, and K. L. Rosenthal. 2001. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J. Immunol. 166:3451-3457. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert, J., M. M. Drehs, and J. M. Weinberg. 2001. Topical imiquimod for acyclovir-unresponsive herpes simplex virus 2 infection. Arch. Dermatol. 137:1015-1017. [PubMed] [Google Scholar]
- 15.Harandi, A. M., B. Svennerholm, J. Holmgren, and K. Eriksson. 2001. Interleukin-12 (IL-12) and IL-18 are important in innate defense against genital herpes simplex virus type 2 infection in mice but are not required for the development of acquired gamma interferon-mediated protective immunity. J. Virol. 75:6705-6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison, C. J., R. L. Miller, and D. I. Bernstein. 2001. Reduction of recurrent HSV disease using imiquimod alone or combined with a glycoprotein vaccine. Vaccine 19:1820-1826. [DOI] [PubMed] [Google Scholar]
- 17.Holterman, A. X., K. Rogers, K. Edelmann, D. M. Koelle, L. Corey, and C. B. Wilson. 1999. An important role for major histocompatibility complex class I-restricted T cells, and a limited role for gamma interferon, in protection of mice against lethal herpes simplex virus infection. J. Virol. 73:2058-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson, E., C. Rask, M. Fredriksson, K. Eriksson, C. Czerkinsky, and J. Holmgren. 1998. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect. Immun. 66:514-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura, H., M. Shibata, K. Kuzushima, K. Nishikawa, Y. Nishiyama, and T. Morishima. 1990. Detection and direct typing of herpes simplex virus by polymerase chain reaction. Med. Microbiol. Immunol. 179:177-184. [DOI] [PubMed] [Google Scholar]
- 20.Kinghorn, G. R. 1996. Limiting the spread of genital herpes. Scand. J. Infect. Dis. Suppl. 100:20-25. [PubMed] [Google Scholar]
- 21.Klinman, D. M., S. Kamstrup, D. Verthelyi, I. Gursel, K. J. Ishii, F. Takeshita, and M. Gursel. 2000. Activation of the innate immune system by CpG oligodeoxynucleotides: immunoprotective activity and safety. Springer Semin. Immunopathol. 22:173-183. [DOI] [PubMed] [Google Scholar]
- 22.Krieg, A. M., A. K. Yi, S. Matson, T. J. Waldschmidt, G. A. Bishop, R. Teasdale, G. A. Koretzky, and D. M. Klinman. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374:546-549. [DOI] [PubMed] [Google Scholar]
- 23.Krieg, A. M. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709-760. [DOI] [PubMed] [Google Scholar]
- 24.Krug, A., S. Rothenfusser, V. Hornung, B. Jahrsdorfer, S. Blackwell, Z. K. Ballas, S. Endres, A. M. Krieg, and G. Hartmann. 2001. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur. J. Immunol. 31:2154-2163. [DOI] [PubMed] [Google Scholar]
- 25.Krug, A., A. Towarowski, S. Britsch, S. Rothenfusser, V. Hornung, R. Bals, T. Giese, H. Engelmann, S. Endres, A. M. Krieg, and G. Hartmann. 2001. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur. J. Immunol. 31:3026-3037. [DOI] [PubMed] [Google Scholar]
- 26.Langenberg, A. G. M., R. L. Burke, S. F. Adair, R. Sekulovich, M. Tigges, C. L. Dekker, and L. Corey. 1995. A recombinant glycoprotein vaccine for herpes simplex type 2: safety and efficacy. Ann. Intern. Med. 122:889-898. [DOI] [PubMed] [Google Scholar]
- 27.Leung, D. T., and S. L. Sacks. 2000. Current recommendations for the treatment of genital herpes. Drugs 60:1329-1352. [DOI] [PubMed] [Google Scholar]
- 28.Liu, T., K. M. Khanna, B. N. Carriere, and R. L. Hendricks. 2001. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J. Virol. 75:11178-11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCluskie, M. J., R. D. Weeratna, P. J. Payette, and H. L. Davis. 2001. The potential of CpG oligodeoxynucleotides as mucosal adjuvants. Crit. Rev. Immunol. 21:103-120. [PubMed] [Google Scholar]
- 30.McDermott, M. R., L. J. Brais, and M. J. Evelegh. 1990. Mucosal and systemic antiviral antibodies in mice inoculated intravaginally with herpes simplex virus type 2. J. Gen. Virol. 71:1497-1504. [DOI] [PubMed] [Google Scholar]
- 31.Magone, M. T., C. C. Chan, L. Beck, S. M. Whitcup, and E. Raz. 2000. Systemic or mucosal administration of immunostimulatory DNA inhibits early and late phases of murine allergic conjunctivitis. Eur. J. Immunol. 30:1841-1850. [DOI] [PubMed] [Google Scholar]
- 32.Malanchere-Bres, E., P. J. Payette, M. Mancini, P. Tiollais, H. L. Davis, and M.-L. Michel. 2001. CpG oligodeoxynucleotides with hepatitis B surface antigen (HbsAg) for vaccination in HbsAg-transgenic mice. J. Virol. 75:6482-6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshall, J. D., S. Abtahi, J. J. Eiden, S. Tuck, R. Milley, F. Haycock, M. J. Reid, A. Kagey, P. S. Creticos, L. M. Lichtenstein, and G. Van Nest. 2001. Immunostimulatory sequence DNA linked to the Amb a 1 allergen promotes T(H)1 cytokine expression while downregulating T(H)2 cytokine expression in PBMCs from human patients with ragweed allergy. J. Allergy Clin. Immunol. 108:191-197. [DOI] [PubMed] [Google Scholar]
- 34.Messina, J. P., G. S. Gilkeson, and D. S. Pisetsky. 1991. Stimulation of in vitro murine lymphocyte proliferation by bacterial DNA. J. Immunol. 147:1759-1764. [PubMed] [Google Scholar]
- 35.Mikloska, Z., and A. L. Cunningham. 2001. Alpha and gamma interferons inhibit herpes simplex virus type 1 infection and spread in epidermal cells after axonal transmission. J. Virol. 75:11821-11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller, R. L., L. M. Imbertson, M. J. Reiter, and J. F. Gerster. 1999. Treatment of primary herpes simplex virus infection in guinea pigs by imiquimod. Antivir. Res. 44:31-42. [DOI] [PubMed] [Google Scholar]
- 37.Milligan, G. N., and D. I. Bernstein. 1995. Analysis of herpes simplex virus-specific T cells in the murine female genital tract following genital infection with herpes simplex virus type 2. Virology 212:481-489. [DOI] [PubMed] [Google Scholar]
- 38.Milligan, G. N., and D. I. Bernstein. 1997. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology 229:259-268. [DOI] [PubMed] [Google Scholar]
- 39.Milligan, G. N., D. I. Bernstein, and N. Bourne. 1998. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J. Immunol. 160:6093-6100. [PubMed] [Google Scholar]
- 40.Milligan, G. N., N. Bourne, and K. L. Dudley. 2001. Role of polymorphonuclear leukocytes in resolution of HSV-2 infection of the mouse vagina. J. Reprod. Immunol. 49:49-65. [DOI] [PubMed] [Google Scholar]
- 41.Miyazaki, D., G. Liu, L. Clark, and S. J. Ono. 2000. Prevention of acute allergic conjunctivitis and late-phase inflammation with immunostimulatory DNA sequences. Investig. Ophthalmol. Vis. Sci. 41:3850-3855. [PubMed] [Google Scholar]
- 42.Myers, M. G., D. I. Bernstein, C. J. Harrison, and L. R. Stanberry. 1988. Herpes simplex virus glycoprotein treatment of recurrent genital herpes reduces cervicovaginal virus shedding in guinea pigs. Antivir. Res. 10:83-88. [DOI] [PubMed] [Google Scholar]
- 43.Parr, M. B., and E. L. Parr. 1997. Protective immunity against HSV-2 in the mouse vagina. J. Reprod. Immunol. 36:77-92. [DOI] [PubMed] [Google Scholar]
- 44.Roman, M., E. MartinOrozco, J. S. Goodman, M. D. Nguyen, Y. Sato, A. Ronaghy, R. S. Kornbluth, D. D. Richman, D. A. Carson, and E. Raz. 1997. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat. Med. 3:849-854. [DOI] [PubMed] [Google Scholar]
- 45.Sato, Y., M. Roman, H. Tighe, D. Lee, M. Corr, M. D. Nguyen, G. J. Silverman, M. Lotz, D. A. Carson, and E. Raz. 1996. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science 273:352-354. [DOI] [PubMed] [Google Scholar]
- 46.Smith, J. R., A. M. Thackray, and R. Bujdoso. 2001. Reduced herpes simplex virus type 1 latency in Flt-3 ligand-treated mice is associated with enhanced numbers of natural killer and dendritic cells. Immunology 102:352-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spruance, S. L., S. K. Tyring, M. H. Smith, and T. C. Meng. 2001. Application of a topical immune response modifier, resiquimod gel, to modify the recurrence rate of recurrent genital herpes: a pilot study. J. Infect. Dis. 184:196-200. [DOI] [PubMed] [Google Scholar]
- 48.Stanberry, L. R., E. R. Kern, J. T. Richards, T. M. Abbott, and J. C. Overall, Jr. 1982. Genital herpes in guinea pigs: pathogenesis of the primary infection and description of recurrent disease. J. Infect. Dis. 146:397-404. [DOI] [PubMed] [Google Scholar]
- 49.Straus, S. E., L. Corey, R. L. Burke, B. Savarese, G. Barnum, P. Krause, R. G. Kost, J. L. Meier, R. Sekulovich, S. F. Adair, and C. L. Dekker. 1994. Placebo-controlled trial of vaccination with recombinant glycoprotein D of herpes simplex virus type 2 for immunotherapy of genital herpes. Lancet 343:1460-1463. [DOI] [PubMed] [Google Scholar]
- 50.Straus, S. E., A. Wald, R. G. Kost, R. McKenzie, A. G. M. Langenberg, P. Hohman, J. Lekstrom, E. Cox, M. Nakamura, R. Sekulovich, A. Izu, C. Dekker, and L. Corey. 1997. Immunotherapy of recurrent genital herpes with recombinant herpes simplex virus type 2 glycoproteins D and B: results of a placebo-controlled vaccine trial. J. Infect. Dis. 176:1129-1134. [DOI] [PubMed] [Google Scholar]
- 51.Takeshita, F., C. A. Leifer, I. Gursel, K. J. Ishii, S. Takeshita, M. Gursel, and D. M. Klinman. 2001. Cutting edge: role of Toll-like receptor 9 in CpG DNA-induced activation of human cells. J. Immunol. 167:3555-3558. [DOI] [PubMed] [Google Scholar]
- 52.Tanigawa, M., J. E. Bigger, M. Y. Kanter, and S. S. Atherton. 2000. Natural killer cells prevent direct anterior-to-posterior spread of herpes simplex virus type 1 in the eye. Investig. Ophthalmol. Vis. Sci. 41:132-137. [PubMed] [Google Scholar]
- 53.Tighe, H., K. Takabayashi, D. Schwartz, G. Van Nest, S. Tuck, J. J. Eiden, A. Kagey-Sobotka, P. S. Creticos, L. M. Lichtenstein, H. L. Spiegelberg, and E. Raz. 2000. Conjugation of immunostimulatory DNA to the short ragweed allergen amb a 1 enhances its immunogenicity and reduces its allergenicity. J. Allergy Clin. Immunol. 106:124-134. [DOI] [PubMed] [Google Scholar]
- 54.Tokunaga, T., O. Yano, E. Kuramoto, Y. Kimura, T. Yamamoto, T. Kataoka, and S. Yamamoto. 1992. Synthetic oligonucleotides with particular base sequences from the cDNA encoding proteins of Mycobacterium bovis BCG induce interferons and activate natural killer cells. Microbiol. Immunol. 36:55-66. [DOI] [PubMed] [Google Scholar]
- 55.Tyring, S. K., I. I. Arany, M. A. Stanley, M. H. Stoler, M. A. Tomai, R. L. Miller, M. L. Owens, and M. H. Smith. 1998. Mechanism of action of imiquimod 5% cream in the treatment of anogenital warts. Prim. Care Update Obstet. Gyn. 5:151-152. [DOI] [PubMed] [Google Scholar]
- 56.Vasilakos, J. P., R. M. Smith, S. J. Gibson, J. M. Lindh, L. K. Pederson, M. J. Reiter, M. H. Smith, and M. A. Tomai. 2000. Adjuvant activities of immune response modifier R-848: comparison with CpG ODN. Cell Immunol. 204:64-74. [DOI] [PubMed] [Google Scholar]
- 57.Vollstedt, S., M. Franchini, G. Alber, M. Ackermann, and M. Suter. 2001. Interleukin-12- and gamma interferon-dependent innate immunity are essential and sufficient for long-term survival of passively immunized mice infected with herpes simplex virus type 1. J. Virol. 75:9596-9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wald, A., A. G. Langenberg, K. Link, A. E. Izu, R. Ashley, T. Warren, S. Tyring, J. M. Douglas, Jr., and L. Corey. 2001. Effect of condoms on reducing the transmission of herpes simplex virus type 2 from men to women. JAMA 285:3100-3106. [DOI] [PubMed] [Google Scholar]