Abstract
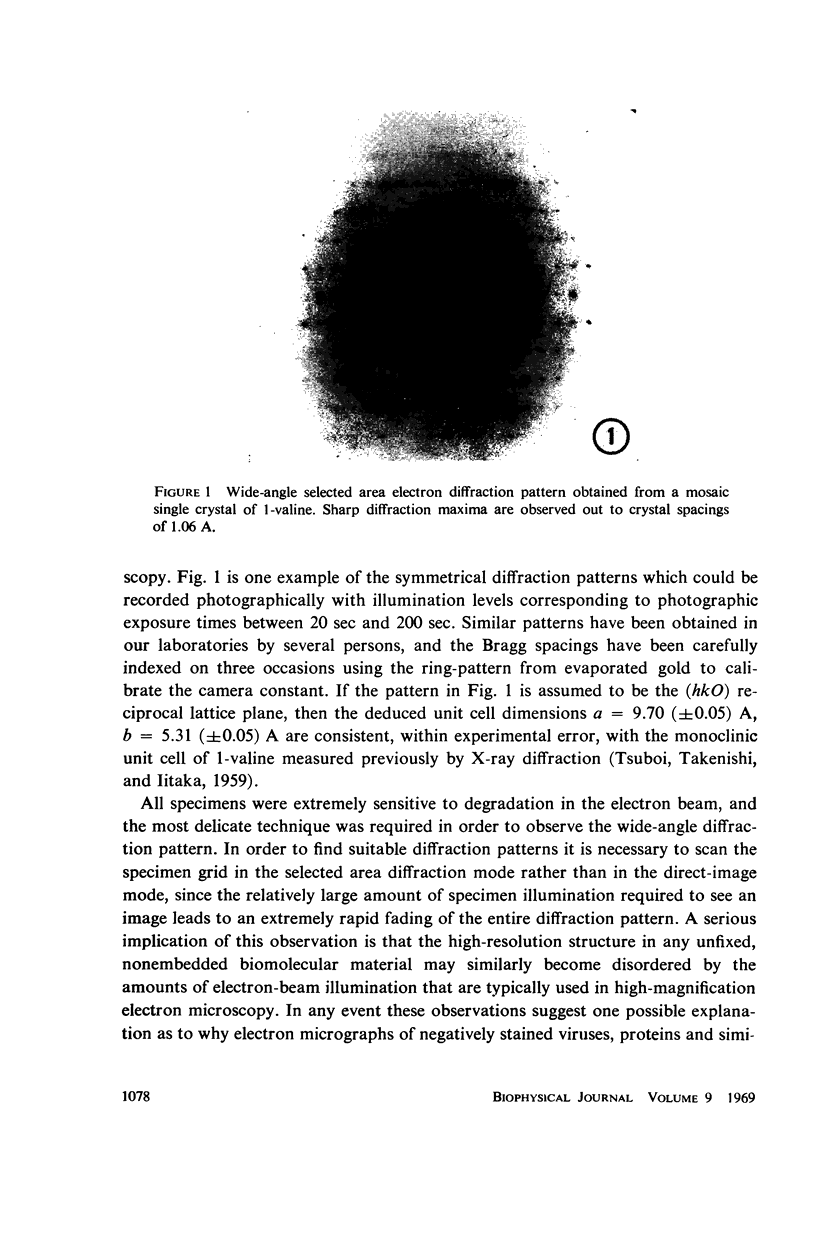
Three methods by which electron diffraction may be applied to problems in electron microscopy are discussed from a fundamental point of view, and experimental applications with biological specimens are demonstrated for each case. It is shown that wide-angle electron diffraction provides valuable information for evaluating specimen damage that can occur either during specimen preparation or while in the electron beam. Dark-field electron microscopy can be used both to enhance the image contrast and to provide highly restricted and therefore highly specific information about the object. Low-angle electron diffraction provides quantitative information about the object structure in the range from 20 A to ∼ 1000 A. Lowangle electron diffraction also demonstrates the important role of Fourier contrast with biological specimens, which are usually characterized by structural features with dimensions of 20 A or larger.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fischbach F. A., Anderegg J. W. An x-ray scattering study of ferritin and apoferritin. J Mol Biol. 1965 Dec;14(2):458–473. doi: 10.1016/s0022-2836(65)80196-6. [DOI] [PubMed] [Google Scholar]
- Glaeser R. M., Hayes T., Mel H., Tobias C. Membrane structure of OsO4-fixed erythrocytes viewed "face on" by electron microscope techniques. Exp Cell Res. 1966 Jun;42(3):467–477. doi: 10.1016/0014-4827(66)90260-6. [DOI] [PubMed] [Google Scholar]
- HUXLEY H. E. X-ray analysis and the problem of muscle. Proc R Soc Lond B Biol Sci. 1953 Mar 11;141(902):59–62. doi: 10.1098/rspb.1953.0017. [DOI] [PubMed] [Google Scholar]
- Haas D. J. Preliminary studies on the denaturation of cross-linked lysozyme crystals. Biophys J. 1968 May;8(5):549–555. doi: 10.1016/S0006-3495(68)86507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. T., Ferrier R. P. Biological applications of electron diffraction. J Ultrastruct Res. 1967 Dec;21(5):361–377. doi: 10.1016/s0022-5320(67)80146-1. [DOI] [PubMed] [Google Scholar]
- Pease D. C. Eutectic ethylene glycol and pure propylene glycol as substituting media for the dehydration of frozen tissue. J Ultrastruct Res. 1967 Nov;21(1):75–97. doi: 10.1016/s0022-5320(67)80007-8. [DOI] [PubMed] [Google Scholar]
- Pease D. C. The preservation of unfixed cytological detail by dehydration with "inert" agents. J Ultrastruct Res. 1966 Feb;14(3):356–378. doi: 10.1016/s0022-5320(66)80054-0. [DOI] [PubMed] [Google Scholar]
- SAGE H. J., SINGER S. J. The properties of bovine pancreatic ribonuclease in ethylene glycol solution. Biochemistry. 1962 Mar;1:305–317. doi: 10.1021/bi00908a018. [DOI] [PubMed] [Google Scholar]
- TANFORD C., BUCKLEY C. E., 3rd, DE P. K., LIVELY E. P. Effect of ethylene glycol on the conformation of gama-globulin and beta-lactoglobulin. J Biol Chem. 1962 Apr;237:1168–1171. [PubMed] [Google Scholar]
- VALENTINE R. C. SUB-UNITS OF THE CATALASE MOLECULE SEEN BY ELECTRON MICROSCOPY. Nature. 1964 Dec 26;204:1262–1264. doi: 10.1038/2041262b0. [DOI] [PubMed] [Google Scholar]