Abstract
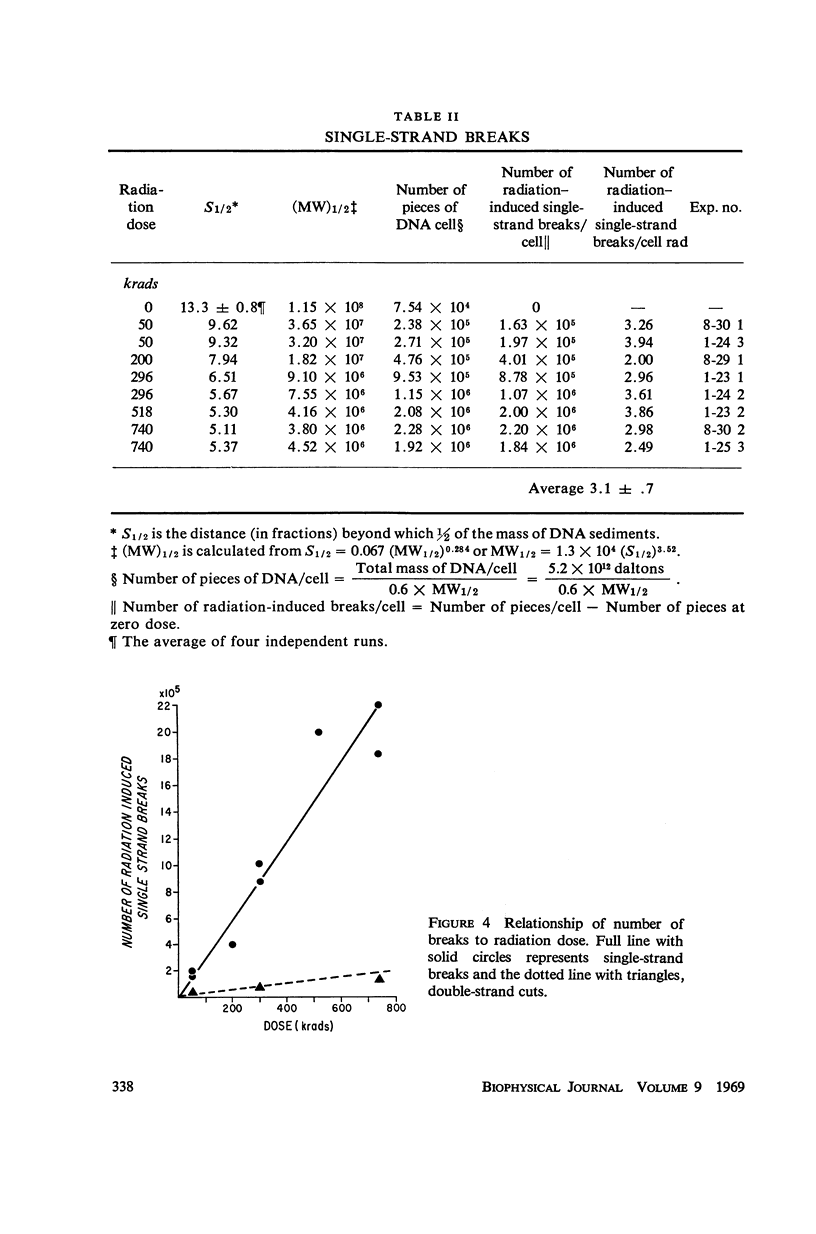
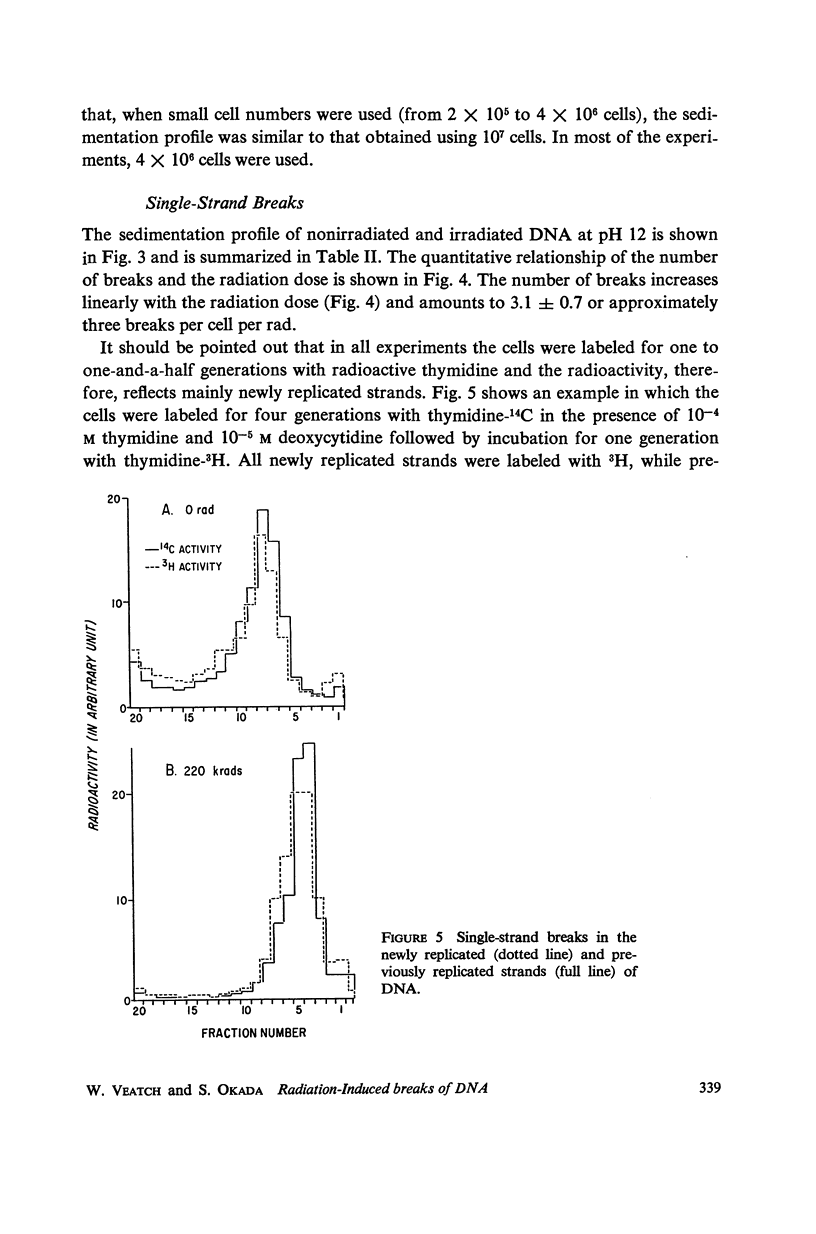
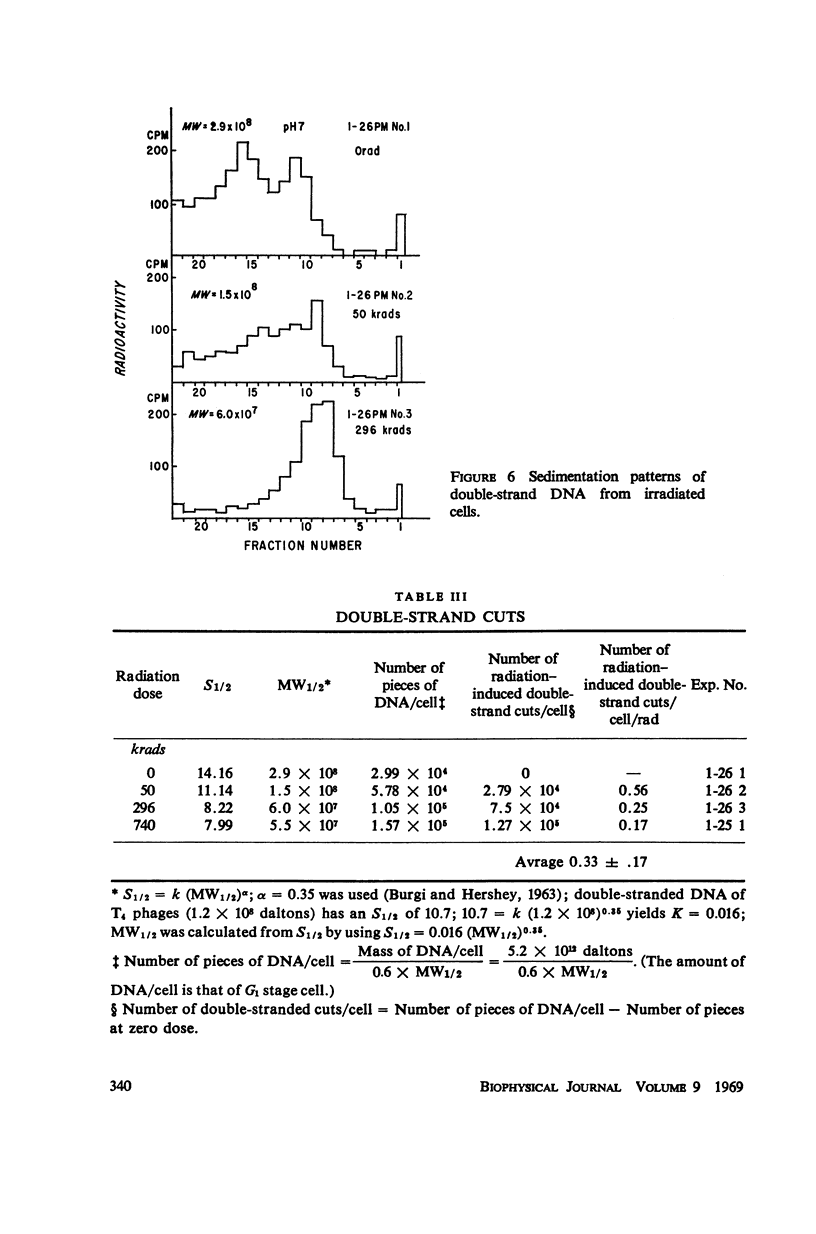
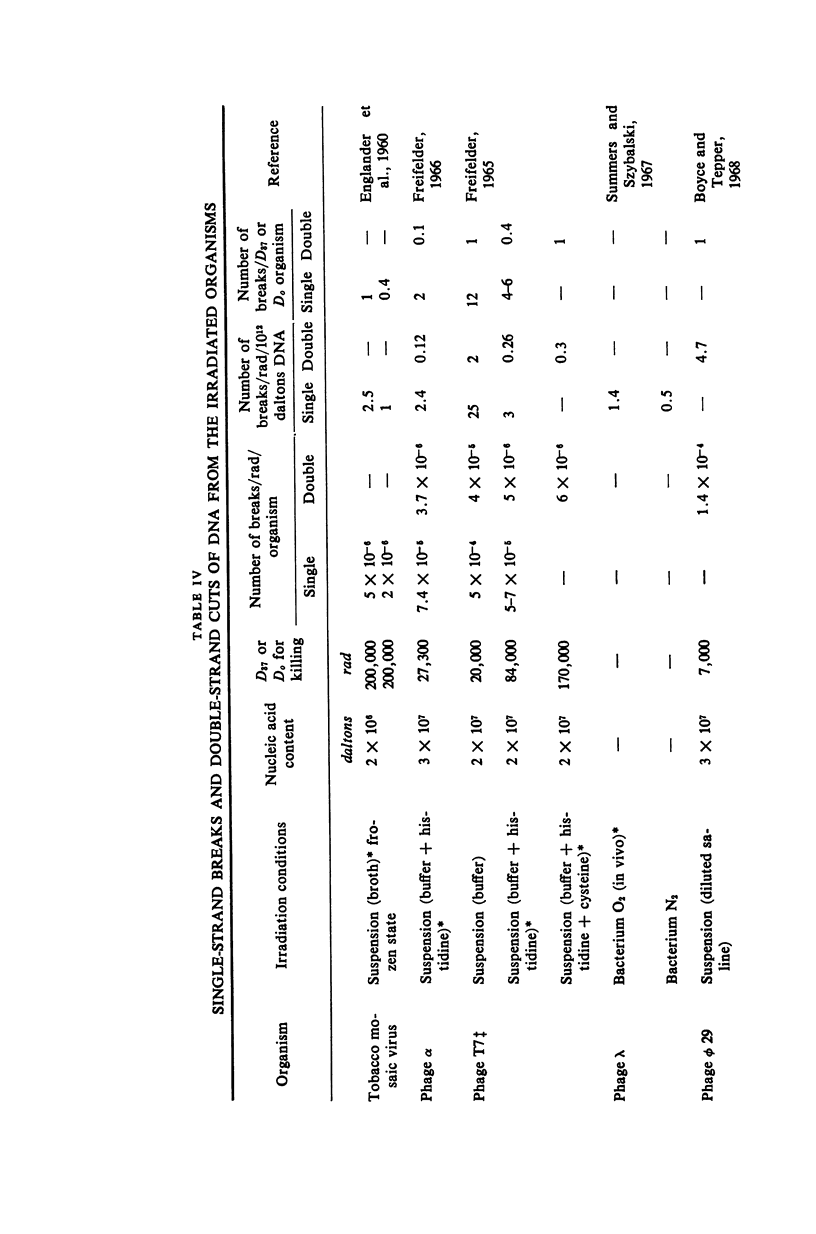
Mouse leukemic cells (L5178Y) in suspension culture were irradiated and the extent of single-strand breaks and double-strand cuts of DNA was estimated by sucrose gradient centrifugation. The radiation produced 3.0 single-strand breaks per cell (G1 stage) per rad and approximately 0.3 double-strand breaks per cell (G1 stage) per rad.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achey P. M., Whitfield V. G. Influence of anoxia on radiation-induced breaks in the Escherichia coli chromosome. J Bacteriol. 1968 Mar;95(3):1180–1181. doi: 10.1128/jb.95.3.1180-1181.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce R. P., Tepper M. X-ray-induced single-strand breaks and joining of broken strands in superinfecting lambda DNA in Escherichia coli lysogenic for lambda. Virology. 1968 Feb;34(2):344–351. doi: 10.1016/0042-6822(68)90245-6. [DOI] [PubMed] [Google Scholar]
- Burki H. J., Okada S. Effective specific activity dilution in labeled DNA of cultured mammalian cells (L5178Y). Biophys J. 1969 Feb;9(2):122–126. doi: 10.1016/S0006-3495(69)86373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLE A., HUMPHREY R. M., DEWEY W. C. LOW-VOLTAGE ELECTRON BEAM IRRADIATION OF NORMAL AND 5-BROMOURIDINE DEOXYRIBOSIDE-TREATED L-P59 MOUSE FIBROBLAST CELLS IN VITRO. Nature. 1963 Aug 24;199:780–782. doi: 10.1038/199780a0. [DOI] [PubMed] [Google Scholar]
- COLLYNS B., OKADA S., SCHOLES G., WEISS J. J., WHEELER C. M. CHAIN SCISSION AND HYDROGEN BOND BREAKAGE ON IRRADIATION OF DNA. Radiat Res. 1965 Jul;25:526–536. [PubMed] [Google Scholar]
- DJORDJEVIC B., SZYBALSKI W. Genetics of human cell lines. III. Incorporation of 5-bromo- and 5-iododeoxyuridine into the deoxyribonucleic acid of human cells and its effect on radiation sensitivity. J Exp Med. 1960 Sep 1;112:509–531. doi: 10.1084/jem.112.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLANDER S. W., BUZZELL A., LAUFFER M. A. The relationship between inactivation of tobacco mosaic virus by x-rays and breakage of nucleic acid. Biochim Biophys Acta. 1960 Jun 3;40:385–392. doi: 10.1016/0006-3002(60)91378-0. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Mechanism of inactivation of coliphage T7 by x-rays. Proc Natl Acad Sci U S A. 1965 Jul;54(1):128–134. doi: 10.1073/pnas.54.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Rate of production of single-strand breaks in DNA by x-irradiation in situ. J Mol Biol. 1968 Jul 28;35(2):303–309. doi: 10.1016/s0022-2836(68)80026-9. [DOI] [PubMed] [Google Scholar]
- Kaplan H. S. DNA-strand scission and loss of viability after x irradiation of normal and sensitized bacterial cells. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1442–1446. doi: 10.1073/pnas.55.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett J. T., Caldwell I., Dean C. J., Alexander P. Rejoining of x-ray induced breaks in the DNA of leukaemia cells. Nature. 1967 May 20;214(5090):790–792. doi: 10.1038/214790a0. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Interruptions in single strands of the DNA in slime mold and other organisms. Biophys J. 1967 May;7(3):309–317. doi: 10.1016/S0006-3495(67)86590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Okada S. Replicating units (replicons) of DNA in cultured mammalian cells. Biophys J. 1968 May;8(5):650–664. doi: 10.1016/S0006-3495(68)86513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAGNI G., SZYBALSKI W. Molecular radiobiology of human cells lines. II. Effects of thymidine replacement by halogenated analogues on cell inactivation by decay of incorporated radiophosphorus. J Mol Biol. 1962 May;4:338–346. doi: 10.1016/s0022-2836(62)80014-x. [DOI] [PubMed] [Google Scholar]
- Summers W. C., Szybalski W. Gamma-irradiation of deoxyribonucleic acid in dilute solutions. II. Molecular mechanisms responsible for inactivation of phage, its transfecting DNA, and of bacterial transforming activity. J Mol Biol. 1967 Jun 14;26(2):227–235. doi: 10.1016/0022-2836(67)90293-8. [DOI] [PubMed] [Google Scholar]
- Watanabe I., Okada S. Effects of temperature on growth rate of cultured mammalian cells (L5178Y). J Cell Biol. 1967 Feb;32(2):309–323. doi: 10.1083/jcb.32.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]



