Abstract
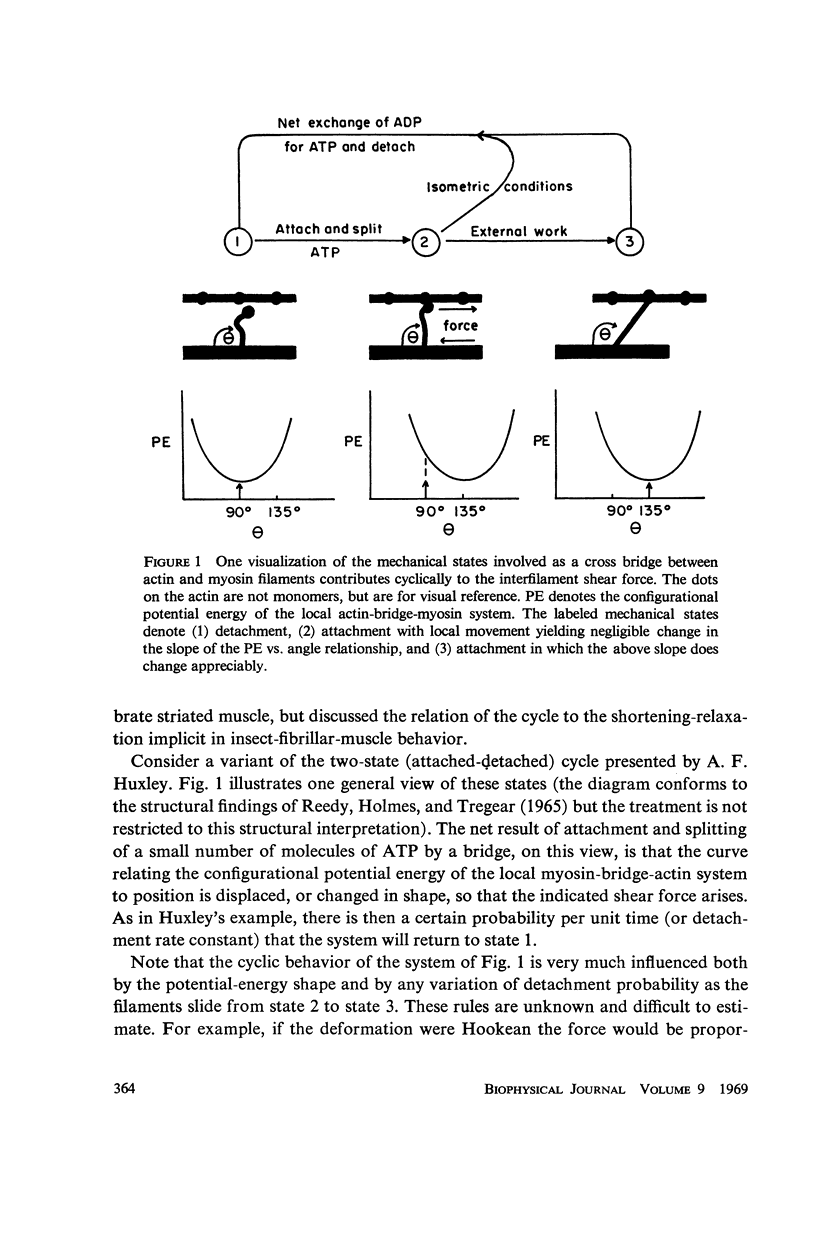
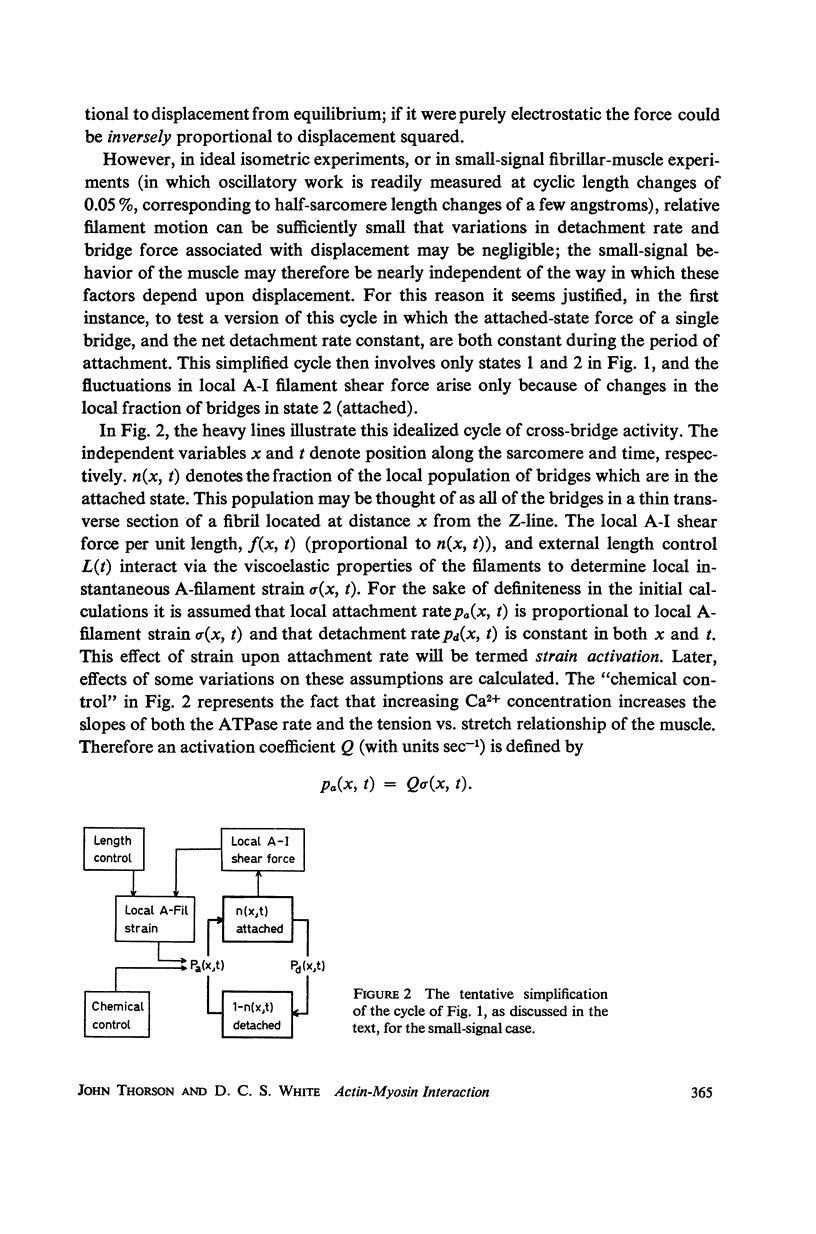
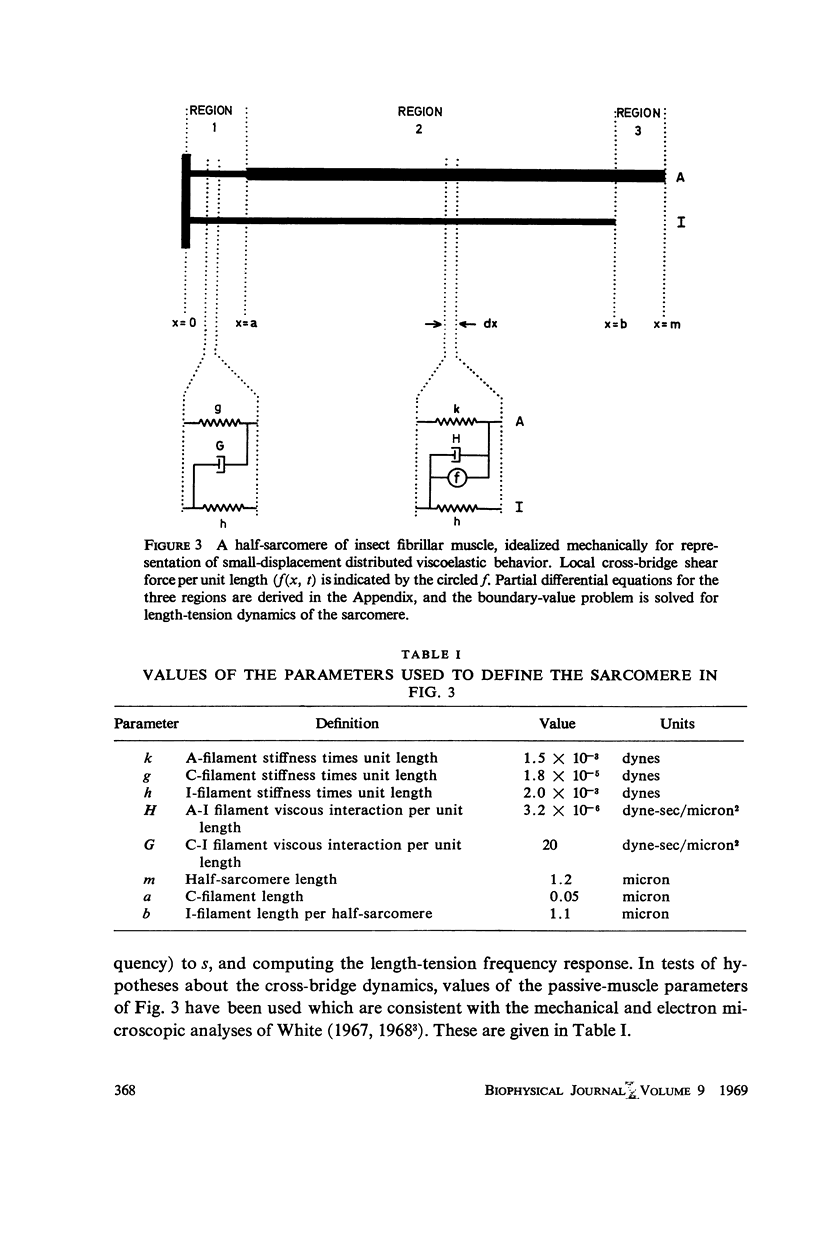
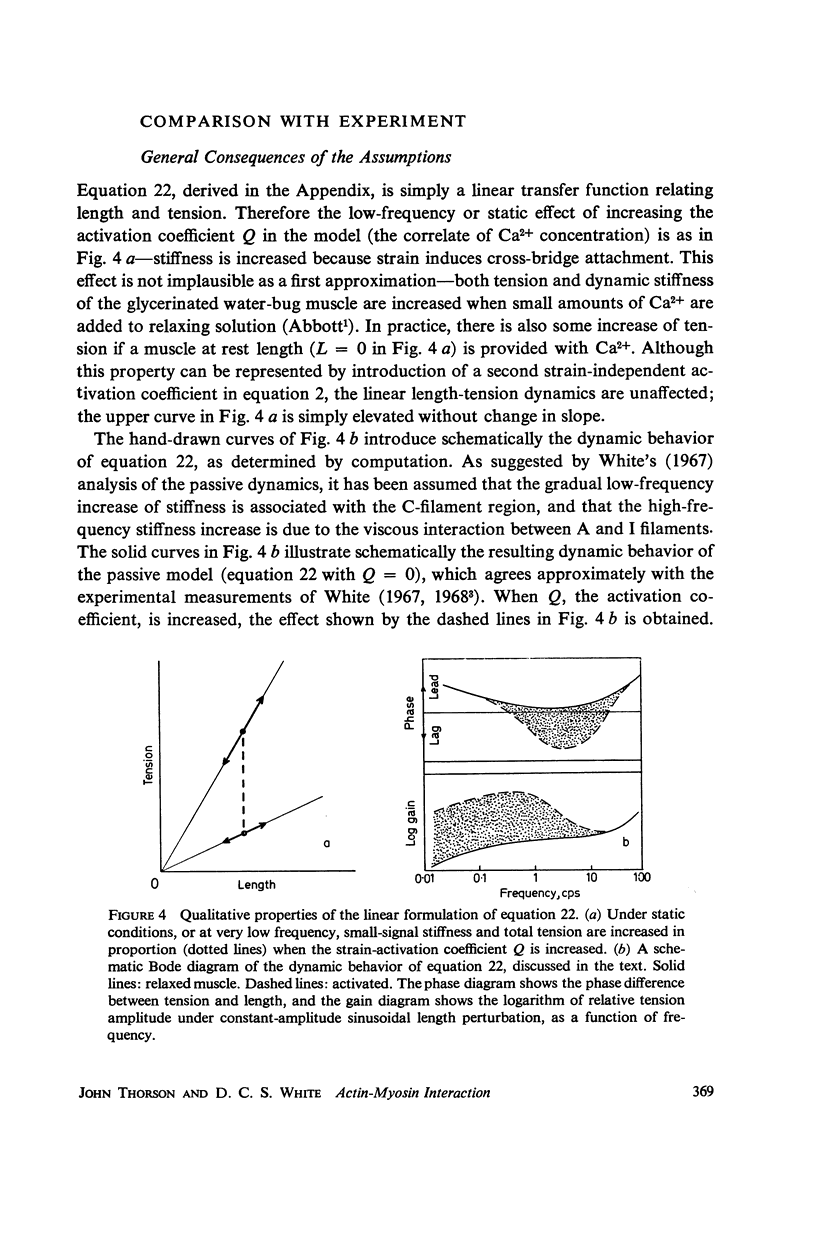
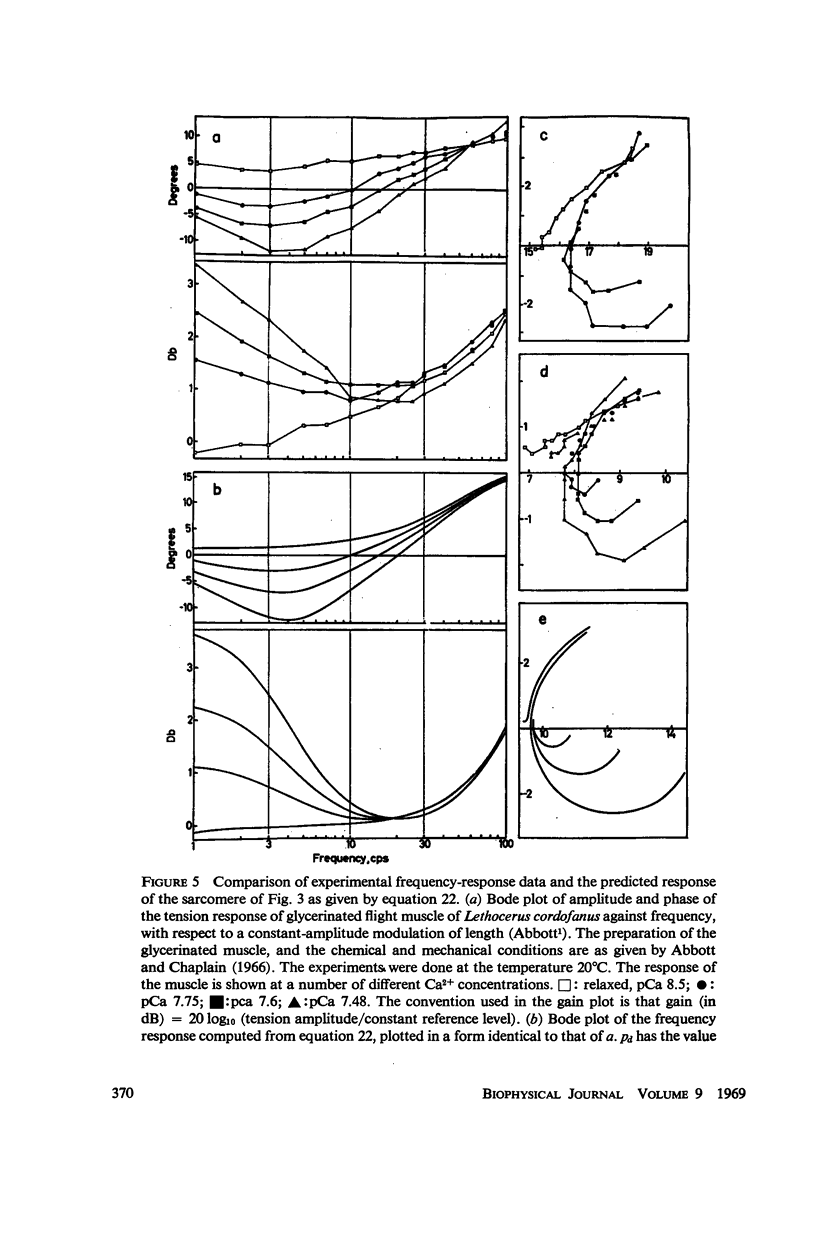
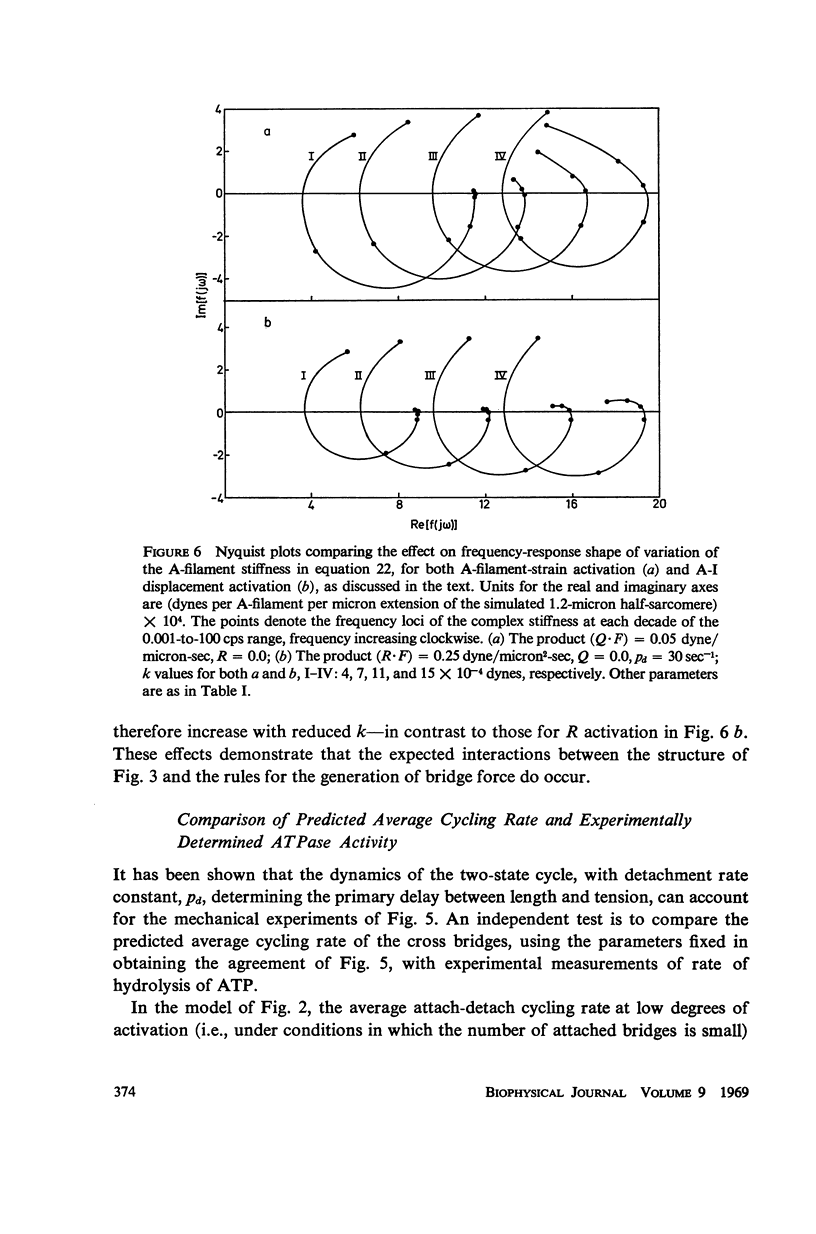
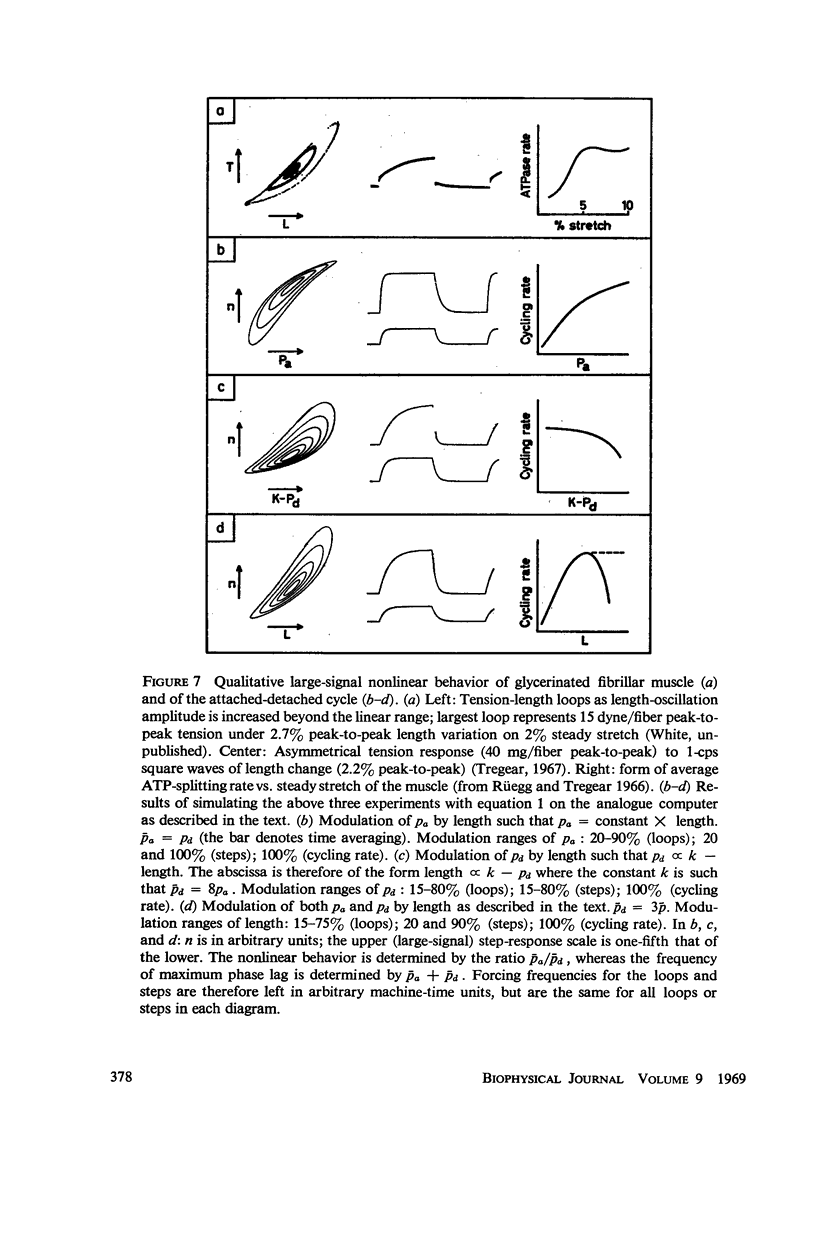
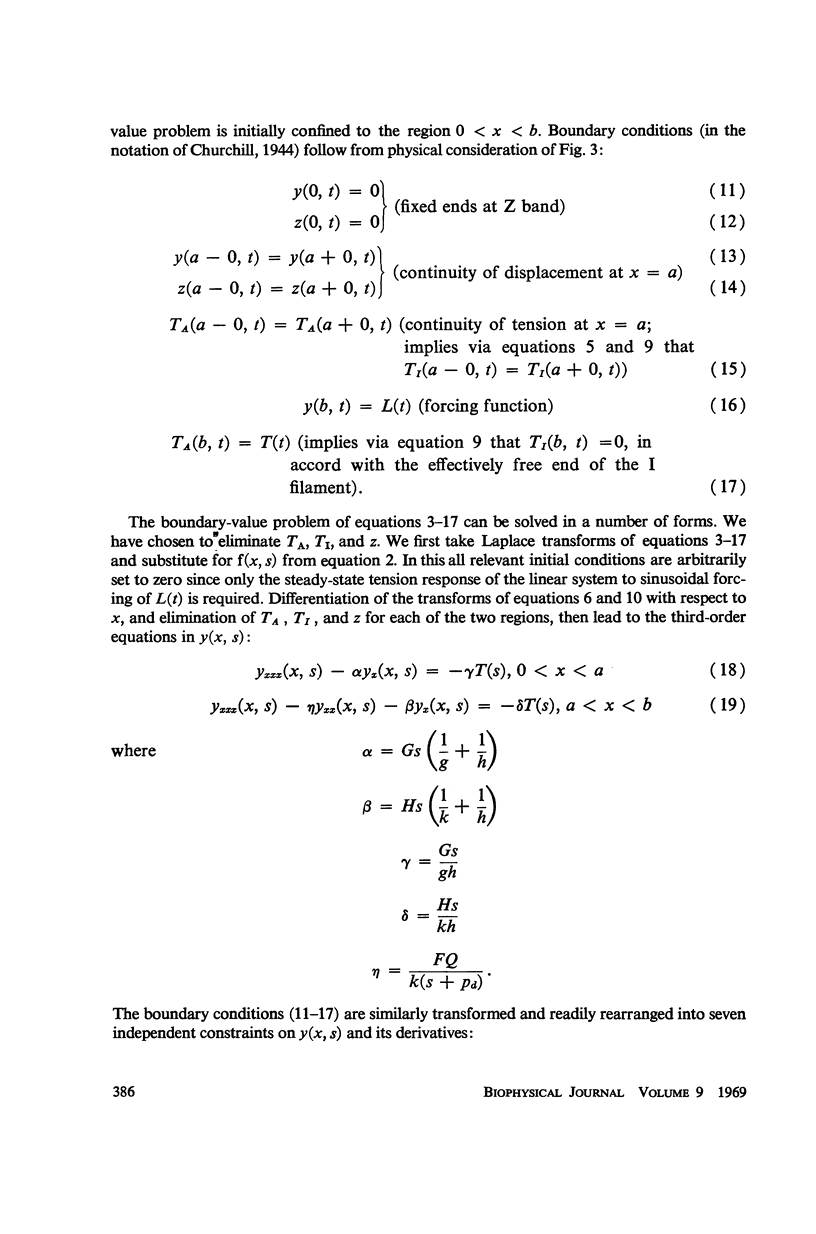
In this paper we suggest and test a specific hypothesis relating the attachment-detachment cycle of cross bridges between actin (I) and myosin (A) filaments to the measured length-tension dynamics of active insect fibrillar flight muscle. It is first shown that if local A-filament strain perturbs the rate constants in the cross-bridge cycle appropriately, then exponentially delayed tension changes can follow imposed changes of length; the latter phenomenon is sufficient for the work-producing property of fibrillar muscle, as measured with small-signal forcing of length and at low Ca2+ concentration, and possibly for related effects described recently in frog striated muscle. It is not clear a priori that the above explanation of work production by fibrillar muscle will remain tenable when the viscoelastic complexity of the heterogeneous sarcomere is taken into account. However, White's (1967) recent mechanical and electron microscope study of the passive dynamics of glycerinated fibrillar muscle has produced a model of the distributed viscoeleastic structure sufficiently explicit that alternative schemes for cross-bridge force generation in this muscle can now be tested more critically than previously. Therefore, we derive and solve third-order partial-differential equations which relate local interfilament shear forces associated with the perturbed cross-bridge cycles to the over-all length-tension dynamics of an idealized sarcomere. We then show (a) that the starting hypothesis can account approximately for the small-signal dynamics of glycerinated muscle in the work-producing state over two decades of frequency and (b) that the rate constants for cross-bridge formation and breakage, restricted solely by fitting of the model to the mechanical data, determine a cycling rate of cross bridges in the model compatible with recent measurements of ATP hydrolysis rate vs. stretch in this muscle. Finally, the formulation is extended tentatively to the large-signal nonlinear case, and shown to compare favorably with previous suggestions for the origin of the work-producing dynamics of fibrillar flight muscle.
Full text
PDF






















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott R. H., Chaplain R. A. Preparation and properties of the contractile element of insect fibrillar muscle. J Cell Sci. 1966 Sep;1(3):311–330. doi: 10.1242/jcs.1.3.311. [DOI] [PubMed] [Google Scholar]
- Ashhurst D. E. Z-line of the flight muscle of belostomatid water bugs. J Mol Biol. 1967 Jul 28;27(2):385–389. doi: 10.1016/0022-2836(67)90027-7. [DOI] [PubMed] [Google Scholar]
- Chaplain R. A., Abbott R. H., White D. C. Indication for an allosteric effect of ADP on actomyosin gels and glycerinated fibres from insect fibrillar flight muscle. Biochem Biophys Res Commun. 1965 Oct 26;21(2):89–93. doi: 10.1016/0006-291x(65)90091-4. [DOI] [PubMed] [Google Scholar]
- Chaplain R. A. The effect of Ca2+ and fibre elongation on the activation of the contractile mechanism of insect fibrillar flight muscle. Biochim Biophys Acta. 1967 Mar 8;131(2):385–392. doi: 10.1016/0005-2728(67)90152-1. [DOI] [PubMed] [Google Scholar]
- Chaplain R. A., Tregear R. T. The mass of myosin per cross-bridge in insect fibrillar flight muscle. J Mol Biol. 1966 Nov 14;21(2):275–280. doi: 10.1016/0022-2836(66)90098-2. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- HUXLEY H. E. The double array of filaments in cross-striated muscle. J Biophys Biochem Cytol. 1957 Sep 25;3(5):631–648. doi: 10.1083/jcb.3.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingels N. P., Thompson N. P. An electrokinematic theory of muscle contraction. Nature. 1966 Sep 3;211(5053):1032–1035. doi: 10.1038/2111032a0. [DOI] [PubMed] [Google Scholar]
- MACHIN K. E., PRINGLE J. W. The physiology of insect fibrillar muscle. III. The effect of sinusoidal changes of length on a beetle flight muscle. Proc R Soc Lond B Biol Sci. 1960 Jun 14;152:311–330. doi: 10.1098/rspb.1960.0041. [DOI] [PubMed] [Google Scholar]
- MIHALYI E., SZENT-GYORGYI A. G. Trypsin digestion of muscle proteins. III. Adenosinetriphosphatase activity and actinbinding capacity of the digested myosin. J Biol Chem. 1953 Mar;201(1):211–219. [PubMed] [Google Scholar]
- Maruyama K., Pringle J. W., Tregear R. T. The calcium sensitivity of ATPase activity of myofibrils and actomyosins from insect flight and leg muscles. Proc R Soc Lond B Biol Sci. 1968 Feb 27;169(1016):229–240. doi: 10.1098/rspb.1968.0008. [DOI] [PubMed] [Google Scholar]
- Pringle J. W. The excitation and contraction of the flight muscles of insects. J Physiol. 1949 Mar 15;108(2):226–232. doi: 10.1113/jphysiol.1949.sp004326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedy M. K., Holmes K. C., Tregear R. T. Induced changes in orientation of the cross-bridges of glycerinated insect flight muscle. Nature. 1965 Sep 18;207(5003):1276–1280. doi: 10.1038/2071276a0. [DOI] [PubMed] [Google Scholar]
- SPENCER M., WORTHINGTON C. R. A hypothesis of contraction in striated muscle. Nature. 1960 Jul 30;187:388–391. doi: 10.1038/187388a0. [DOI] [PubMed] [Google Scholar]



