Abstract
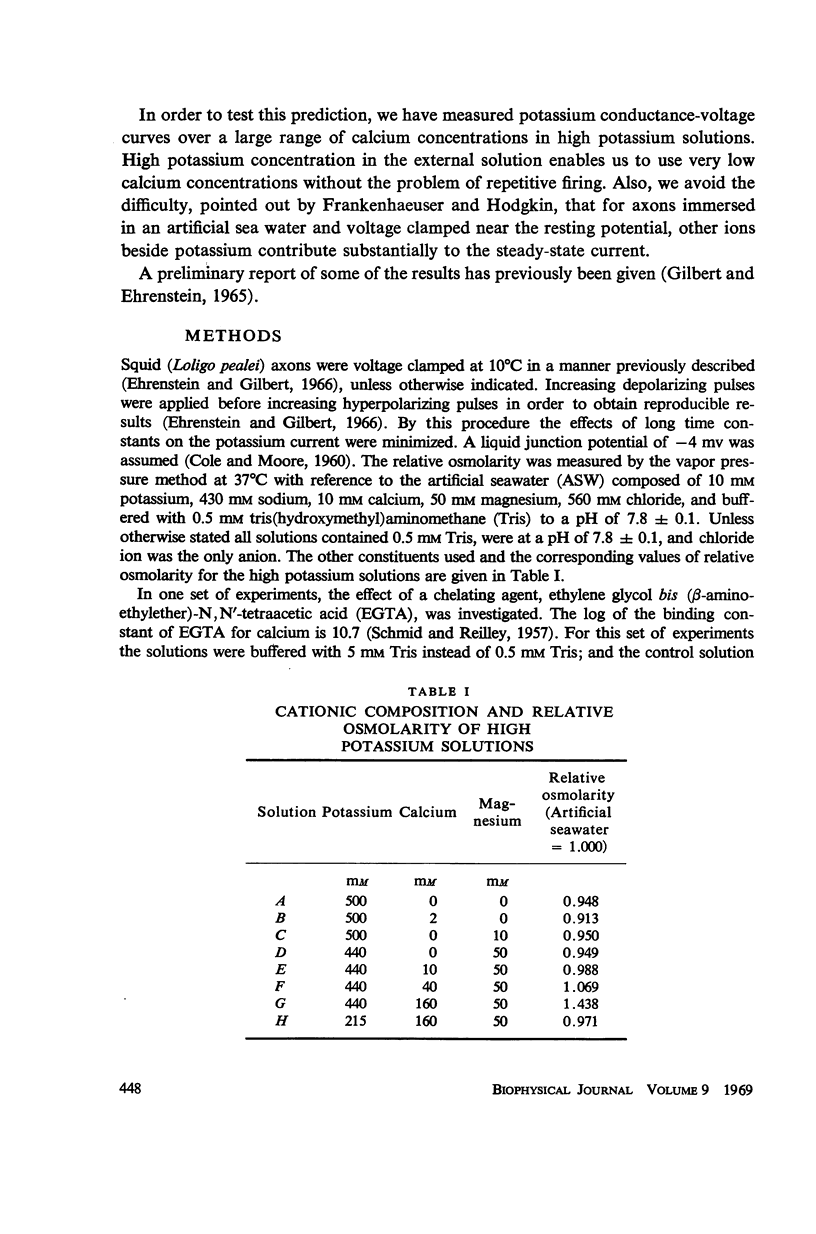
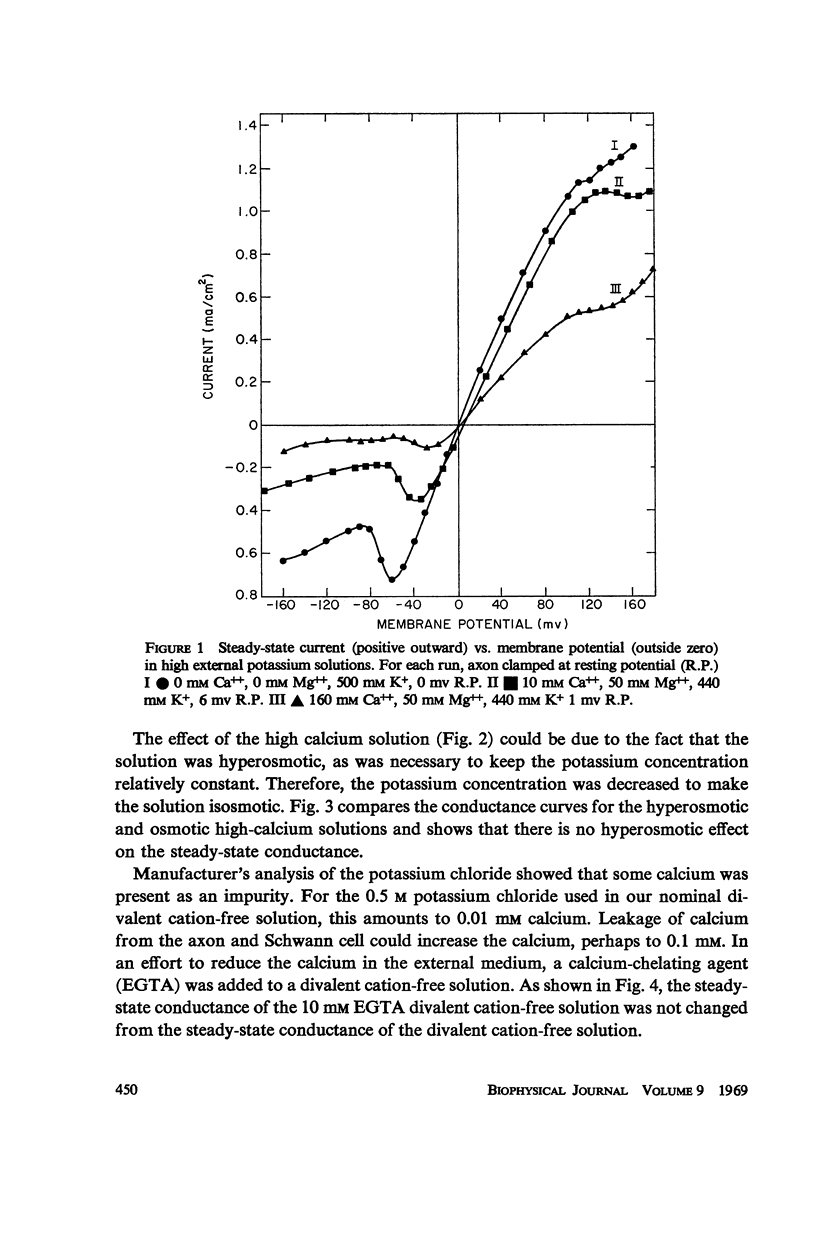
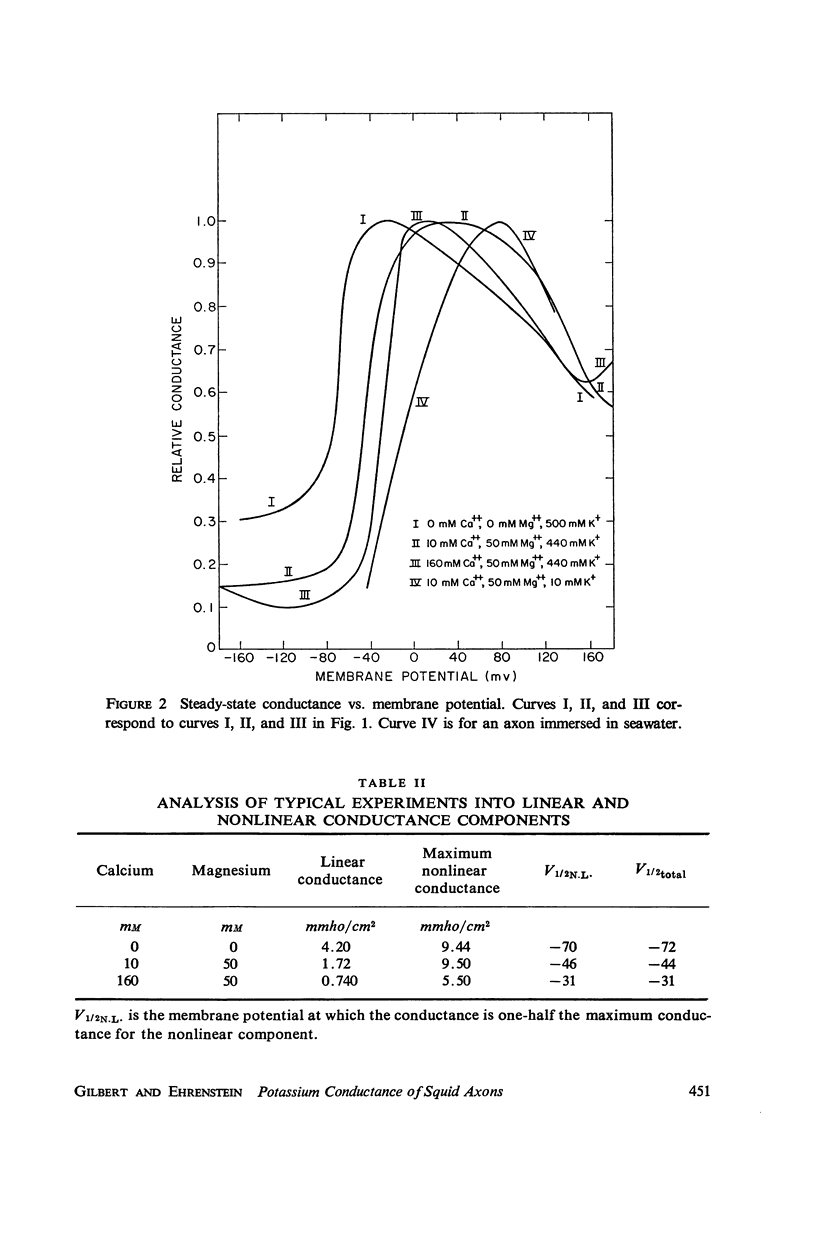
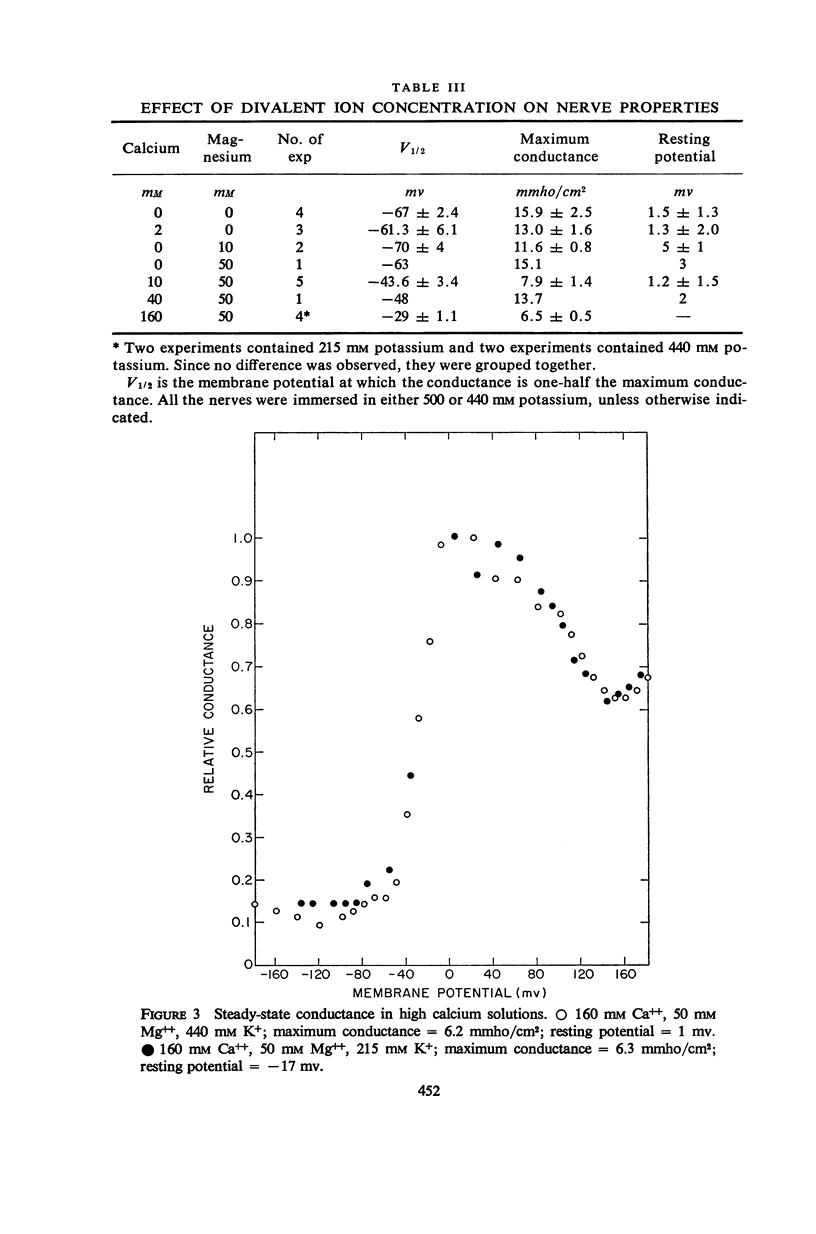
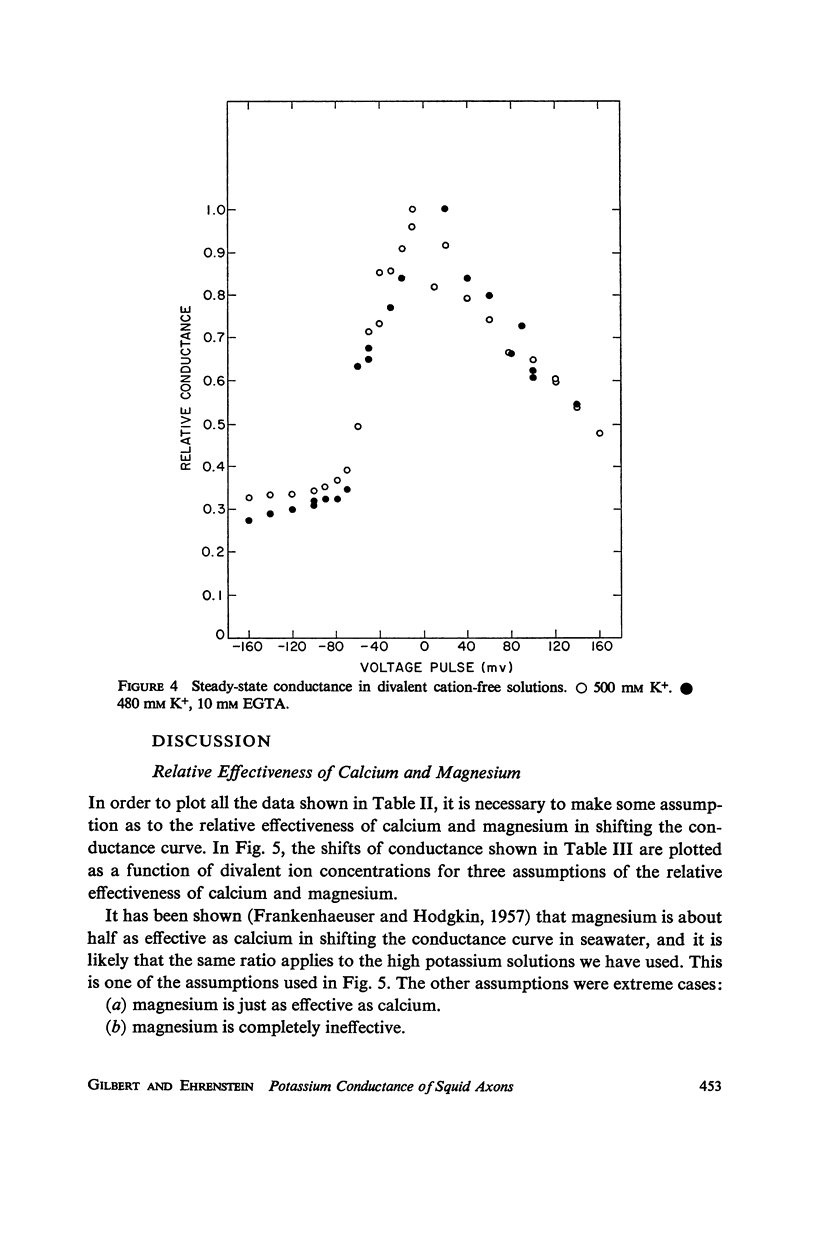
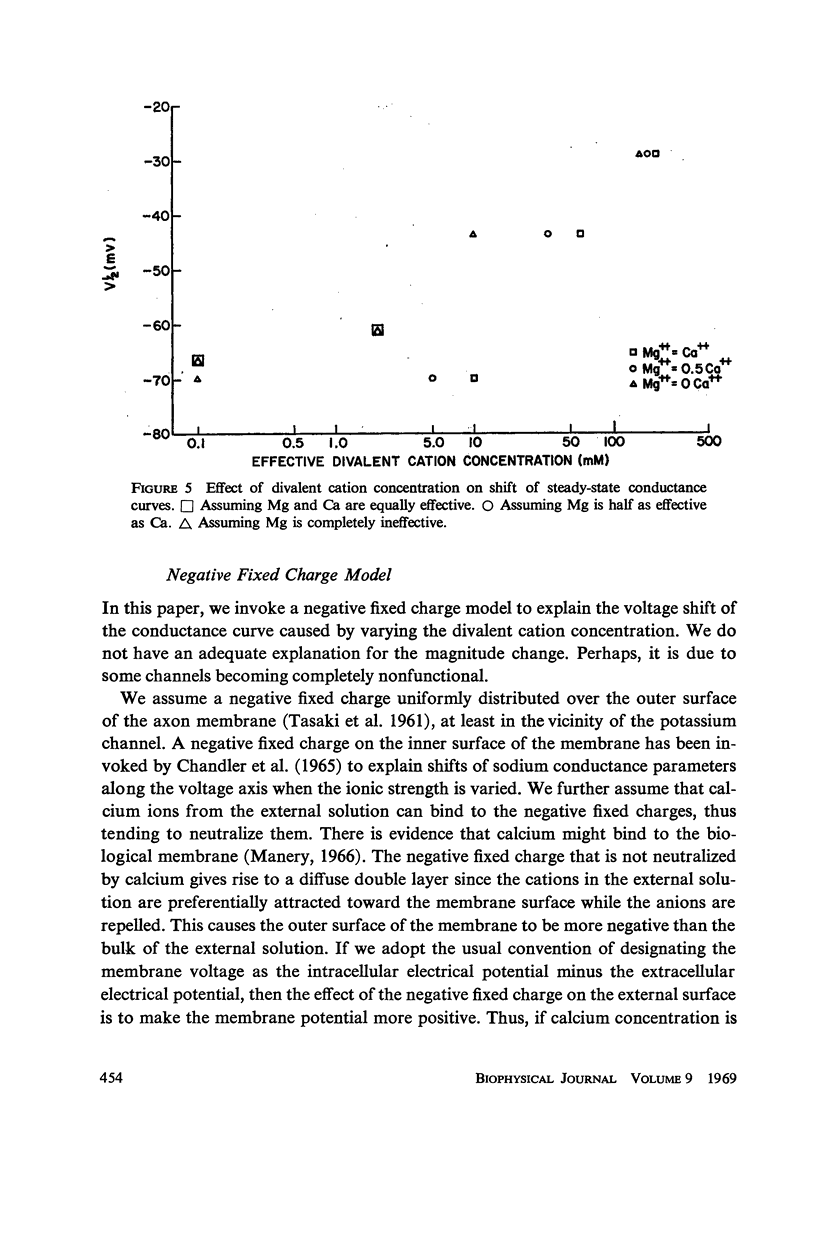
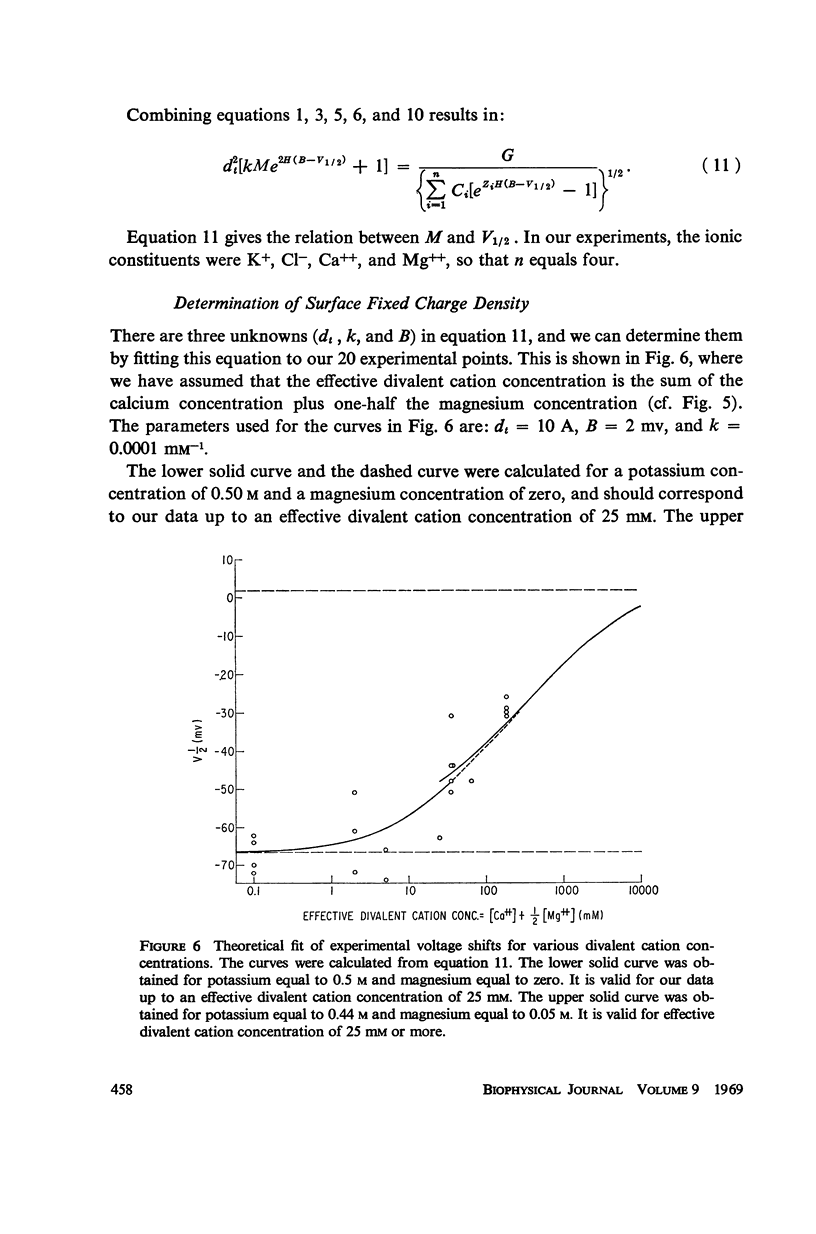
Potassium conductance-voltage curves have been determined for a squid axon in high external potassium solution for a wide range of divalent cation concentrations. A decrease in divalent ion concentration shifts the conductance-voltage curve along the voltage axis in the direction of more hyperpolarized voltages by as much as 9 mv for an e-fold change in concentration. When the divalent ion concentration is less than about 5 mM, a further decrease does not cause a significant shift of the conductance-voltage curve. These results can be explained by assuming that on the outer surface of the membrane there is a negative fixed charge which can bind calcium ions, and that the axon is sensitive to the resulting double-layer potential. From our data, the best value for charge density was found to be one electronic charge per 120 square angstroms, and a lower limit to be one electronic charge per 280 square angstroms.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaustein M. P., Goldman D. E. The action of certain polyvalent cations on the voltage-clamped lobster axon. J Gen Physiol. 1968 Mar;51(3):279–291. doi: 10.1085/jgp.51.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLE K. S., MOORE J. W. Liquid junction and membrane potentials of the squid giant axon. J Gen Physiol. 1960 May;43:971–980. doi: 10.1085/jgp.43.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole K. S. Zeta potential and discrete vs. uniform surface charges. Biophys J. 1969 Mar;9(3):465–469. doi: 10.1016/S0006-3495(69)86397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAN K. Electrokinetic studies of marine ova; the effect of salts on the zeta potential of the eggs of Strongylocentrotus pulcherrimus. Biol Bull. 1947 Dec;93(3):267–273. [PubMed] [Google Scholar]
- Ehrenstein G., Gilbert D. L. Slow changes of potassium permeability in the squid giant axon. Biophys J. 1966 Sep;6(5):553–566. doi: 10.1016/S0006-3495(66)86677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elul R. Fixed charge in the cell membrane. J Physiol. 1967 Apr;189(3):351–365. doi: 10.1113/jphysiol.1967.sp008173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. The effect of calcium on the myelinated nerve fibre. J Physiol. 1957 Jul 11;137(2):245–260. doi: 10.1113/jphysiol.1957.sp005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenhaeuser B., Lännergren J. The effect of calcium on the mechanical response of single twitch muscle fibres of Xenopus laevis. Acta Physiol Scand. 1967 Mar;69(3):242–254. doi: 10.1111/j.1748-1716.1967.tb03518.x. [DOI] [PubMed] [Google Scholar]
- GRAHAME D. C. The electrical double layer and the theory of electrocapillarity. Chem Rev. 1947 Dec;41(3):441–501. doi: 10.1021/cr60130a002. [DOI] [PubMed] [Google Scholar]
- Goldman L. The effects of some ions on the membrane potential of the giant axon of Myxicola. J Cell Physiol. 1968 Feb;71(1):33–42. doi: 10.1002/jcp.1040710106. [DOI] [PubMed] [Google Scholar]
- Goodford P. J. An interaction between potassium and sodium in the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Sep;186(1):11–26. doi: 10.1113/jphysiol.1966.sp008017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J Gen Physiol. 1967 Jan;50(3):583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Charges and potentials at the nerve surface. Divalent ions and pH. J Gen Physiol. 1968 Feb;51(2):221–236. doi: 10.1085/jgp.51.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JULIAN F. J., MOORE J. W., GOLDMAN D. E. Current-voltage relations in the lobster giant axon membrane under voltage clamp conditions. J Gen Physiol. 1962 Jul;45:1217–1238. doi: 10.1085/jgp.45.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecar H., Ehrenstein G., Binstock L., Taylor R. E. Removal of potassium negative resistance in perfused squid giant axons. J Gen Physiol. 1967 Jul;50(6):1499–1515. doi: 10.1085/jgp.50.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manery J. F. Effects of Ca ions on membranes. Fed Proc. 1966 Nov-Dec;25(6):1804–1810. [PubMed] [Google Scholar]
- Rojas E., Atwater I. An experimental approach to determine membrane charges in squid giant axons. J Gen Physiol. 1968 May;51(5 Suppl):131S+–131S+. [PubMed] [Google Scholar]
- Segal J. R. Surface charge of giant axons of squid and lobster. Biophys J. 1968 Apr;8(4):470–489. doi: 10.1016/S0006-3495(68)86501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TASAKI I., TEORELL T., SPYROPOULOS C. S. Movement of radioactive tracers across squid axon membrane. Am J Physiol. 1961 Jan;200:11–22. doi: 10.1152/ajplegacy.1961.200.1.11. [DOI] [PubMed] [Google Scholar]



