Abstract
The interaction of viruses with host cell receptors is the initial step in viral infection and is an important determinant of virus host range, tissue tropism, and pathogenesis. The complement regulatory protein decay-accelerating factor (DAF/CD55) is an attachment receptor for enterovirus 70 (EV70), a member of the Picornaviridae, commonly associated with an eye infection in humans known as acute hemorrhagic conjunctivitis. In early work, the EV70 receptor on erythrocytes, responsible for its hemagglutinating activity, was shown to be sensitive to neuraminidase, implying an essential role for sialic acid in virus attachment. Here, we extend these results to show that cell surface sialic acid is required for EV70 binding to nucleated cells susceptible to virus infection and that sialic acid binding is important in productive infection. Through the use of site-directed mutagenesis to eliminate the single N-linked glycosylation site of DAF and of a chimeric receptor protein in which the O-glycosylated domain of DAF was replaced by a region of the HLA-B44 molecule, a role in EV70 binding for the sialic acid residues of DAF was excluded, suggesting the existence of at least one additional, sialylated EV70-binding factor at the cell surface. Treatment of cells with metabolic inhibitors of glycosylation excluded a role for the N-linked oligosaccharides of glycoproteins but suggested that O-linked glycosylation is important for EV70 binding.
Enterovirus 70 (EV70) is an unusual human pathogen of the Enterovirus genus of the family Picornaviridae. Unlike the majority of human enteroviruses, EV70 apparently does not replicate in the enteric tract but rather is primarily associated with an infection of the eye commonly referred to as acute hemorrhagic conjunctivitis (AHC) (for a review, see reference 49). Since the emergence of the virus in western Africa in 1969, it has been responsible for two pandemics and numerous smaller outbreaks and has been implicated in roughly 100 million cases of AHC; most recently, occurrences have been reported in India (24, 47), Japan (44), and Israel (38). In rare instances, a neurological illness that resembles acute poliomyelitis follows, usually within several weeks of the onset of the conjunctivitis (13), suggesting that the tissue tropism of EV70 also includes the central nervous system (CNS). It has also been observed that, whereas the majority of human enteroviruses are restricted in vitro to replication in cells of human or primate origin, EV70 replicates with various efficiencies in cells derived from a wide variety of mammalian species (50).
The first step in a viral infection is the binding of the virus to a specific cell surface receptor. This step is often an important determinant of virus host range, tissue tropism, and pathogenesis (for a review see reference 34) and may contribute to the molecular basis for the unusual tissue tropism and host range of EV70. Previous work in our laboratory demonstrated that EV70 binds to the complement control protein decay-accelerating factor (DAF/CD55) on susceptible cells (18). Although DAF is expressed in the epithelia of the eye and in the CNS (the sites of EV70 replication) (26), it is also widely expressed in other tissues in vivo, and thus the fact that EV70 binds to DAF cannot alone account for the tissue tropism of the virus. Furthermore, DAF is utilized as an attachment receptor by a range of human enteroviruses, whose patterns of tropism and pathogenesis vary widely (11). For many of these DAF-binding viruses, additional molecules, possibly associated with DAF at the cell surface or involved at a post-DAF binding stage, are required for virus entry; these accessory molecules may confer a more limited tissue specificity (11). Early work on EV70 receptor utilization indicated that sialic acid, a terminal, negatively charged sugar molecule commonly found on cell surface glycoproteins and glycolipids, was required for viral hemagglutination of human erythrocytes (46). However, the significance of this interaction to the life cycle of the virus is unclear. Here, we extend these observations by demonstrating that sialic acid is required for EV70 binding to susceptible nucleated cells and that this binding is important for productive infection. Further, we demonstrate that critical sialic acid residues for EV70 binding do not reside in either the N-linked or O-linked glycosylation domains of DAF, implicating additional factors in EV70 attachment and entry.
MATERIALS AND METHODS
Cells, cell culture, and viruses.
HeLa cells were obtained from the National Institute of Allergy and Infectious Diseases AIDS Research and Reference Reagent Program (Bethesda, Md.). U-937 cells (human histiocytic lymphoma) and NIH 3T3 (murine fibroblast) cells were provided by L. Filion and E. G. Brown (Department of Biochemistry, Microbiology and Immunology, University of Ottawa, Ottawa, Canada), respectively. NIH 3T3 cells constitutively expressing human DAF have been described previously (39) and were from O. B. Spiller (Department of Medicinal Biochemistry, University of Wales College of Medicine, Cardiff, United Kingdom). Chinese hamster ovary (CHO) cells constitutively expressing human DAF or a DAF/HLA-B44 chimera (amino acid residues 1 through 257 of DAF fused to the carboxyl terminus of HLA-B44 from amino acid 66 to the end of the sequence) were obtained from B. Nowicki (Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston, Tex.) and have also been previously described (9). HeLa cells, NIH 3T3 cells and derivatives, and CHO cells and derivatives were maintained in Eagle's minimal essential medium containing Earle's salts, as previously described (18); U-937 cells were grown in RPMI 1640 medium containing 10% fetal bovine serum. All media and supplements were from Invitrogen Life Technologies. EV70, strain J 670/71, was obtained from M. Hatch and M. Pallansch (Centers for Disease Control and Prevention, Atlanta, Ga.) and was passaged in LLC-MK2 cells (rhesus monkey [Macaca mulatta] kidney) as described previously (18). Echovirus 11 (E11), strain Gregory, was from S. Lee (National Centre for Enteroviruses, Halifax, Nova Scotia, Canada) and was also passaged in LLC-MK2 cells. Vaccinia virus strain vTF7-3 was purchased from the American Type Culture Collection (Manassas, Va.) and propagated in CV-1 cells (African green monkey [Cercopithecus aethiops] kidney).
Virus binding assay.
Adherent cells were detached by incubation in 15 mM EDTA in Tris-buffered saline (TBS). For each replicate, 5 × 105 cells were washed once in TBS and a nonsaturating amount (1,000 to 10,000 cpm) of 35S-radiolabeled EV70 or 35S-E11 in serum-free medium (prepared as in reference 18) was added to the cells. After incubation at 33 (EV70) or 37°C (E11) for 1 h, the cells were pelleted and the supernatant was removed. The cells were washed once with serum-free medium and pelleted again, and the resulting supernatant was pooled with that obtained previously; this pooled supernatant represented the unbound fraction of input virus. The cells, with associated virus particles, representing the bound fraction of input virus, were resuspended, and samples were analyzed by liquid scintillation counting.
Neuraminidase treatment of cells.
Cells were processed as in the virus binding assay described above. Prior to the addition of 35S-labeled virus, cells were incubated in protease-free Vibrio cholerae neuraminidase (Roche) in 150 μl of serum-free medium for 30 min at 37°C and then washed twice with TBS.
Elimination of the N-linked glycosylation site of DAF.
A mutagenic positive-sense primer (5′-ATCTGCCTTAAGGGCAGTCAATGGTCAGATATTGAAGAGTTCTGCAGTCGTAGCTGCGAGGTG-3′) spanning nucleotides 291 to 353 of the DAF cDNA (numbering as in reference 25) was synthesized; it was designed to introduce an A-to-G mutation at position 337, altering the derived amino acid sequence from Asn to Ser at position 61 of the mature polypeptide (N61S). This primer also incorporated the unique AflII site in the DAF cDNA. The negative-sense primer, 5′-GTTGGTGGGACCTTGGAA-3′, incorporated a unique PpuMI restriction site and spanned residues 919 to 937 of the DAF cDNA. The resulting PCR amplicon was inserted between the AflII and PpuMI sites in the wild-type DAF cDNA sequence.
Transient expression of DAF and DAF N61S.
DAF and DAF N61S sequences were inserted between the NcoI and EcoRI sites of the pCITE-2a(+) vector (Novagen). Twelve-well tissue culture dishes were seeded with NIH 3T3 cells and transfected with the different constructs with Lipofectamine reagent (Invitrogen Life Technologies) according to the manufacturer's instructions. After 24 h, cells were infected with vaccinia virus strain vTF7-3, encoding the T7 RNA polymerase (12), at a multiplicity of infection of 15 PFU/cell. After an additional 24 h, cells were detached from the culture dishes. A fraction of the cells was processed for 35S-labeled virus binding, as described above, while the remaining fraction was used to measure transgene expression by flow cytometry, as described previously (17). Briefly, separate analyses were performed using monoclonal antibodies EVR1 (complement control protein domain 1 [CCP1] specific) (18), 11D7 (CCP1 specific) (9), IF7 (CCP2 specific) (5), and 8D11 (CCP4 specific) (9) as primary antibodies, each at a final concentration of 0.2 μg/ml. 11D7 and 8D11 were from W. Rosse (Duke University Medical Center, Durham, N.C.), and IF7 was from R. Finberg (Dana-Farber Cancer Institute, Boston, Mass.). Goat anti-mouse immunoglobulin G (heavy plus light chains) conjugated to fluorescein isothiocyanate (Roche) was used as the secondary antibody (diluted 1:1,000 from the supplied stock). Virus binding data were normalized for DAF construct expression by using the average of the mean fluorescence intensities for all of the monoclonal antibodies used; expression levels of wild-type DAF and DAF N61S varied by twofold or less.
Use of metabolic inhibitors of glycosylation.
To inhibit N-linked glycosylation, cells were incubated in culture medium containing 0.2 μg of tunicamycin (Sigma)/ml for 24 h prior to assaying virus binding. Benzyl N-acetyl-α-d-galactosaminide (benzyl GalNAc; Sigma), included in culture medium at a final concentration of 3 mM for 48 h prior to 35S-labeled virus binding, was used to inhibit O-linked glycosylation.
Immunoblot analysis.
Adherent cells were detached by incubation in 15 mM EDTA in TBS and washed twice. To release DAF from the cell surface, cells were resuspended in phosphatidylinositol-specific phospholipase C (PI-PLC) buffer (RPMI 1640 medium, 0.2% bovine serum albumin, 50 μM 2-mercaptoethanol, 10 mM HEPES, 0.1% sodium azide) containing 6 U of PI-PLC/ml from Bacillus cereus (Sigma) and 1% (vol/vol) broad-specificity protease inhibitor cocktail (Sigma). Following incubation at 37°C for 4 h, cells were pelleted and samples of the supernatant were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting. DAF was visualized by incubation in rabbit anti-human DAF polyclonal immunoglobulin G (1:500 dilution; Research Diagnostics Inc.), followed by protein A-alkaline phosphatase conjugate (1:1,250 dilution; Sigma) and a colorimetric substrate (5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium; Promega).
RESULTS
Sialic acid is involved in EV70 binding to and infection of susceptible cell lines.
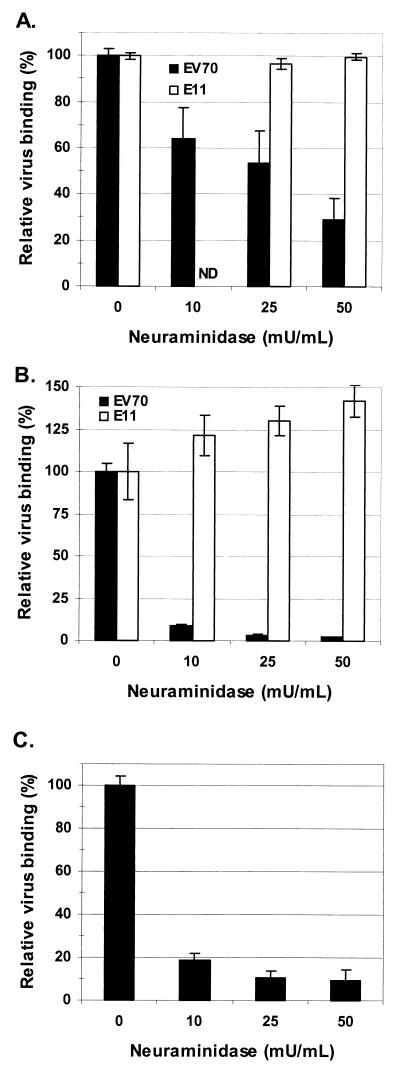
Previous results presented by Utagawa et al. (46) implicated sialic acid as an essential component of the EV70 erythrocyte receptor, as evaluated by viral hemagglutination inhibition. Here, we were interested to extend these findings by testing whether sialic acid is involved in EV70 attachment to nucleated cells that are susceptible to EV70 infection. Binding to all cell lines we examined was found to be neuraminidase sensitive, although to various extents. As shown in Fig. 1A, EV70 binding to HeLa cells decreased in a dose-dependent manner as a result of preincubation of the cells with the broad-specificity, protease-free V. cholerae neuraminidase. E11 binding to HeLa cells under the same conditions was measured as a control; the results of Utagawa et al. (46) indicated that E11-induced hemagglutination was not dependent on sialic acid, and it has been repeatedly shown that, like EV70, E11 recognizes DAF as a cellular receptor (5, 21, 29, 31). As expected, E11 binding to HeLa cells was not affected by neuraminidase treatment (Fig. 1A), indicating that the observed inhibition of EV70 binding is specific and not due to a general decrease in the ability of the cells to bind virus. EV70 binding to HeLa cells was also found to be inhibited, to a lesser extent, by preincubation of the virus with free sialic acid in the range of 5 to 20 mg/ml (data not shown), further indicating a specific role for sialic acid in virus binding and arguing against the presence of a contaminating, receptor-destroying enzymatic activity in the neuraminidase preparation. EV70 binding to U-937 cells was particularly sensitive to neuraminidase pretreatment (Fig. 1B), whereas E11 binding was not adversely affected; in fact, E11 binding appeared to be enhanced by the removal of cell surface sialic acid, possibly rendering virus binding sites on DAF more accessible. Expression of human DAF confers EV70 binding activity on the receptor-negative murine cell line NIH 3T3 and renders the cells susceptible to EV70 infection (17, 18). As shown in Fig. 1C, this DAF-dependent EV70 binding activity is also inhibited by pretreatment of the cells with neuraminidase. To rule out the possibility that neuraminidase treatment inhibited a critical postbinding event required for virus retention at the cell surface, virus binding experiments following neuraminidase treatment were also carried out at 4°C; similar results were obtained (data not shown). In addition to those cell lines shown in Fig. 1, experiments in our laboratory have found that EV70 binding to a wide range of leukocyte cell lines, most of which support EV70 replication, is highly susceptible to neuraminidase pretreatment (A. Haddad et al., unpublished data).
FIG. 1.
Neuraminidase pretreatment inhibits EV70 binding to several susceptible cell lines. Cells were incubated in the presence of the indicated concentrations of neuraminidase for 30 min at 37°C. After being washed, cells were incubated for 1 h with 35S-labeled EV70 (33°C) or E11 (37°C) (2,000 to 10,000 cpm). Virus binding data are presented as the means ± standard deviations for binding to treated cells relative to binding to untreated cells (i.e., 0 mU of neuraminidase/ml) from at least three experiments. (A) EV70 and E11 binding to HeLa cells. Untreated cells bound 33 and 67% of input EV70 and E11, respectively. (B) EV70 and E11 binding to U-937 cells. Untreated cells bound 51 and 16% of input EV70 and E11, respectively. (C) EV70 binding to NIH 3T3 cells constitutively expressing human DAF. Untreated cells bound 30% of input EV70. ND, not determined.
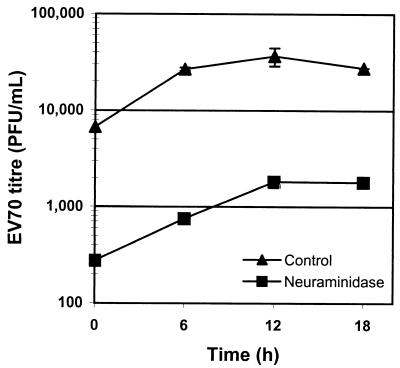
In an effort to determine if the EV70 binding mediated by cell surface sialic acid leads to the productive infection of cells, U-937 cells were incubated with neuraminidase prior to EV70 infection; U-937 cells were used due to the sensitivity of the EV70 binding activity of this cell line to neuraminidase pretreatment (Fig. 1B). As shown in Fig. 2, the titer of EV70 released by neuraminidase-treated cells was 1 order of magnitude lower than the titer of EV70 released by untreated cells at all time points, demonstrating that binding to cell surface sialic acid by EV70 is an important step in cell entry. Infection of HeLa cells was also inhibited by neuraminidase pretreatment, although to a lesser extent (data not shown).
FIG. 2.
Neuraminidase pretreatment inhibits EV70 infection of U-937 cells. Cells were incubated in 50 mU of neuraminidase/ml for 30 min at 37°C. After being washed twice with TBS, cells were infected with EV70 at a multiplicity of infection of 5 PFU/cell and incubated at 33°C. Aliquots were removed at the indicated times, and EV70 titers ± standard deviations for duplicate experiments were determined by plaque assay on LLC-MK2 cells.
Sialic acid residues of DAF are not involved in EV70 binding.
DAF has been identified as a HeLa cell receptor for EV70 (18) and is known to possess both a single complex-type N-linked glycosylation site and a heavily sialylated, serine- and threonine-rich O-glycosylated domain (9, 23). The expression of DAF on receptor-negative NIH 3T3 cells resulted in an EV70 binding activity that was sensitive to neuraminidase (Fig. 1C). Taken together, the above observations suggested that sialic acid residues involved in EV70 binding to cells might be localized on the DAF molecule.
DAF is a member of the family of proteins called regulators of complement activation and consists of four CCP domains mounted on a rod-like, serine- and threonine-rich O-glycosylated region, bound to the outer leaflet of the plasma membrane by a glycosylphosphatidylinositol anchor (22). The site of EV70 binding on DAF has been localized to the N-terminal CCP domain, CCP1, with evidence also suggesting a role for CCP2 (17); the single N-linked glycosylation site of DAF is located in the same region, at the interface of CCP1 and -2 (9). Therefore, it was hypothesized that sialic acid residues in the complex-type N-linked oligosaccharide may contribute to EV70 binding to DAF. To test this hypothesis, a derivative of DAF was prepared by site-directed mutagenesis, altering the asparagine residue to serine in the N-linked glycosylation consensus sequence (Asn-X-Ser), resulting in Ser-Arg-Ser. When expressed in the receptor-negative cell line NIH 3T3, this mutant form of DAF (DAF N61S) was observed to bind EV70 as efficiently as the wild-type control (Fig. 3A). Additional experiments showed that the EV70 binding activity of cells expressing DAF N61S was sensitive to neuraminidase, similar to that of cells expressing wild-type DAF (Fig. 3B), further establishing that the sialic acid residues critical for EV70 binding are not a component of the N-linked glycan of DAF.
FIG. 3.
(A) Elimination of the N-linked carbohydrate of DAF has no effect on EV70 binding. An A-to-G mutation was introduced into DAF cDNA, resulting in an Asn-to-Ser substitution (N61S) in the DAF polypeptide. Vector-only control (vector), wild-type (DAF), and mutant (DAF N61S) constructs were transfected into the EV70 receptor-negative cell line NIH 3T3. 35S-EV70 binding was determined after 48 h and normalized for DAF construct expression as determined by flow cytometry (see Materials and Methods). (B) EV70 binding to both wild-type and mutant DAF is sensitive to neuraminidase. Transfection and virus binding were performed as for panel A, except that cells were incubated in serum-free medium (control) or in serum-free medium containing 50 mU of neuraminidase/ml for 30 min at 37°C before 35S-EV70 binding. For both panels, data are presented as means ± standard deviations for at least three experiments.
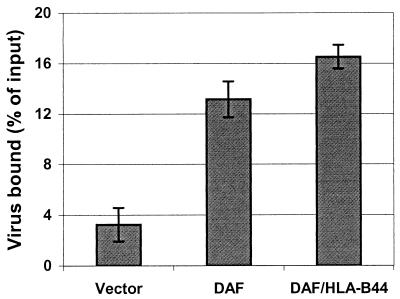
As mentioned above, the DAF molecule also contains a heavily sialylated O-glycosylated region which projects the four CCP domains above the plasma membrane. This region has been demonstrated to serve an essential but nonspecific spacer function in the complement regulatory function of DAF (9) and also plays a role in the ability of DAF to function as a receptor for echovirus 7 (7) and for certain strains of pathogenic Escherichia coli bearing Dr or related adhesins (28). Although it seemed unlikely that sialic acid residues in this domain, located at a distance from the identified EV70 binding site at the amino terminus of the molecule, would play a direct role in the binding of the virus, it was imperative to test this possibility. Therefore, the EV70 binding activity of a chimeric construct (DAF/HLA-B44; described in reference 9) consisting of the four CCP domains of DAF mounted on the membrane-proximal transmembrane and cytoplasmic domains of the HLA-B44 molecule was examined. Figure 4 shows that the replacement of the O-glycosylated domain of DAF had no significant effect on the EV70 binding activity of the molecule, indicating that this region and its associated sialic acid residues play no direct and necessary role in virus binding.
FIG. 4.
The O-glycosylated domain of DAF is not directly involved in EV70 binding. CHO cells constitutively expressing wild-type DAF or a chimeric construct consisting of the four CCP domains of DAF fused to the majority of the HLA-B44 molecule (DAF/HLA-B44) and control cells transfected with vector alone were assayed for EV70 binding. Virus binding results were normalized for DAF construct expression level.
O-linked, but not N-linked, oligosaccharides are involved in EV70 binding.
Having excluded the possibility that sialic acid residues on the DAF molecule are critical for EV70 binding to cells, we decided to evaluate more generally the role of N-linked and O-linked carbohydrate moieties of cell surface glycoproteins in EV70 binding. The effect of specific inhibitors of glycosylation on the ability of cells to bind EV70 was therefore assessed.
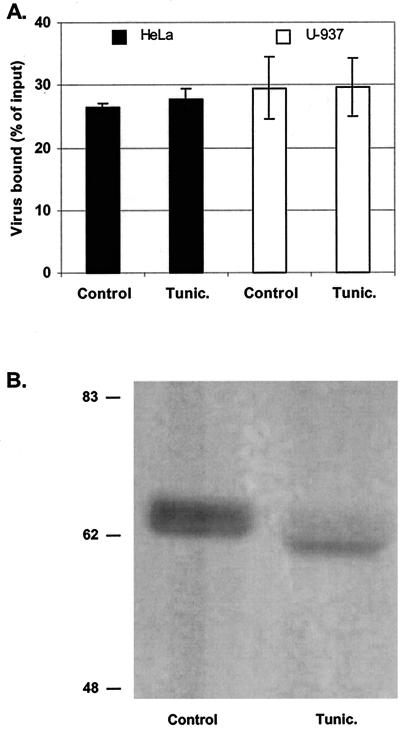
Tunicamycin is commonly used to test the role of N-linked glycoproteins in cellular processes; it specifically inhibits the transfer of N-acetylglucosamine to dolichol monophosphate, a very early step in the synthesis of the high-mannose core oligosaccharide (10). HeLa cells and U-937 cells were cultivated in the presence of 0.2 μg of tunicamycin/ml for 24 h and then processed for EV70 binding. As shown in Fig. 5, tunicamycin treatment had no effect on EV70 binding to either cell line. To verify that tunicamycin was effective at inhibiting N-linked glycosylation under these conditions, an immunoblot of DAF cleaved from the surface of intact cells by the action of PI-PLC was prepared. DAF released from HeLa cells cultivated in the presence of tunicamycin showed a decrease in relative molecular weight compared to DAF released from untreated control cells that was consistent with the loss of its single N-linked carbohydrate moiety, as reported previously (23) (Fig. 5B).
FIG. 5.
N-linked glycans are not involved in EV70 attachment. (A) HeLa and U-937 cells, as indicated, were incubated in cell culture medium alone (control) or in cell culture medium containing 0.2 μg of tunicamycin (tunic.)/ml for 24 h at 37°C prior to 35S-EV70 binding. Data are presented as means ± standard deviations for three experiments. (B) Immunoblot analysis of DAF released from tunicamycin-treated and untreated HeLa cells by digestion with PI-PLC. The apparent molecular weights (in thousands) of prestained protein markers are shown at the left.
To investigate the role of the O-linked carbohydrates of cell surface glycoproteins in EV70 binding, cells were cultivated in the presence of benzyl GalNAc. Benzyl GalNAc inhibits N-acetyl-α-d-galactosaminyltransferase, which catalyzes the first step in the biosynthesis of O-linked glycans (20). HeLa cells treated with benzyl GalNAc showed an approximately 75% decrease in EV70 binding compared to untreated control cells (Fig. 6A), and U-937 cells showed a greater-than-50% decrease in EV70 binding relative to untreated controls (Fig. 6B). To verify that the observed effect on EV70 binding was specific and not due to the toxicity of the inhibitor, E11 binding was once again measured as a control. No significant change in E11 binding to HeLa cells treated with benzyl GalNAc was observed (Fig. 6A), and E11 binding to U-937 cells was somewhat enhanced by the treatment (Fig. 6B). This enhancement parallels the increase in E11 binding to U-937 cells treated with neuraminidase (Fig. 1B) and is consistent with a role for sialic acid, or oligosaccharides more generally, in masking virus binding sites. As a further control, the amounts of DAF expressed at the surfaces of treated and untreated cells were assessed by immunoblotting and were found to be equivalent (Fig. 6C); this analysis also confirmed that the size of DAF released from benzyl GalNAc-treated cells was consistent with the inhibition of O-linked glycosylation.
FIG. 6.
Disruption of O-linked glycosylation inhibits EV70 binding. (A) HeLa cells were incubated in the presence or absence of 3 mM benzyl GalNAc for 48 h at 37°C prior to 35S-EV70 or 35S-E11 binding. (B) Same as panel A with U-937 cells in place of HeLa cells. For panels A and B, data are presented as means ± standard deviations for three experiments. (C) Immunoblot analysis of DAF released from benzyl GalNAc-treated and untreated HeLa cells by digestion with PI-PLC. The apparent molecular weights (in thousands) of prestained protein markers are shown at the left.
DISCUSSION
Sialic acid binding has been observed for a wide variety of viruses, including both DNA and RNA viruses, and with both enveloped and nonenveloped virions. Included among these are orthomyxoviruses (43), respiroviruses (42), certain strains of animal rotaviruses (27), polyomavirus (4), transmissible gastroenteritis coronavirus (19), certain strains of subgroup D adenoviruses (1), reoviruses (2), and several members of the family Parvoviridae (16) (for each reference, see also the references cited therein). The use of sialic acid as a receptor among the Picornaviridae, including encephalomyocarditis virus (15), human rhinovirus 87 (HRV87) (45), Theiler's murine encephalomyelitis virus (TMEV) (51), and bovine enterovirus 261 (41), has also been previously described. (Interestingly, a recent study of the genetic relationships among HRV prototype strains has shown that HRV87 clusters separately from the remaining HRVs and close to EV70 [32].) Binding to sialic acid may also have some parallels with the widespread use of another class of ubiquitous anionic cell surface carbohydrates, glycosaminoglycans, and in particular heparan sulfate, in cell attachment by a variety of viruses and other pathogens (40).
Recent work in several laboratories has advanced our understanding of the role of sialic acid binding in host cell infection and pathogenesis. Barton et al. (2) recently demonstrated that reovirus binding to sialic acid increases the avidity of cell attachment and enhances productive infection, possibly by providing a locus for the initial virus-cell interaction in a multistep adhesion-strengthening strategy. Sialic acid binding is then followed by higher-affinity binding to a secondary receptor critical for virus entry, the junction adhesion molecule (3). Within such a model, the capacity to bind cell surface sialic acid may be an important determinant of cell and tissue tropism in situations where the secondary entry receptor is expressed at low levels or where infection of cells proceeds in an environment of rapidly moving body fluid, such as blood, lymph, or tears. Virus binding to sialic acid may also activate cellular signaling pathways and thus may have important effects downstream of virus attachment on cellular metabolism and the outcome of infection. For example, reovirus binding to cell surface sialic acid is an important mediator in the activation of nuclear factor κB (NF-κB) and in the induction of apoptosis (8).
For TMEV, the capacity to bind sialic acid appears to have a pronounced effect on viral pathogenesis. Infection with certain strains of TMEV results in viral persistence in the CNS, accompanied by demyelination, whereas other strains follow a rapid, acute course of infection. Differential binding to sialic acid residues on the cell surface appears to account for this striking difference in the in vivo outcomes, with sialic acid binding being a unique property of the demyelinating, persistent group (51). The sialic acid binding activity of TGEV contributes to the pathogenicity of this virus as well; in addition to a possible role for sialic acid binding in enhancing cell attachment and entry, it has been speculated that the binding of sialylated macromolecules to the virus surface may increase the stability of the virus against detergent-like bile salts encountered in the gastrointestinal tract (19).
In each of the above examples, the ability to elucidate the role of the sialic acid binding activity in infection and pathogenesis was made possible by the availability of virus strains differing in their capacities to bind sialic acid. What role, if any, the sialic acid binding activity of EV70 plays in vivo is unknown. In the present study, the prototype J 670/71 strain of EV70 was employed; the sialic acid binding activity of other EV70 strains has not been evaluated.
EV70 is the only human enterovirus to date observed to require cell surface sialic acid for attachment; sialic acid binding was also found to be important for infection of susceptible cells, as was shown previously for bovine enterovirus 261 (41). The data presented here also indicate that the absence of the sialic acid residues contained in the single, complex-type N-linked oligosaccharide or in the heavily sialylated O-linked oligosaccharides of DAF did not significantly reduce virus binding to transfected cells expressing the receptor variants. Taken together, these results suggest that at least one additional sialylated receptor molecule, apart from DAF, is involved in EV70 attachment and infection. The requirement for an additional receptor is consistent with previously reported findings for other DAF-binding human enteroviruses, which include certain variants or isolates of coxsackie B virus serotypes 1, 3, and 5 (6, 33, 35), coxsackievirus A21 (CAV21) (36), and various echovirus serotypes (5, 48). Several of these viruses have been shown to require additional factors for cell entry and infection; coxsackievirus B3 and CAV21 require the expression of coxsackievirus-adenovirus receptor (37) and intercellular adhesion molecule 1 (36), respectively. Several of the DAF-binding echoviruses may also require accessory molecules in addition to DAF for cell attachment and infection (29, 30). In these cases, it has been proposed that DAF serves as an initial, reversible, low-affinity virus attachment site that facilitates or enhances binding to a higher-affinity entry factor, which may also support virus infection in the absence of DAF. A version of this model may be applicable to the binding of EV70 to susceptible cells, in which the interaction of the virus with DAF may be required for initial cell adhesion, followed by binding to the putative secondary factor in a sialic acid-dependent manner. For example, for both HeLa cells and NIH 3T3 cells expressing human DAF, EV70 binding is dependent on the presence of both DAF and sialic acid (17) (Fig. 1 and 3), although, as shown here, the sialic acid residues of DAF itself are not required. The identity of an accessory factor and whether it may contain sialic acid residues required for EV70 binding and infection are the subjects of ongoing work in our laboratory.
More broadly, the data presented here also rule out a direct or an indirect role in EV70 attachment for the oligosaccharides underlying the sialic acid residues of DAF. The interaction of DAF with other human enteroviruses has also generally been found to be independent of N-linked and O-linked glycosylations (7, 30, 31). Only for CAV21 has a role for a nonpeptide modification of DAF been suggested; here, hemagglutination inhibition by soluble DAF variants lacking the N-linked glycan was ineffective and hemagglutination inhibition by a partially N-glycosylated construct containing all four CCPs of DAF was significantly less effective for CAV21 than for all other viruses tested (31). Interestingly, CAV21 is the only DAF-binding enterovirus apart from EV70 that has been found to interact with the N-terminal CCP1 of DAF (36); however, the interactions of the two viruses with DAF appear to differ with respect to the requirement for the N-linked oligosaccharide. It has been suggested that the ability to bind DAF evolved independently in different clusters of human enteroviruses (31), and, since EV70 and CAV21 are representatives of separate clusters, it may not be surprising that the two viruses interact with DAF in distinct ways.
The results obtained with metabolic inhibitors of protein glycosylation, tunicamycin and benzyl GalNAc, may provide preliminary information about the nature of the sialylated molecular species involved in EV70 binding. The absence of an effect of tunicamycin treatment suggests that the N-linked oligosaccharides of glycoproteins, along with any associated sialic acid residues, are dispensable for EV70 attachment. In contrast, disruption of O-linked glycosylation inhibited EV70 binding. O-linked glycans of cell surface glycoproteins are often found clustered together in mucin-like domains (as is the case with DAF), where they confer an extended, rod-like structure to the polypeptide chain (14). Thus, the inhibition of virus binding by benzyl GalNAc treatment may be due to a direct effect on virus binding to sialic acid associated with O-linked oligosaccharides on the cell surface, although it is also possible that interfering with O-glycosylation perturbed the conformation of a virus-binding factor in such a way as to prevent EV70 interaction. Whereas it is clear that benzyl GalNAc inhibits O-linked glycosylation of proteins (20), its effects on glycolipid glycosylation have not been reported; experiments assessing the role of sialylated glycolipids in EV70 binding are under way.
For many viruses that bind sialic acid, further details of the specificity of the interaction, regarding the type of linkage between the sialic acid and the underlying oligosaccharide or the size and location of the acyl chain, have been elucidated. Such data can be obtained through the use of specific neuraminidases and sialyltransferases, of lectins with specificity for particular neuraminic acids or linkages, or of mutant cell lines. The V. cholerae neuraminidase used in the present work has a broad substrate specificity and as such was suitable for an initial evaluation of the role of sialic acid in EV70 binding; future work will characterize more precisely the specificity of the sialic acid species and linkage recognized by EV70.
Acknowledgments
We thank P. Urvil and B. Nowicki for kindly supplying the CHO cells expressing the DAF/HLA-B44 chimera and controls, O. B. Spiller for providing the NIH 3T3 cells expressing human DAF, and A. Khan for assistance.
This work was supported by the Natural Sciences and Engineering Research Council of Canada.
REFERENCES
- 1.Arnberg, N., A. H. Kidd, K. Edlund, F. Olfat, and G. Wadell. 2000. Initial interactions of subgenus D adenoviruses with A549 cellular receptors: sialic acid versus αv integrins. J. Virol. 74:7691-7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barton, E. S., J. L. Connolly, J. C. Forrest, J. D. Chappell, and T. S. Dermody. 2001. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J. Biol. Chem. 276:2200-2211. [DOI] [PubMed] [Google Scholar]
- 3.Barton, E. S., J. C. Forrest, J. L. Connolly, J. D. Chappell, Y. Liu, F. J. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441-451. [DOI] [PubMed] [Google Scholar]
- 4.Bauer, P. H., C. Cui, T. Stehle, S. C. Harrison, J. A. DeCaprio, and T. L. Benjamin. 1999. Discrimination between sialic acid-containing receptors and pseudoreceptors regulates polyomavirus spread in the mouse. J. Virol. 73:5826-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergelson, J. M., M. Chan, K. R. Solomon, N. F. St. John, H. Lin, and R. W. Finberg. 1994. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc. Natl. Acad. Sci. USA 91:6245-6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergelson, J. M., J. F. Modlin, W. Wieland-Alter, J. A. Cunningham, R. L. Crowell, and R. W. Finberg. 1997. Clinical coxsackievirus B isolates differ from laboratory strains in their interaction with two cell surface receptors. J. Infect. Dis. 175:697-700. [DOI] [PubMed] [Google Scholar]
- 7.Clarkson, N. A., R. Kaufman, D. M. Lublin, T. Ward, P. A. Pipkin, P. D. Minor, D. J. Evans, and J. W. Almond. 1995. Characterization of the echovirus 7 receptor: domains of CD55 critical for virus binding. J. Virol. 69:5497-5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connolly, J. L., E. S. Barton, and T. S. Dermody. 2001. Reovirus binding to cell surface sialic acid potentiates virus-induced apoptosis. J. Virol. 75:4029-4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coyne, K. E., S. E. Hall, S. Thompson, M. A. Arce, T. Kinoshita, T. Fujita, D. J. Anstee, W. Rosse, and D. M. Lublin. 1992. Mapping of epitopes, glycosylation sites, and complement regulatory domains in human decay accelerating factor. J. Immunol. 149:2906-2913. [PubMed] [Google Scholar]
- 10.Duksin, D., and W. C. Mahoney. 1982. Relationship of the structure and biological activity of the natural homologues of tunicamycin. J. Biol. Chem. 257:3105-3109. [PubMed] [Google Scholar]
- 11.Evans, D. J., and J. W. Almond. 1998. Cell receptors for picornaviruses as determinants of cell tropism and pathogenesis. Trends Microbiol. 6:198-202. [DOI] [PubMed] [Google Scholar]
- 12.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 83:8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins, P. G. 1982. Enteroviral conjunctivitis and its neurological complications. Arch. Virol. 73:91-101. [DOI] [PubMed] [Google Scholar]
- 14.Jentoft, N. 1990. Why are proteins O-glycosylated? Trends Biochem. Sci. 15:291-294. [DOI] [PubMed] [Google Scholar]
- 15.Jin, Y. M., I. U. Pardoe, A. T. Burness, and T. I. Michalak. 1994. Identification and characterization of the cell surface 70-kilodalton sialoglycoprotein(s) as a candidate receptor for encephalomyocarditis virus on human nucleated cells. J. Virol. 68:7308-7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaludov, N., K. E. Brown, R. W. Walters, J. Zabner, and J. A. Chiorini. 2001. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J. Virol. 75:6884-6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karnauchow, T. M., S. Dawe, D. M. Lublin, and K. Dimock. 1998. Short consensus repeat domain 1 of decay-accelerating factor is required for enterovirus 70 binding. J. Virol. 72:9380-9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karnauchow, T. M., D. L. Tolson, B. A. Harrison, E. Altman, D. M. Lublin, and K. Dimock. 1996. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55). J. Virol. 70:5143-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krempl, C., M. L. Ballesteros, G. Zimmer, L. Enjuanes, H. D. Klenk, and G. Herrler. 2000. Characterization of the sialic acid binding activity of transmissible gastroenteritis coronavirus by analysis of haemagglutination-deficient mutants. J. Gen. Virol. 81:489-496. [DOI] [PubMed] [Google Scholar]
- 20.Kuan, S. F., J. C. Byrd, C. Basbaum, and Y. S. Kim. 1989. Inhibition of mucin glycosylation by aryl-N-acetyl-alpha-galactosaminides in human colon cancer cells. J. Biol. Chem. 264:19271-19277. [PubMed] [Google Scholar]
- 21.Lea, S. M., R. M. Powell, T. McKee, D. J. Evans, D. Brown, D. I. Stuart, and P. A. van der Merwe. 1998. Determination of the affinity and kinetic constants for the interaction between the human virus echovirus 11 and its cellular receptor, CD55. J. Biol. Chem. 273:30443-30447. [DOI] [PubMed] [Google Scholar]
- 22.Lublin, D. M., and J. P. Atkinson. 1989. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu. Rev. Immunol. 7:35-58. [DOI] [PubMed] [Google Scholar]
- 23.Lublin, D. M., J. Krsek-Staples, M. K. Pangburn, and J. P. Atkinson. 1986. Biosynthesis and glycosylation of the human complement regulatory protein decay-accelerating factor. J. Immunol. 137:1629-1635. [PubMed] [Google Scholar]
- 24.Maitreyi, R. S., L. Dar, A. Muthukumar, M. Vajpayee, I. Xess, R. B. Vajpayee, P. Seth, and S. Broor. 1999. Acute hemorrhagic conjunctivitis due to enterovirus 70 in India. Emerg. Infect. Dis. 5:267-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medof, M. E., D. M. Lublin, V. M. Holers, D. J. Ayers, R. R. Getty, J. F. Leykam, J. P. Atkinson, and M. L. Tykocinski. 1987. Cloning and characterization of cDNAs encoding the complete sequence of decay-accelerating factor of human complement. Proc. Natl. Acad. Sci. USA 84:2007-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medof, M. E., E. I. Walter, J. L. Rutgers, D. M. Knowles, and V. Nussenzweig. 1987. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J. Exp. Med. 165:848-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendez, E., S. Lopez, M. A. Cuadras, P. Romero, and C. F. Arias. 1999. Entry of rotaviruses is a multistep process. Virology 263:450-459. [DOI] [PubMed] [Google Scholar]
- 28.Nowicki, B., A. Hart, K. E. Coyne, D. M. Lublin, and S. Nowicki. 1993. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of a cell-cell interaction. J. Exp. Med. 178:2115-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powell, R. M., V. Schmitt, T. Ward, I. Goodfellow, D. J. Evans, and J. W. Almond. 1998. Characterization of echoviruses that bind decay accelerating factor (CD55): evidence that some haemagglutinating strains use more than one cellular receptor. J. Gen. Virol. 79:1707-1713. [DOI] [PubMed] [Google Scholar]
- 30.Powell, R. M., T. Ward, D. J. Evans, and J. W. Almond. 1997. Interaction between echovirus 7 and its receptor, decay-accelerating factor (CD55): evidence for a secondary cellular factor in A-particle formation. J. Virol. 71:9306-9312. (Erratum, 72:890, 1998.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powell, R. M., T. Ward, I. Goodfellow, J. W. Almond, and D. J. Evans. 1999. Mapping the binding domains on decay accelerating factor (DAF) for haemagglutinating enteroviruses: implications for the evolution of a DAF-binding phenotype. J. Gen. Virol. 80:3145-3152. [DOI] [PubMed] [Google Scholar]
- 32.Savolainen, C., S. Blomqvist, M. N. Mulders, and T. Hovi. 2002. Genetic clustering of all 102 human rhinovirus prototype strains: serotype 87 is close to human enterovirus 70. J. Gen. Virol. 83:333-340. [DOI] [PubMed] [Google Scholar]
- 33.Schmidtke, M., H. C. Selinka, A. Heim, B. Jahn, M. Tonew, R. Kandolf, A. Stelzner, and R. Zell. 2000. Attachment of coxsackievirus B3 variants to various cell lines: mapping of phenotypic differences to capsid protein VP1. Virology 275:77-88. [DOI] [PubMed] [Google Scholar]
- 34.Schneider-Schaulies, J. 2000. Cellular receptors for viruses: links to tropism and pathogenesis. J. Gen. Virol. 81:1413-1429. [DOI] [PubMed] [Google Scholar]
- 35.Shafren, D. R., R. C. Bates, M. V. Agrez, R. L. Herd, G. F. Burns, and R. D. Barry. 1995. Coxsackieviruses B1, B3, and B5 use decay accelerating factor as a receptor for cell attachment. J. Virol. 69:3873-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shafren, D. R., D. J. Dorahy, R. A. Ingham, G. F. Burns, and R. D. Barry. 1997. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J. Virol. 71:4736-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shafren, D. R., D. T. Williams, and R. D. Barry. 1997. A decay-accelerating factor-binding strain of coxsackievirus B3 requires the coxsackievirus-adenovirus receptor protein to mediate lytic infection of rhabdomyosarcoma cells. J. Virol. 71:9844-9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shulman, L. M., Y. Manor, R. Azar, R. Handsher, A. Vonsover, E. Mendelson, S. Rothman, D. Hassin, T. Halmut, B. Abramovitz, and N. Varsano. 1997. Identification of a new strain of fastidious enterovirus 70 as the causative agent of an outbreak of hemorrhagic conjunctivitis. J. Clin. Microbiol. 35:2145-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiller, O. B., I. G. Goodfellow, D. J. Evans, J. W. Almond, and B. P. Morgan. 2000. Echoviruses and coxsackie B viruses that use human decay-accelerating factor (DAF) as a receptor do not bind the rodent analogues of DAF. J. Infect. Dis. 181:340-343. [DOI] [PubMed] [Google Scholar]
- 40.Spillmann, D. 2001. Heparan sulfate: anchor for viral intruders? Biochimie 83:811-817. [DOI] [PubMed] [Google Scholar]
- 41.Stoner, G. D., B. Williams, A. Kniazeff, and M. B. Shimkin. 1973. Effect of neuraminidase pretreatment on the susceptibility of normal and transformed mammalian cells to bovine enterovirus 261. Nature 245:319-320. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki, T., A. Portner, R. A. Scroggs, M. Uchikawa, N. Koyama, K. Matsuo, Y. Suzuki, and T. Takimoto. 2001. Receptor specificities of human respiroviruses. J. Virol. 75:4604-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki, Y., T. Ito, T. Suzuki, R. E. Holland, Jr., T. M. Chambers, M. Kiso, H. Ishida, and Y. Kawaoka. 2000. Sialic acid species as a determinant of the host range of influenza A viruses. J. Virol. 74:11825-11831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uchio, E., K. Yamazaki, H. Ishikawa, I. Matsunaga, Y. Asato, K. Aoki, and S. Ohno. 1999. An epidemic of acute haemorrhagic conjunctivitis caused by enterovirus 70 in Okinawa, Japan, in 1994. Graefe's Arch. Clin. Exp. Ophthalmol. 237:568-572. [DOI] [PubMed] [Google Scholar]
- 45.Uncapher, C. R., C. M. DeWitt, and R. J. Colonno. 1991. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology 180:814-817. [DOI] [PubMed] [Google Scholar]
- 46.Utagawa, E. T., K. Miyamura, A. Mukoyama, and R. Kono. 1982. Neuraminidase-sensitive erythrocyte receptor for enterovirus type 70. J. Gen. Virol. 63:141-148. [DOI] [PubMed] [Google Scholar]
- 47.Wairagkar, N. S., S. S. Gogate, and A. S. Labhsetwar. 1999. Investigation of an epidemic of acute haemorrhagic conjunctivitis in Pune, India. J. Commun. Dis. 31:41-43. [PubMed] [Google Scholar]
- 48.Ward, T., P. A. Pipkin, N. A. Clarkson, D. M. Stone, P. D. Minor, and J. W. Almond. 1994. Decay-accelerating factor CD55 is identified as the receptor for echovirus 7 using CELICS, a rapid immuno-focal cloning method. EMBO J. 13:5070-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wright, P. W., G. H. Strauss, and M. P. Langford. 1992. Acute hemorrhagic conjunctivitis. Am. Fam. Physician 45:173-178. [PubMed] [Google Scholar]
- 50.Yoshii, T., K. Natori, and R. Kono. 1977. Replication of enterovirus 70 in non-primate cell cultures. J. Gen. Virol. 36:377-384. [DOI] [PubMed] [Google Scholar]
- 51.Zhou, L., Y. Luo, Y. Wu, J. Tsao, and M. Luo. 2000. Sialylation of the host receptor may modulate entry of demyelinating persistent Theiler's virus. J. Virol. 74:1477-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]