Abstract
The latent membrane protein-1 (LMP-1) of Epstein-Barr virus (EBV) contributes to the proliferation of infected B lymphocytes by signaling through its binding to cellular signaling molecules. It apparently mimics members of the tumor necrosis factor receptor family, in particular, CD40, by binding a similar set of cellular molecules as does CD40. LMP-1 differs dramatically in its structure from CD40. LMP-1 has six membrane-spanning domains as opposed to CD40's one. LMP-1 also differs from CD40 in its apparent independence of a ligand for its signaling. We have examined the role of LMP-1's membrane-spanning domains in its signaling. Their substitution with six membrane-spanning domains from the LMP-2A protein of EBV yields a derivative which neither coimmunoprecipitates with LMP-1 nor signals to increase the activity of NF-κB as does wild-type LMP-1. These observations indicate that LMP-1 has specific sequences in its membrane-spanning domains required for these activities. LMP-1's first and sixth membrane-spanning domains have multiple leucine residues potentially similar to leucine-heptad motifs that can mediate protein-protein interactions in membranes (Gurezka et al., J. Biol. Chem. 274:9265-9270, 1999). Substitution of seven leucines in LMP-1's sixth membrane-spanning domain has no effect on its function, whereas similar substitutions in its first membrane-spanning domain yielded a derivative which aggregates as does wild-type LMP-1 but has only 3% of wild-type's ability to signal through NF-κB. Importantly, this derivative complements a mutant of LMP-1 with wild-type membrane-spanning domains but no carboxy-terminal signaling domain. These findings together indicate that the membrane-spanning domains of LMP-1 contribute multiple functions to its signaling.
The latent membrane protein-1 (LMP-1) shares multiple features with members of the tumor necrosis factor receptor (TNFR) family. LMP-1 is required for the efficient maintenance of proliferation of B lymphocytes infected by Epstein-Barr virus (EBV) (23). CD40, the member of the TNFR family that LMP-1 most resembles, is also involved in the induction of proliferation of B lymphocytes. Activation of CD40 and IL-4 receptors induces B-cell proliferation in culture (1). LMP-1 can partially restore the wild-type phenotype of mice deficient in CD40 (35). Multiple biochemical properties of LMP-1 are similar to those of activated members of the TNFR family. Some portion of LMP-1 homes to the plasma membrane; much homes to detergent-insoluble membranes, and all of it turns over rapidly (21, 26, 27). Both CD40 and LMP-1 activate NF-κB-, AP-1-, and STAT-mediated transcription by binding TNFR-associated factors (TRAFs) and/or tumor necrosis factor receptor associated death domain-containing protein (TRADD) and janus activated kinase-3 (JAK3) (2, 3, 6, 7, 10, 11, 17-20, 22, 30).
One major difference between LMP-1 and CD40 is a requirement for ligand. CD40 requires its ligand to become activated; LMP-1 apparently does not. It has been proposed that LMP-1's amino terminus and six-transmembrane domains support ligand-independent signaling of LMP-1 by assembling a complex embedded in membrane containing multiple molecules of LMP-1 (12). In support of this notion differentially tagged derivatives of LMP-1's amino terminus and transmembrane domains coimmunoprecipitate in cell lysates (12). The composition of this complex containing LMP-1 molecules directly or indirectly in contact is not known, and it will therefore be referred to as an “aggregate.” In addition, proteins that contain LMP-1's amino terminus and membrane-spanning domains when fused to the carboxy-terminal signaling domain of TNFR-1, TNFR-2, and CD40 signal in the absence of their ligands (9, 12, 15). Conversely, proteins that contain the extracellular ligand binding domains of various cell surface receptors fused to LMP-1's carboxy terminus activate that carboxy terminus in a ligand-dependent manner (9, 12). Recently, it has been demonstrated that some TNF receptor family members are found on the cell surface as preformed complexes due to their pre-ligand association domain (PLAD) (4). Studies with chemical cross-linkers and fluorescent resonance energy transfer support the interpretation that these receptors are found as preformed trimers on the cell surface (4). Although TNFR-1 is an aggregate, its signaling is inactive in the absence of ligand. It is likely that TNFR-1's ligand contributes allosteric modifications, functions other than aggregation, to induce TNFR-1's signaling. In support of this idea, treatment of some TNFRs with their ligands induce their association with lipid rafts (16, 36). Localization to lipid rafts positively facilitates receptors' signaling. Based on TNFRs as a model, LMP-1's amino terminus and transmembrane domains would be predicted to encode functions other than aggregation that are required for LMP-1's signaling.
To define better the role of LMP-1's amino terminus and membrane-spanning domains in LMP-1's signaling, we generated and analyzed mutants that substitute LMP-1's amino terminus and transmembrane domains with those of LMP-2A's amino terminus and first six transmembrane domains. LMP-2A is an EBV-encoded protein having 12 membrane-spanning domains which, unlike LMP-1, affects signaling by binding cellular tyrosine kinases and ubiquitin ligases (8, 37). The regions of LMP-2A that we used to replace LMP-1's amino terminus and membrane-spanning domains have <25% amino acid sequence identity to those of LMP-1 (24, 25). The substitution mutants that replace LMP-1's transmembrane domains with those of LMP-2A fail to activate NF-κB-mediated transcription efficiently and fail to coimmunoprecipitate with wtLMP-1. These observations demonstrate that there are specific residues in LMP-1's transmembrane domains required for LMP-1's activation of NF-κB and aggregation.
Because we found there are specific sequences in LMP-1's transmembrane domains required for its signaling and aggregation not found in LMP-2A's, we searched for motifs that mediate protein-protein interactions present in LMP-1's transmembrane-spanning domains but absent in the first six membrane-spanning domains of LMP-2A. Gurezka et al. (14) have found that proteins which have clusters of leucines within their membrane spanning domains similar to the sequence, LLXXLLXLLXXLLXLL, can self-assemble. They identified LMP-1 of EBV as having this pattern. We found that LMP-1's first and sixth transmembrane-spanning domains, in particular, contain leucines potentially similar to these leucine-heptad motifs. To test whether these clusters of leucines are required for LMP-1's signaling, we generated derivatives that changed seven of the leucines to alanines in the first, sixth or both the first and sixth transmembrane-spanning domains of LMP-1. The mutants with substitutions in the first membrane-spanning domain, SubLZLMP-1 (substituted in putative leucine zippers 1 and 6) and SubLZ1LMP-1 (substituted in putative leucine zipper 1) fail to activate NF-κB efficiently. Surprisingly, SubLZLMP-1 coimmunoprecipitates with wtLMP-1, and differentially tagged derivatives of it colocalize in cells and colocalize with wtLMP-1 in cells. Importantly, the signaling defect in SubLZLMP-1 can be complemented with a derivative of LMP-1 that lacks its carboxy-terminal signaling domain but has wild-type membrane-spanning domains. These observations indicate that LMP-1's amino terminus and membrane-spanning domains contribute a function other than aggregation that is required to support LMP-1's efficient signaling.
MATERIALS AND METHODS
Cell culture.
Cell line 293, a human embryonic kidney cell line, was obtained form the American Type Culture Collection (CRL 1573) and grown in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum. All cell culture media was supplemented with 200 U per ml of penicillin and 200 μg of streptomycin/ml, and all cells were grown at 37°C in a humidified 5% CO2 atmosphere.
Construction of recombinant DNAs.
The wild-type LMP-1 was constructed by moving the LMP-1 cDNA from the B958 stain of EBV into PSG5 (Stratagene). LMPGFP and LMPRFP are derivatives of LMP-1 that fuse enhanced green fluorescent protein (EGFP) or red fluorescent protein (RFP) DsRed (Clontech) to the carboxy terminus of LMP-1. The following were constructed by replacing the indicated regions of LMP-1 with products derived by PCR or with oligonucleotides. NL2ALMP-1 is a derivative of wild-type LMP-1 and was constructed by replacing nucleotides encoding amino acids (aa) 1 to 25 of LMP-1 with the nucleotides encoding aa 1 to 125 of LMP-2A from the B958 strain of EBV. NL1LMP-1 is a derivative of wild-type LMP-1 and was constructed by replacing nucleotides encoding aa 25 to 180 of LMP-1 with the nucleotides encoding aa 126 to 280 of LMP-2A. LMP-2ALMP-1 is a derivative of wild-type LMP-1 and was constructed by replacing nucleotides encoding aa 1 to 180 of LMP-1 with the nucleotides encoding aa 1 to 280 of LMP-2A. HALMP-2A LMP-1 has a hemagglutinin (HA) epitope (YPYDVPDYA) substituted in its amino terminus. SubLZ1LMP-1 is a derivative of LMP-1 and was constructed by replacing the nucleotides encoding leucines 29, 30, 32, 33, 36, 37, and 40 with those encoding alanines. SubLZ6LMP-1 is a derivative of LMP-1 and was constructed by replacing the nucleotides encoding 167,168, 171, 172, 174, 175, and 178 with those encoding alanines. SubLZLMP-1 combines the substitutions of leucines with alanines found in SubLZ1LMP-1 and SubLZ6LMP-1. HASubLZLMPΔC and SubLZLMPΔC-GFP are derivatives of SubLZLMP-1 that substitute two EE (EYMPMEV) epitopes or EGFP, respectively, for the C terminus of LMP-1 (aa 190 to 386). SubLZLMPGFP and SubLZLMPRFP are derivatives of SubLZLMP-1 with EGFP or DsRed fused to C terminus of LMP-1. HALMPΔC-GFP is a derivative of LMP-1 that substitutes EGFP for the C terminus of LMP-1 (aa 190 to 386). HASubLZLMPΔC and HALMPΔC-GFP each have a HA epitope (YPYDVPDYA) inserted between LMP-1's residues 2 and 3.
Coimmunoprecipitation.
A total of 5.0 × 106 293 cells were transfected with expression plasmids for LMP-1 and its derivatives via calcium phosphate precipitation (13). The amount of plasmid DNA transfected was varied to normalize the expression levels of different derivatives of LMP-1. At 48 h after transfection the cells were lysed in 500 μl of 1× radioimmunoprecipitation assay (RIPA) buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 150 mM NaCl, and protease inhibitors). Cell debris was pelleted by centrifugation at 14,000 rpm for 15 min at 4°C. Then, 100 μl of lysate was saved and used to detect the level of each protein in the lysate. Next, 400 μl of lysate was precleared with 50 μl of 50% slurry of protein A-agarose and immunoprecipitated for 2 h at 4°C with 100 μl of anti-LMP-1 antiserum or antiLMP-1 monoclonal antibodies cs1 to cs4. The samples were washed four times with 1× RIPA and boiled for 5 min in 50 μl of 1× sample buffer (1% SDS, 0.1% bromophenol blue, 10% glycerol, 100 mM dithiothreitol). The samples were separated by electrophoresis through a SDS-polyacrylamide gel electrophoresis (PAGE) gel, transferred to nitrocellulose, probed with either mouse anti-HA at 1:1,000, rabbit anti-LMP-1 at 1:200, or rabbit anti-GFP at 1:250 and the appropriate, secondary antibody conjugated to alkaline phosphatase or labeled with 35S.
SDS-PAGE and quantitative Western blot analysis.
The GSTLMP-1 fusion protein used to quantify the number of molecules of LMP-1, contains LMP-1's C terminus, the epitopes for the anti-LMP-1 antiserum, and was described previously (31). LMP-1, its derivatives, and GSTLMP-1 were resolved by electrophoresis through a 10% polyacrylamide gel. Proteins resolved by SDS-PAGE were transferred to nitrocellulose and blocked with Blotto (1% nonfat dry milk and 0.05% Tween 20 in phosphate-buffered saline) for 20 min. Blots were probed with affinity-purified polyclonal anti-LMP-1 antiserum that recognizes epitopes in the carboxy terminus of LMP-1 at a 1:200 dilution. The blots were probed with the corresponding anti-rabbit antibodies (Kirkegaard Perry) conjugated to biotin at a 1:2,000 dilution and 35S-labeled streptavidin (Amersham) at a 1:1,000 dilution (0.5 μCi per blot). Alternatively, for detection with alkaline phosphatase, the secondary anti-rabbit antibody was conjugated with alkaline phosphatase. The blots were probed for 45 min with each antibody and with streptavidin at room temperature. The blots were then washed once with Blotto for 10 min at room temperature and exposed to a phosphorimager screen (Molecular Dynamics). The level of protein expression was quantified by using ImageQuant software (Molecular Dynamics).
Assay for NF-κB activity.
The assay for NF-κB activity was described previously (28) with a few modifications. In short, 50 to 80% confluent 10-cm or six-well dishes of 293 cells were transfected via calcium phosphate precipitation. The precipitate was made as a 1 ml of slurry and all of it was used for a10 cm dish or 250 μl of it was use for 1-well of a six-well dish. One milliliter of precipitate contains 50 ng of a NF-κB-luciferase reporter which contained four copies of an NF-κB responsive element upstream of luciferase (p1242), 20 ng of an expression vector for renilla luciferase (TKRluc; Promega), and/or 1 μg of an expression plasmid for EGFP or RFP. The DNA was brought up to a concentration of 30 μg/ml with an empty vector and expression vectors encoding LMP-1 or its derivatives. At 48 h after transfection the cells were harvested. The 10-cm dishes were split, and half the cells were used for SDS-PAGE and/or Western analysis. Approximately, 105 cells from the 10-cm dish or one well of a six-well dish was lysed in passive lysis buffer (Promega) and assayed for light emission on a monolight 3010 luminometer. All transfection efficiencies were normalized to the levels of renilla, GFP, or RFP, and the fold induction refers to the fold induction over the empty vector alone.
Microscopy.
All microscopy was preformed on a Bio-Rad MRC 1024 laser scanning confocal microscope equipped with a mixed gas (argon-krypton) laser operated by 24-bit Lasersharp software, allowing simultaneous display of red and green signals. 293 cells were plated on 18-by-18-mm coverslips and imaged live or fixed as indicated. Cells were fixed with 4.0% neutral formalin for 20 min at room temperature and mounted on slides with 1 eyedrop full of vectashield (Vector labs). Cells were imaged for GFP and RFP. Where more than one fluorophore was used each was displayed separately and simultaneously merged to minimize nonspecific excitation of overlapping fluorophores.
RESULTS
LMP-1's transmembrane domains contain specific residues required for its efficient activation of NF-κB-mediated transcription.
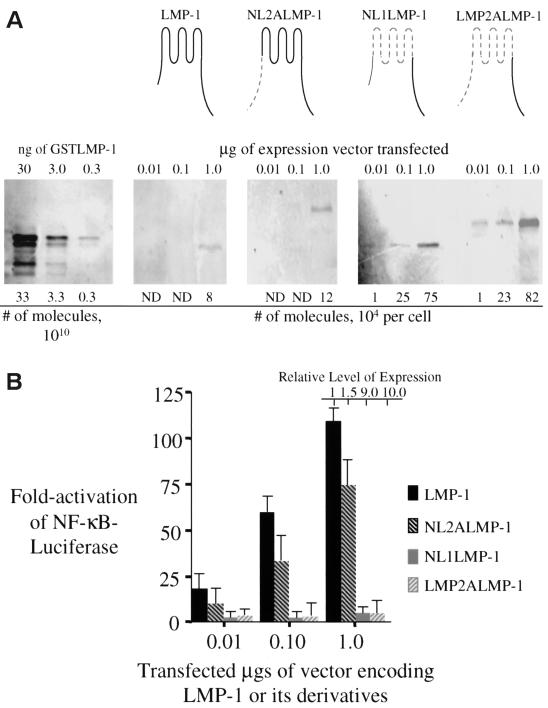
LMP-1 can induce more than a 100-fold activation of NF-κB-mediated transcription when a vector encoding it is introduced into 293 cells (Fig. 1). To test whether specific residues in LMP-1's amino terminus or transmembrane-spanning domain are required for its activation of NF-κB-mediated transcription, we substituted LMP-1's amino terminus and transmembrane-spanning domains, including its intracellular and extracellular loops with LMP-2A's amino terminus and first six transmembrane-spanning domains and its intracellular and extracellular loops (Fig. 1). The amino terminus and transmembrane domains of LMP-1 share less than 25% amino acid sequence identity with the structurally analogous regions of LMP-2A with which they were replaced. LMP-2A affects its signaling by binding cellular tyrosine kinases and ubiquitin ligases through its amino terminus (8, 37). LMP-1 and its derivatives were tested for their ability to activate NF-κB-mediated transcription on a per-molecule basis. The derivative of LMP-1, NL2ALMP-1, that substitutes only LMP-1's amino terminus with that of LMP-2A's activates similar levels of NF-κB-mediated transcription as does LMP-1 (Fig. 1). Interestingly, the derivatives of LMP-1, NL1LMP-1, and LMP-2ALMP-1 that contain LMP-2A's first six transmembrane domains accumulated to higher levels in cells than does LMP-1 and activate less than 3% the activity of NF-κB-mediated transcription as does LMP-1 on a per-molecule basis (Fig. 1A and B). There are multiple, possible reasons for the failure of these latter derivatives to signal which include an inappropriate localization, an inability to aggregate, or an inability to associate with necessary factors within the cell. However, their failure to signal efficiently contrasts with LMP-1 and indicates that there are specific residues in LMP-1's transmembrane-spanning domains or intracellular and extracellular loops that are required for its efficient activation of NF-κB-mediated transcription. We searched for potential protein motifs in the regions substituted in the nonfunctional derivatives of LMP-1 to explain their lack of function. Based on the studies of Gurezka et al. (14), we found clusters of leucines in LMP-1's first and sixth transmembrane-spanning domains which are similar to potential leucine-heptad motifs known to mediate protein-protein interactions in membranes. To test whether these potential motifs are required for LMP-1's signaling, we generated derivatives that changed seven of the leucines in these clusters to alanines in the first, sixth or both the first and sixth transmembrane-spanning domains of LMP-1 (Fig. 2A) .
FIG. 1.
LMP-1 and its derivatives with substitutions in its amino terminus and membrane-spanning domains were tested for their ability to activate NF-κB-mediated transcription. (A) Quantitative Western blots were performed to measure the levels of expression of LMP-1 (aa 1 to 386 [depicted in black]), NL2ALMP-1 (LMP-1's N terminus substituted with LMP-2A's N terminus [depicted by gray dashed lines]), NL1ALMP-1 (LMP-1's six transmembrane domain, including its intracellular and extracellular loops substituted with LMP-2A's first six transmembrane domains, including the intracellular and extracellular loops [depicted by gray dashed lines]), and LMP-2ALMP-1 (LMP-1's N terminus and transmembrane domains substituted with LMP-2A's N terminus and transmembrane domain, including its intracellular and extracellular loops [depicted by gray dashed lines]) after the vectors encoding them and one for GFP were introduced into 293 cells. A total of 105 GFP-positive cells transfected with the indicated amount of expression vector for each expression plasmid was lysed and separated electrophoretically by SDS-PAGE. The samples were transferred to a nitrocellulose membrane and then probed with a rabbit anti-LMP-1 antibody, a secondary biotinylated goat anti-rabbit antibody, and 35S-labeled streptavidin. The samples were visualized and quantified by phosphorimage analysis. The number of molecules of LMP-1 and its derivatives were calculated from known amounts of GSTLMP-1 assayed on the same blot. (B) The stimulation of NF-κB activity in 293 cells transfected with the amount of expression vectors indicated for LMP-1, NL1LMP-1, NL2ALMP-1, and LMP-2ALMP-1 was measured with an NF-κB responsive luciferase reporter. All transfections were normalized to renilla luciferase levels or to the number of GFP-positive cells. The expression of each protein relative to that of wtLMP-1 is shown and was determined from Fig. 1A. The fold activation of firefly luciferase over cells transfected with pSG5 alone is shown. The relative light units (RLUs) in these experiments varied from ∼5.0 × 103 to ∼2.0 × 104 in cells transfected with empty vector and up to ∼3.0 × 106 in the presence of expression vectors for LMP-1 or its derivatives. Dividing the fold activation by the relative level of expression for LMP-1 and each of its derivatives gives the fold activation on a per molecule basis for each of the proteins tested. The data represent the average ± the standard deviation for three separate experiments with two measurements each.
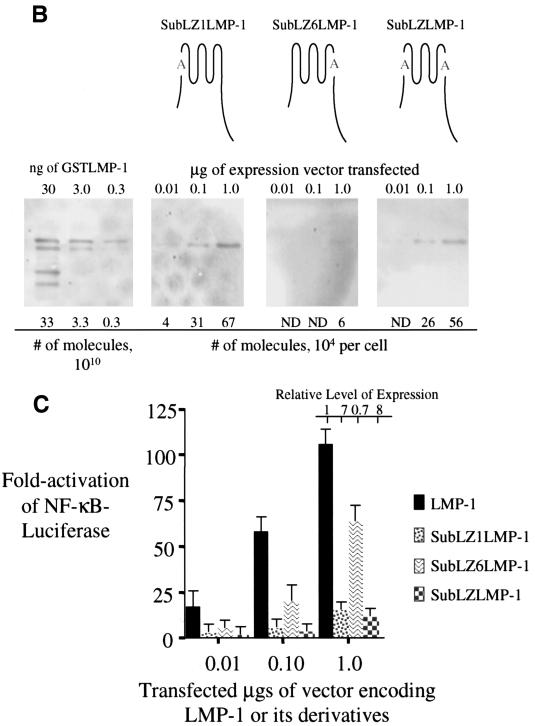
FIG. 2.
Substitution of seven of the leucines for alanines in a potential leucine-heptad motif found in LMP-1's first transmembrane domain impairs its efficient activation of NF-κB-mediated transcription. (A) Shown are the amino acids from residues 20 to 50 and 156 to 186 of LMP-1 that encompass its first and sixth membrane-spanning domains. The sequences beneath each with alanines at the starred leucines represent the substitutions introduced into derivatives of LMP-1 at the first and sixth membrane-spanning domains. These substitutions are within the potential leucine heptad motif, LLXXLLXLLXXLLXLL, identified by Gurezka et al. (14). (B) Quantitative Western blots were performed to assay the expression of SubLZ1LMP-1 (LMP-1 with its leucines 29, 30, 32, 33, 36, 37, and 40 in its first transmembrane substituted with alanines [shown with the letter A in gray]), SubLZ6LMP-1 (LMP-1 with its leucines 167, 168, 171, 172, 174, 175, and 178 in its sixth transmembrane domains substituted with alanines [shown with the letter A in gray]), and SubLZLMP-1 (LMP-1 with its leucines substituted with alanines 29, 30, 32, 33, 36, 37, 40, 167, 168, 171, 172, 174, 175, and 178 [shown with the two letter A's in gray]) as described in legend for Fig. 1A. (C) The stimulation of NF-κB activity in 293 cells transfected with expression vectors for LMP-1, SubLZ1LMP-1, SubLZ6LMP-1, and SubLZLMP-1 was measured with an NF-κB responsive luciferase reporter as described in the legend to Fig. 1B. The RLUs in these experiments varied from ∼5.0 × 103 to ∼2.0 × 104 in cells transfected with empty vector and up to ∼3.0 × 106 in the presence of expression vectors for LMP-1 or its derivatives. Dividing the fold activation by the relative level of expression for LMP-1 and its derivatives gives the fold activation on a per-molecule basis for each of the proteins tested. The data represent the average ± the standard deviation for three separate experiments with two measurements each.
SubLZ1LMP-1 and SubLZLMP-1 both contain substitutions of leucines with alanines in LMP-1's first transmembrane domain and activate NF-κB-mediated transcription inefficiently. SubLZ1LMP-1 and SubLZLMP-1 both accumulate in cells to higher levels than does LMP-1 and activate ca. 3% of the NF-κB activity, as does LMP-1 on a per molecule basis (Fig. 2A and C). SubLZ6LMP-1 accumulates in cells to similar levels as does LMP-1 and activates similar levels (when measured on a per-molecule basis) of NF-κB's activity as does LMP-1 (Fig. 2B and C). These experiments demonstrate that LMP-1's first transmembrane domain contains leucine residues that are required for LMP-1's efficient activation of NF-κB's activity.
Failure to aggregate does not explain SubLZLMP-1's inefficient signaling.
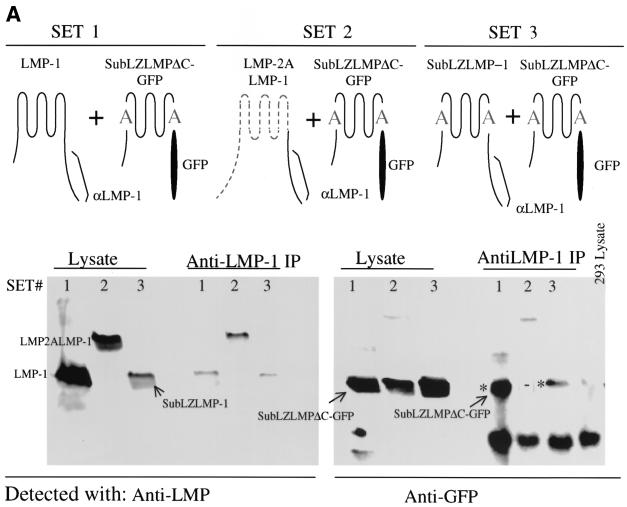
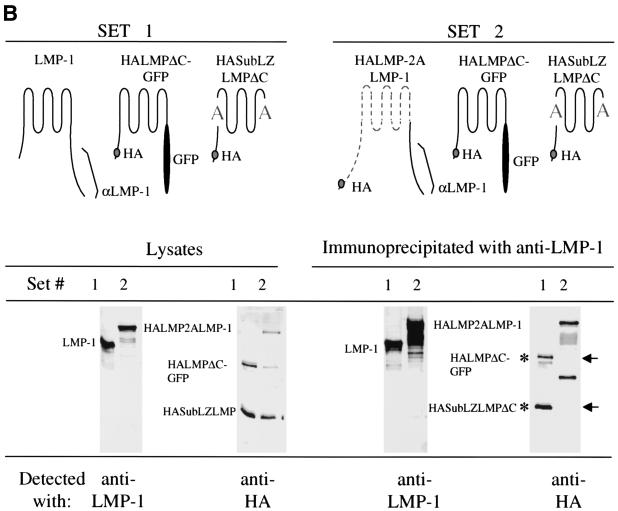
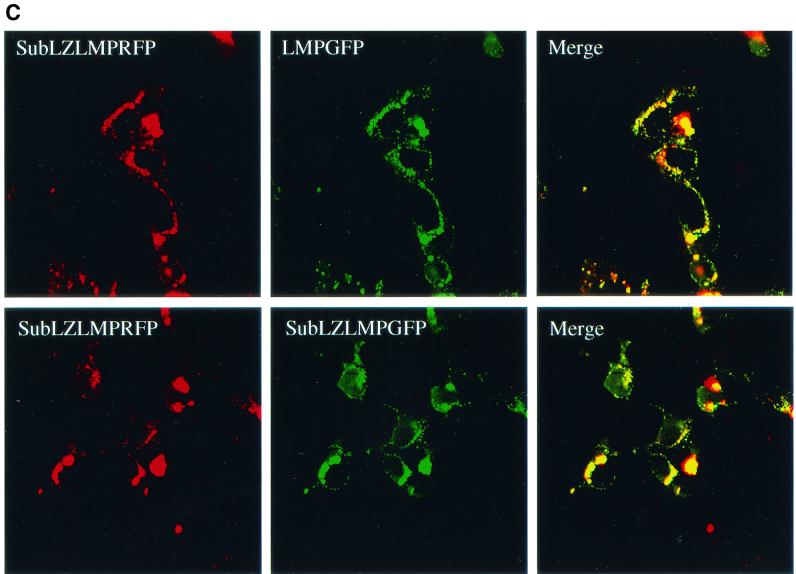
One possible explanation for SubLZ1LMP-1's and SubLZLMP-1's inefficient signaling would be a failure to aggregate. To test this possibility SubLZLMP-1 was tested for its ability to coimmunoprecipitate with a differently tagged derivative of itself. SubLZLMPΔC-GFP coimmunoprecipitates with wtLMP-1 and SubLZLMP-1 but not with a derivative of LMP-1 that contains LMP-2A's first six transmembrane domains, LMP2LMP-1 (Fig. 3A ). We also tested whether an HA-tagged derivative of SubLZMP-1 with it carboxy terminus deleted (to delete the epitopes used to immunoprecipitate LMP-1), HASubLZLMPΔC, coimmunoprecipitates with LMP-1 as well as does an HA-tagged derivative of LMP-1 with it carboxy terminus substituted with GFP, HALMPΔC-GFP. Both HASubLZLMPΔC and HALMPΔC-GFP coimmunoprecipitate with LMP-1 (Fig. 3B). In addition, neither of these derivatives coimmunoprecipitate with a derivative of LMP-1 that has its amino terminus and membrane-spanning domains substituted with LMP-2A's (Fig. 3B). Furthermore, derivatives of SubLZLMP-1 fused to red and green fluorescent proteins colocalize with each other in cells as does wild-type LMP-1 fused to GFP colocalize with SubLZLMP-1 fused to RFP (Fig. 3C). These experiments demonstrate that the defects in SubLZ1LMP-1's and SubLZLMP-1's signaling cannot be explained by their inability to aggregate. This observation has at least two implications. First, LMP-1's amino terminus and membrane-spanning domains contain other residues than those substituted in SubLZLMP-1 that can mediate LMP-1's aggregation. Second, a function mediated by LMP-1's first membrane-spanning domain other than aggregation is required for LMP-1's efficient activation of NF-κB's activity.
FIG. 3.
A failure to aggregate cannot explain SubLZLMP-1's inefficient signaling. (A) SubLZLMP-1 coimmunoprecipitates with derivatives of itself but not with derivatives of LMP-1 that contain LMP-2A's transmembrane domains. 293 cells were transfected with the expression vectors of set 1, set 2, and set 3. Set 1 contains expression plasmids for LMP-1 and SubLZLMPΔC-GFP which is a derivative of SubLZLMP-1 with its carboxy terminus replaced with GFP. Set 2 contains expression plasmids for LMP-2ALMP-1, which contains LMP-2A's amino terminus, its first six membrane-spanning domains and LMP-1's carboxy terminus, and SubLZLMPΔC-GFP. Set 3 contains expression plasmids for SubLZLMP-1 and SubLZLMPΔC-GFP. Lysates of these cells were probed directly for LMP-1's carboxy terminus or for GFP or immunoprecipitated with an anti-LMP-1 antibody and probed with either antibody. Precipitation of LMP-1 or of SubLZLMP-1 each coimmunoprecipitated SubLZLMPΔC-GFP as indicated by an asterisk. However, precipitation of LMP-2ALMP-1 failed to coimmunoprecipitate SubLZLMPΔC-GFP as indicated by “−”. The lower bands seen in all lanes, including the lane of immunoprecipitated 293 cell lysate, result from an unknown cross-reactivity of the antibodies. (B) Derivatives of SubLZLMP-1 coimmunoprecipitate with wild-type LMP-1, as well as derivatives of LMP-1 with its wild-type transmembrane domains, but not with derivatives of LMP-1 that contain LMP-2A's transmembrane domains. 293 cells were transfected with the expression vectors of set 1 or set 2. Set 1 contains expression plasmids for LMP-1, (i) HALMPΔC-GFP, which contains and an HA epitope tag at its amino terminus, LMP-1's transmembrane-spanning domains, and GFP in place of LMP-1's carboxy terminus and (ii) HASubLZLMPΔC, which contains an HA epitope tag at its amino terminus and LMP-1's transmembrane domains with the substituted residues in SubLZLMP-1 but lacks LMP-1's carboxy terminus. Set 2 contains expression plasmids for (i) HALMP-2ALMP-1, which contains an HA epitope at its amino terminus, LMP-2A's amino terminus, and first six membrane-spanning domains and LMP-1's carboxy terminus, (ii) HALMPΔC-GFP, and (iii) HASubLZLMPΔC. Lysates of these cells were probed directly for LMP-1's carboxy terminus or for HA or immunoprecipitated with an anti-LMP-1 antibody and probed with either antibody. Precipitation of LMP-1 coimmunoprecipitated similar amounts of HALMPΔC-GFP and HASubLZLMPΔC, as indicated by an asterisk. However, precipitation of HALMP-2ALMP-1 failed to coimmunoprecipitate either HALMPΔC-GFP or HASubLZLMPΔC, as indicated by arrows. (C) Confocal microscopy of GFP- and RFP-tagged derivatives of SubLZLMP-1 (bottom panels) and GFP-tagged LMP-1 and RFP-tagged SubLZLMP-1 (top panels). 293 cells were transfected with vectors encoding both SubLZLMPGFP and SubLZLMPRFP or both LMPGFP and SubLZLMPRFP. The colocalization shown in yellow is consistent with these two derivatives aggregating as does wtLMP-1 (21).
A derivative of LMP-1 that has its wild-type transmembrane-spanning domains but cannot signal complements the defect in SubLZLMP-1's activation of NF-κB's activity and decreases SubLZLMP-1's level of expression.
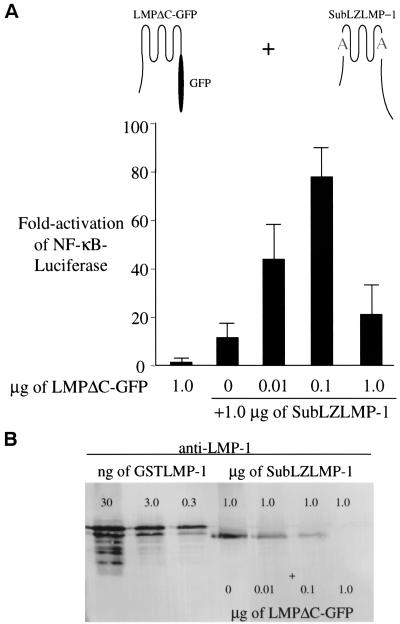
Derivatives of a variety of oligomeric proteins, e.g., β-galactosidase, can complement each other's defects if they retain their ability to associate (29). Because SubLZLMP-1 retains its ability to associate with LMP-1, we tested whether a mutant of LMP-1, LMPΔC-GFP, that cannot signal because it has its carboxy-terminal signaling domain replaced with GFP complements the defect in SubLZLMP-1's activation of NF-κB's activity. Expression of either LMPΔC-GFP and SubLZLMP-1 alone in 293 cells induces only low levels of NF-κB's activity (Fig. 4A). However, when SubLZLMP-1 is expressed in cells with increasing amounts of LMPΔC-GFP the combination of both proteins increases the activation of NF-κB's activity and leads to a decrease in the apparent expression of SubLZLMP-1 (Fig. 4). When 1.0 μg of SubLZLMP-1 is transfected with 100 ng of LMPΔC-GFP, SubLZLMP-1's ability to activate NF-κB's activity is nearly restored to that of wtLMP-1's ability and SubLZLMP-1's level of expression is decreased by at least one-half (Fig. 4A and B). These findings indicate that SubLZLMP-1 directly or indirectly, perhaps via a cellular protein bridge, associates with LMP-1 and that a function of LMP-1's transmembrane domains that is defective in SubLZLMP-1 other than aggregation can be complemented by the expression of the wild-type transmembrane-spanning domains of LMP-1.
FIG. 4.
The inefficient activation of NF-κB-mediated transcription induced by SubLZLMP-1 can be complemented to wild-type levels by expression of a plasmid encoding a derivative of LMP-1 with its wild-type amino terminus and membrane-spanning domains but lacking its carboxy terminus. (A) LMPΔC-GFP complements the SubLZLMP-1 derivative and restores its ability to activate NF-κB-mediated transcription efficiently. The stimulation of NF-κB activity in 293 cells transfected with 1.0 μg of vector encoding LMPΔC-GFP alone or 1.0 μg of SubLZLMP-1 and increasing amounts of LMPΔC-GFP was measured with a luciferase reporter. The RLUs in these experiments varied from ∼6.0 × 103 to up to ∼6.0 × 104 in cells transfected with empty vector to ∼3.0 × 107 in the presence of expression vectors for SubLZLMP-1 and LMPΔC-GFP. LMPΔC-GFP, which is expressed from the cytomegalovirus promoter, is likely to inhibit gene expression at concentrations of DNA higher than 100 ng per transfection (31). The data represent the average ± the standard deviation for three separate experiments with two measurements each. (B) Lysates from 293 cells transfected as in panel A were probed with anti-LMP-1 antiserum and a secondary alkaline phosphatase-conjugated antibody to visualize the level of expression of SubLZLMP-1. The level of SubLZLMP-1 decreases when increasing amounts of LMPΔC-GFP are cotransfected. GSTLMP-1 is used to determine the relative level of expression of LMP-1.
DISCUSSION
This study demonstrates that LMP-1's transmembrane-spanning domains contain specific residues required for LMP-1's activation of NF-κB-mediated transcription. Derivatives of LMP-1 that contain substitutions of LMP-1's transmembrane-spanning domains with LMP-2A's first six transmembrane spanning domains inefficiently activate NF-κB-mediated transcription. A derivative of LMP-1 with LMP-2A's membrane-spanning domains fails to associate with wild-type LMP-1 as measured by coimmunoprecipitation. This experiment demonstrates that there are specific residues in LMP-1's transmembrane domains or intracellular or extracellular loops that are required for its association into a complex with other LMP-1 molecules and is consistent with aggregation being required for LMP-1's activation of NF-κB-mediated transcription. The residues in LMP-1's amino terminus only play a minimal role in LMP-1's activation of NF-κB's activity since they can be replaced by a structurally analogous domain of LMP-2A's with only a small effect on LMP-1's activation of NF-κB's activity. We have found that LMP-1's first and sixth membrane-spanning domains have clusters of leucines, each containing a potential leucine-heptad motif, known to mediate interaction of some proteins embedded within the plasma membrane (14). Substitution of seven leucines with alanines in the presumptive leucine-heptad motifs did not affect LMP-1's ability to coimmunoprecipitate or colocalize in a complex with other derivatives of LMP-1. However, these substitutions in LMP-1's first transmembrane-spanning domain decrease its induction of NF-κB's activity and increase its accumulation at steady state. It is mechanistically revealing that a derivative of LMP-1 that has wild-type transmembrane-spanning domains but cannot signal for lack of its carboxy terminus complements the defect in the derivative of LMP-1 having seven leucines substituted with alanines in its first membrane-spanning domain. This complementation both restores efficient activation of NF-κB's activity and decreases accumulation of the derivative (Fig. 4A and B). These observations are consistent with LMP-1's amino terminus and membrane-spanning domains contributing one or more functions other than aggregation that are required to support LMP-1's efficient signaling.
It is not clear if LMP-1's membrane-spanning domains confer a specific structure to LMP-1 that is required for its signaling. However, given the similarities between LMP-1's and CD40's signaling, it seems likely that LMP-1's membrane-spanning domains do confer such a structure. For instance, some members of the TNFR family are found as preformed trimers on the surface of cells due to their PLAD domain and are activated by a presumably allosteric association of their trimeric ligands (4). Fusion of LMP-1's amino terminus and transmembrane-spanning domains to the cytoplasmic domains of members of the TNFR family can activate their signaling in the absence of their ligands (12, 15). This finding demonstrates the LMP-1's amino terminus and transmembrane-spanning domains can substitute for the extracellular ligand binding domain of TNFR members when bound by ligand. One possible explanation for the defect in SubLZLMP-1's ability to signal is that substitution of the leucines for alanines in LMP-1's first membrane-spanning domain disrupts LMP-1's structure and decreases its affinity for TRAFs and TRADD. The SubLZLMP-1 mutant may genetically separate the domain of LMP-1 that aggregates, in a way similar to that of the PLAD domain of some TNFR family members, from its domain that confers a structure on its carboxy terminus in the absence of ligand that is similar to the structure that TNF ligand confers on TNF receptors when they associate with the ligand-binding domain of TNF receptors. A second possible explanation is that the cluster of leucines in LMP-1's first membrane-spanning domain is a leucine zipper which mediates association with a cellular protein required for LMP-1's signaling.
All of the derivatives of LMP-1 in the present study that fail to signal as efficiently as does wild-type LMP-1 accumulate in cells to higher levels than does wild-type LMP-1. On average, these derivatives accumulate in cells to 10-fold higher levels than does wild-type LMP-1 and activate per molecule 3% of the NF-κB activity as does wild-type LMP-1. The measurements of NF-κB activity have been performed under conditions in which LMP-1 and its derivatives act dose dependently (Fig. 1B, 2C, and 4A), indicating that their activities have not saturated the cells' signaling machinery. The inefficient signaling of the derivatives of LMP-1, even though they accumulate to higher levels than wild-type LMP-1, indicates that they are likely defective in their ability to assemble a signaling complex. These observations are consistent with LMP-1's efficient signaling and short-half life being coupled. This proposed coupling would render LMP-1's signaling similar to that of activated receptors. For instance, EGFR and TNFR-1 are turned over rapidly from the plasma membrane when they are treated with ligand, and their turnover is required for their signaling (33, 34). One tantalizing possibility is that a function of LMP-1's first transmembrane domain that is affected in SubLZLMP-1 is required for both signaling and rapid turnover. For instance, LMP-1's first membrane spanning domain may associate with proteins required for both its targeting to its site for signaling and its rapid turnover. Alternatively, LMP-1's first membrane-spanning domain may not only be required for LMP-1's signaling via TRAFs but may facilitate LMP-1's ubiquitination and turnover. Consistent with this idea TRAFs are not only required for LMP-1's efficient activation of NF-κB but also are ubiquitin ligases (5). TRAF6 in particular is critical for LMP-1's signaling (32) and functions as a ubiquitin ligase. A detailed characterization of SubLZLMP-1 will help to elucidate the role of LMP-1's transmembrane domains in LMP-1's signaling and turnover and will likely illuminate the intricacies of signaling by members of the TNFR family.
Acknowledgments
We thank Richard Longnecker and Wolfgang Hammerschmidt for their gifts of vectors. We thank Teresa Compton, Paul Lambert, and Ngan Lam for critical help in revising the manuscript.
This study was supported by NIH grants CA22443, CA07175, CA09135, and CA70723. K.W. was supported by a Hilldale Fellowship. B.S. is an American Cancer Society Research Professor.
REFERENCES
- 1.Banchereau, J., P. de Paoli, A. Valle, E. Garcia, and F. Rousset. 1991. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science 251:70-72. [DOI] [PubMed] [Google Scholar]
- 2.Berberich, I., G. L. Shu, and E. A. Clark. 1994. Cross-linking CD40 on B cells rapidly activates nuclear factor-κB. J. Immunol. 153:4357-4366. [PubMed] [Google Scholar]
- 3.Brodeur, S. R., G. Cheng, D. Baltimore, and D. Thorley-Lawson. 1997. Localization of the major NF-κB-activating site and the sole TRAF3 binding site of LMP-1 defines two distinct signaling motifs J. Biol. Chem. 272:19777-19784. [DOI] [PubMed] [Google Scholar]
- 4.Chan, F. K., H. J. Chun, L. Zheng, R. M. Siegel, K. L. Bui, and M. J. Lenardo. 2000. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science 288:2351-2354. [DOI] [PubMed] [Google Scholar]
- 5.Deng, L., C. Wang, E. Spencer, L. Yang, A. Braun, J. You, C. Slaughter, C. Pickart, and Z. J. Chen. 2000. Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103:351-361. [DOI] [PubMed] [Google Scholar]
- 6.Devergne, O., E. C. McFarland, G. Mosialos, K. M. Izumi, C. F. Ware, and E. Kieff. 1998. Role of the TRAF binding site and NF-κB activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression. J. Virol. 72:7900-7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eliopoulos, A. G., and L. S. Young. 1998. Activation of the cJun N-terminal kinase (JNK) pathway by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1). Oncogene 16:1731-1742. [DOI] [PubMed] [Google Scholar]
- 8.Engels, N., M. Merchant, R. Pappu, A. C. Chan, R. Longnecker, and J. Wienands. 2001. Epstein-Barr virus latent memberane protein 2A (LMP2A) employs the SLP-65 signaling module. J. Exp. Med. 194:255-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Floettmann, J. E., and M. Rowe. 1997. Epstein-Barr virus latent membrane protein-1 (LMP1) C-terminus activation region 2 (CTAR2) maps to the far C-terminus and requires oligomerisation for NF-κB activation. Oncogene 15:1851-1858. [DOI] [PubMed] [Google Scholar]
- 10.Francis, D. A., J. G. Karras, X. Y. Ke, R. Sen, and T. L. Rothstein. 1995. Induction of the transcription factors NF-κB, AP-1 and NF-AT during B cell stimulation through the CD40 receptor. Int. Immunol. 7:151-161. [DOI] [PubMed] [Google Scholar]
- 11.Gires, O., F. Kohlhuber, E. Kilger, M. Baumann, A. Kieser, C. Kaiser, R. Zeidler, B. Scheffer, M. Ueffing, and W. Hammerschmidt. 1999. Latent membrane protein 1 of Epstein-Barr virus interacts with JAK3 and activates STAT proteins. EMBO J. 18:3064-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gires, O., S. U. Zimber, R. Gonnella, M. Ueffing, G. Marschall, R. Zeidler, D. Pich, and W. Hammerschmidt. 1997. Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. EMBO J. 16:6131-6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham, F. L., J. Smiley, W. C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59-74. [DOI] [PubMed] [Google Scholar]
- 14.Gurezka, R., R. Laage, B. Brosig, and D. Langosch. 1999. A heptad motif of leucine residues found in membrane proteins can drive self-assembly of artificial transmembrane segments. J. Biol. Chem. 274:9265-9270. [DOI] [PubMed] [Google Scholar]
- 15.Hatzivassiliou, E., W. E. Miller, N. Raab-Traub, E. Kieff, and G. Mosialos. 1998. A fusion of the EBV latent membrane protein-1 (LMP1) transmembrane domains to the CD40 cytoplasmic domain is similar to LMP1 in constitutive activation of epidermal growth factor receptor expression, nuclear factor-κB, and stress-activated protein kinase. J. Immunol. 160:1116-1121. [PubMed] [Google Scholar]
- 16.Hostager, B. S., I. M. Catlett, and G. A. Bishop. 2000. Recruitment of CD40 and tumor necrosis factor receptor-associated factors 2 and 3 to membrane microdomains during CD40 signaling. J. Biol. Chem. 275:15392-15398. [DOI] [PubMed] [Google Scholar]
- 17.Huo, L., and T. L. Rothstein. 1995. Receptor-specific induction of individual AP-1 components in B lymphocytes. J. Immunol. 154:3300-3309. [PubMed] [Google Scholar]
- 18.Izumi, K. M., and E. D. Kieff. 1997. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-κB. Proc. Natl. Acad. Sci. USA 94:12592-12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karras, J. G., Z. Wang, L. Huo, D. A. Frank, and T. L. Rothstein. 1997. Induction of STAT protein signaling through the CD40 receptor in B lymphocytes: distinct STAT activation following surface Ig and CD40 receptor engagement. J. Immunol. 159:4350-4355. [PubMed] [Google Scholar]
- 20.Kaye, K. M., O. Devergne, J. N. Harada, K. M. Izumi, R. Yalamanchili, E. Kieff, and G. Mosialos. 1996. Tumor necrosis factor receptor associated factor 2 is a mediator of NF-κB activation by latent infection membrane protein 1, the Epstein-Barr virus transforming protein Proc. Natl. Acad. Sci. USA 93:11085-11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaykas, A., K. Worringer, and B. Sugden. 2001. CD40 and LMP-1 both signal from lipid rafts but LMP-1 assembles a distinct, more efficient signaling complex. EMBO J. 20:2641-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kieser, A., E. Kilger, O. Gires, M. Ueffing, W. Kolch, and W. Hammerschmidt. 1997. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J. 16:6478-6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilger, E., A. Kieser, M. Baumann, and W. Hammerschmidt. 1998. Epstein-Barr virus-mediated B-cell proliferation is dependent upon latent membrane protein 1, which simulates an activated CD40 receptor. EMBO J. 17:1700-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Longnecker, R., and E. Kieff. 1990. A second Epstein-Barr virus membrane protein (LMP2) is expressed in latent infection and colocalizes with LMP1. J. Virol. 64:2319-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longnecker, R., C. L. Miller, B. Tomkinson, X. Q. Miao, and E. Kieff. 1993. Deletion of DNA encoding the first five transmembrane domains of Epstein-Barr virus latent membrane proteins 2A and 2B. J. Virol. 67:5068-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin, J., and B. Sugden. 1991. The latent membrane protein oncoprotein resembles growth factor receptors in the properties of its turnover. Cell Growth Differ. 2:653-700. [PubMed] [Google Scholar]
- 27.Martin, J., and B. Sugden. 1991. Transformation by the oncogenic latent membrane protein correlates with its rapid turnover, membrane localization, and cytoskeletal association. J. Virol. 65:3246-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell, T., and B. Sugden. 1995. Stimulation of NF-κB-mediated transcription by mutant derivatives of the latent membrane protein of Epstein-Barr virus. J. Virol. 69:2968-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossi, F., C. A. Charlton, and H. M. Blau. 1997. Monitoring protein-protein interactions in intact eukaryotic cells by β-galactosidase complementation Proc. Natl. Acad. Sci. USA 94:8405-8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandberg, M., W. Hammerschmidt, and B. Sugden. 1997. Characterization of LMP-1's association with TRAF1, TRAF2, and TRAF3. J. Virol. 71:4649-4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandberg, M. L., A. Kaykas, and B. Sugden. 2000. Latent membrane protein 1 of Epstein-Barr virus inhibits as well as stimulates gene expression. J. Virol. 74:9755-9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schultheiss, U., S. Puschner, E. Kremmer, T. W. Mak, H. Engelmann, W. Hammerschmidt, and A. Kieser. 2001. TRAF6 is a critical mediator of signal transduction by the viral oncogene latent membrane protein 1. EMBO J. 20:5678-5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schutze, S., T. Machleidt, D. Adam, R. Schwandner, K. Wiegmann, M. L. Kruse, M. Heinrich, M. Wickel, and M. Kronke. 1999. Inhibition of receptor internalization by monodansylcadaverine selectively blocks p55 tumor necrosis factor receptor death domain signaling. J. Biol. Chem. 274:10203-10212. [DOI] [PubMed] [Google Scholar]
- 34.Stang, E., L. E. Johannessen, S. L. Knardal, and I. H. Madshus. 2000. Polyubiquitination of the epidermal growth factor receptor occurs at the plasma membrane upon ligand-induced activation. J. Biol. Chem. 275:13940-13947. [DOI] [PubMed] [Google Scholar]
- 35.Uchida, J., T. Yasui, Y. Takaoka-Shichijo, M. Muraoka, W. Kulwichit, N. Raab-Traub, and H. Kikutani. 1999. Mimicry of CD40 signals by Epstein-Barr virus LMP1 in B lymphocyte responses. Science 286:300-303. [DOI] [PubMed] [Google Scholar]
- 36.Vidalain, P. O., O. Azocar, C. Servet-Delprat, C. Rabourdin-Combe, D. Gerlier, and S. Manie. 2000. CD40 signaling in human dendritic cells is initiated within membrane rafts. EMBO J. 19:3304-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winberg, G., L. Matskova, F. Chen, P. Plant, D. Rotin, G. Gish, R. Ingham, I. Ernberg, and T. Pawson. 2000. Latent membrane protein 2A of Epstein-Barr virus binds WW domain E3 protein-ubiquitin ligases that ubiquitinate B-cell tyrosine kinases. Mol. Cell. Biol. 20:8526-8535. [DOI] [PMC free article] [PubMed] [Google Scholar]