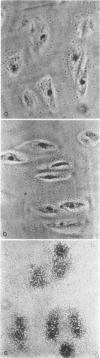
Abstract
A number of hormones somehow modify the turnover of the polysaccharides in a variety of connective tissues. In hypophysectomized animals the turnover of chondroitin sulfate and hyaluronic acid is decreased; when such animals are given growth hormone the turnover of chondroitin sulfate is enhanced but that of hyaluronic acid is unaltered. The effect of parathyroid extracts may be of a dual nature: in some connective tissues there may be an increase in the rate at which chondroitin sulfate is catabolized, in other tissues its synthesis may be stimulated. Thyroxine effectively restores toward normal the depressed synthesis and breakdown of polysaccharides in hypothyroid animals. Estradiol, in addition to inhibiting the resorption of the metaphyses in rats, inhibits the synthesis of chondroitin sulfate in cartilage and aorta. Cortisone too inhibits the synthesis of chondroitin sulfates and hyaluronic acid; its effect on their catabolism is not as striking.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELANGER L. F. Autoradiographic visualization of the entry and transit of S35 methionine and cystine in the soft and hard tissues of the growing rat. Anat Rec. 1956 Mar;124(3):555–579. doi: 10.1002/ar.1091240305. [DOI] [PubMed] [Google Scholar]
- BUDY A. M., URIST M. R., MCLEAN F. C. The effect of estrogens on the growth apparatus of the bones of immature rats. Am J Pathol. 1952 Nov-Dec;28(6):1143–1167. [PMC free article] [PubMed] [Google Scholar]
- CAMPO R. D., DZIEWIATKOWSKI D. D. A consideration of the permeability of cartilage to inorganic sulfate. J Biophys Biochem Cytol. 1961 Feb;9:401–408. doi: 10.1083/jcb.9.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPO R. D., DZIEWIATKOWSKI D. D. Intracellular synthesis of protein-polysaccharides by slices of bovine costal cartilage. J Biol Chem. 1962 Sep;237:2729–2735. [PubMed] [Google Scholar]
- CLARK I., UMBREIT W. W. Effect of cortisone and other steroids upon in vitro synthesis of chondroitin sulfate. Proc Soc Exp Biol Med. 1954 Jul;86(3):558–561. doi: 10.3181/00379727-86-21164. [DOI] [PubMed] [Google Scholar]
- DZIEWIATKOWSKI D. D., BRONNER F., DI FERRANTE N., ARCHIBALD R. M. Some aspects of the metabolism of sulfate-S35 and calcium-45 in the metaphyses of immature rats: influence of beta-estradiol benzoate. J Biophys Biochem Cytol. 1957 Mar 25;3(2):151–160. doi: 10.1083/jcb.3.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
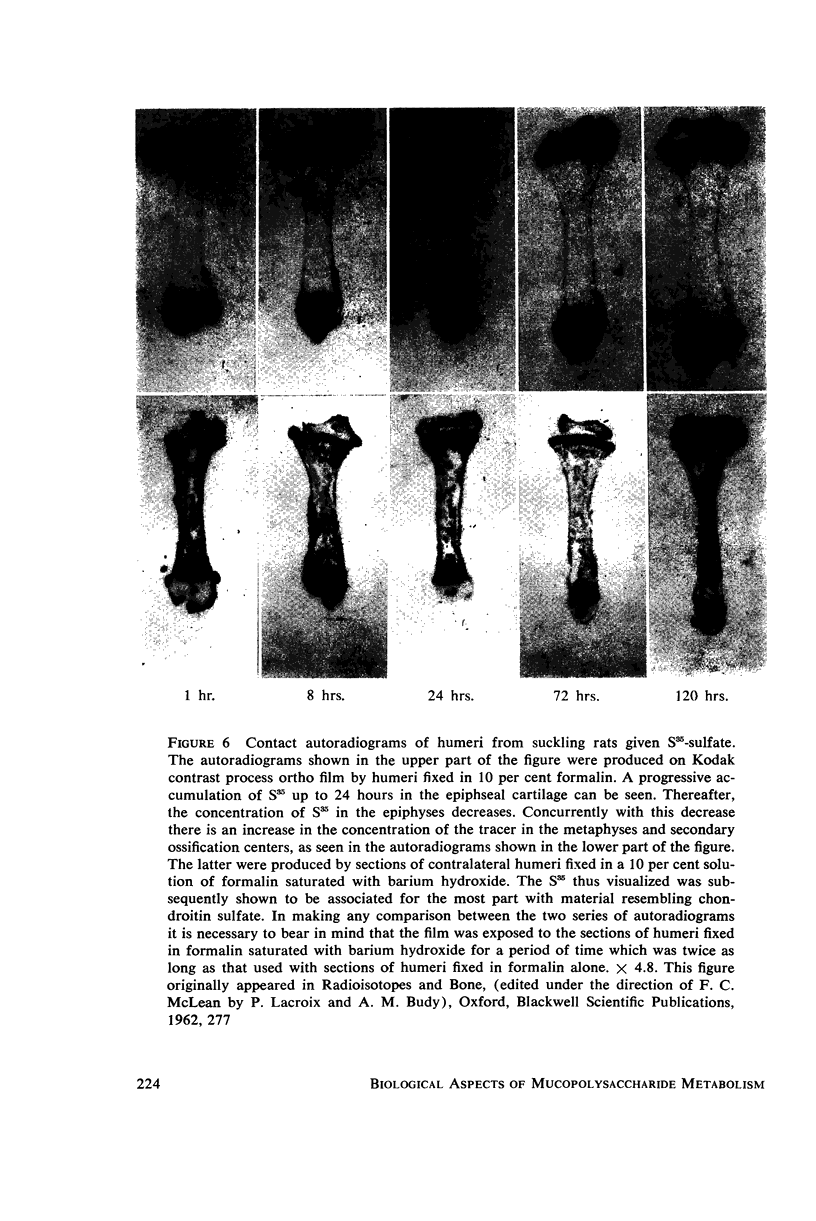
- DZIEWIATKOWSKI D. D., DI FERRANTE N., BRONNER F., OKINAKA G. Turnover of S35-sulfate in epiphyses and diaphyses of suckling rats; nature of the S35-labelled compounds. J Exp Med. 1957 Oct 1;106(4):509–524. doi: 10.1084/jem.106.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DZIEWIATKOWSKI D. D. Intracellular synthesis of chondroitin sulfate. J Cell Biol. 1962 Jun;13:359–364. doi: 10.1083/jcb.13.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DZIEWIATKOWSKI D. D. Radioautographic studies of sulfate-sulfur (S35) metabolism in the articular cartilage and bone of suckling rats. J Exp Med. 1952 May 1;95(5):489–496. doi: 10.1084/jem.95.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
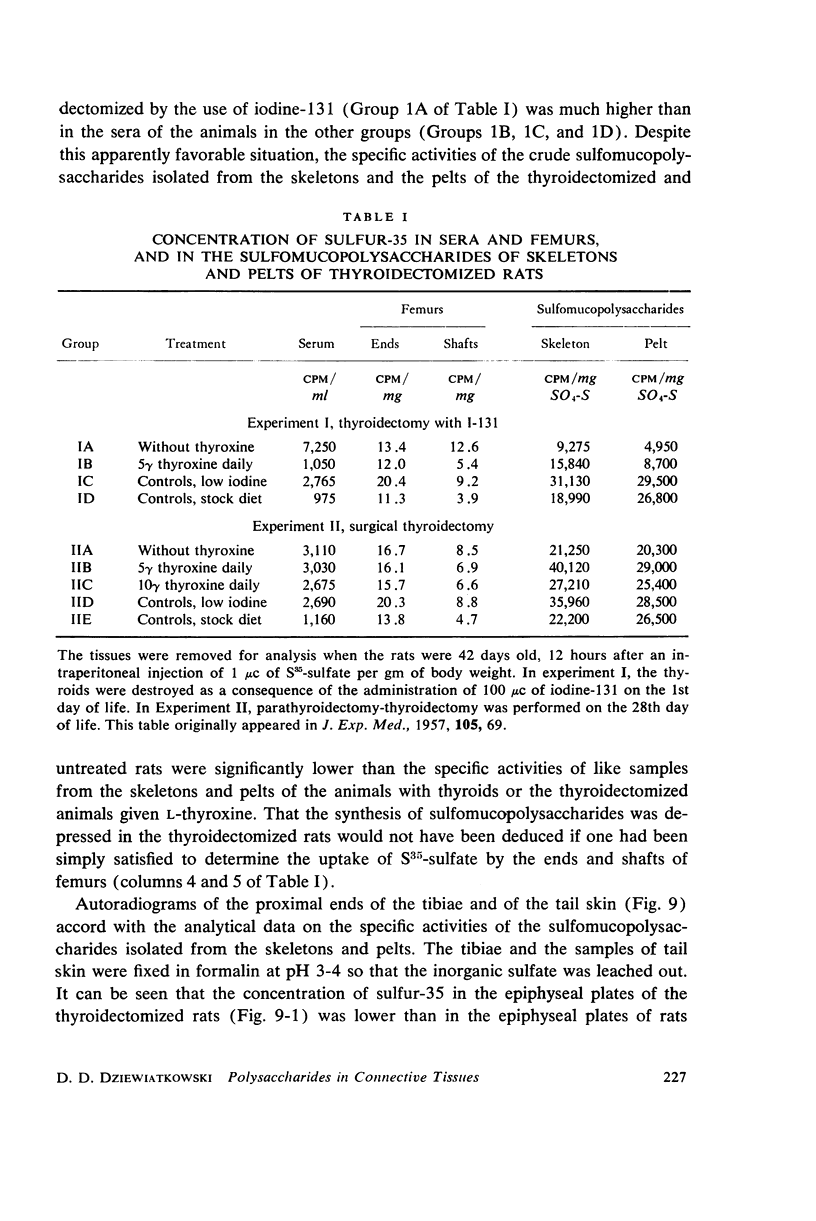
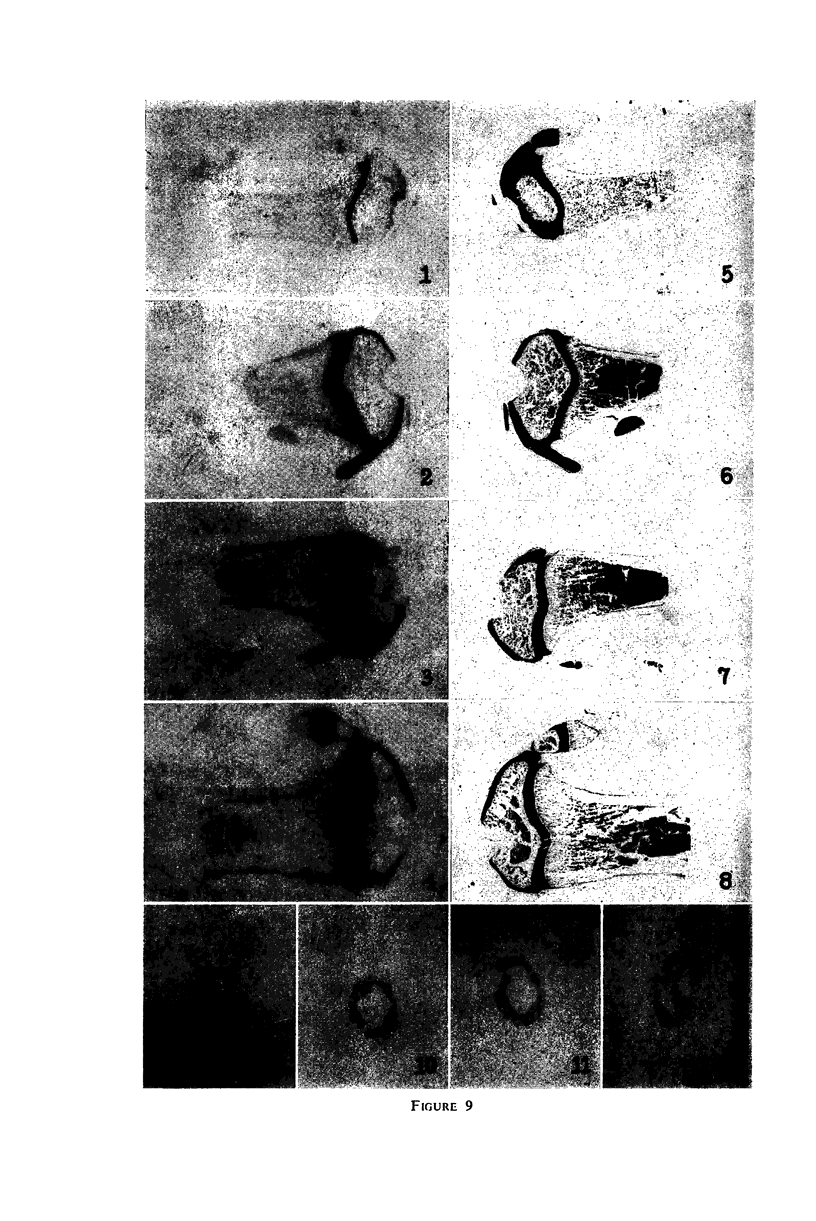
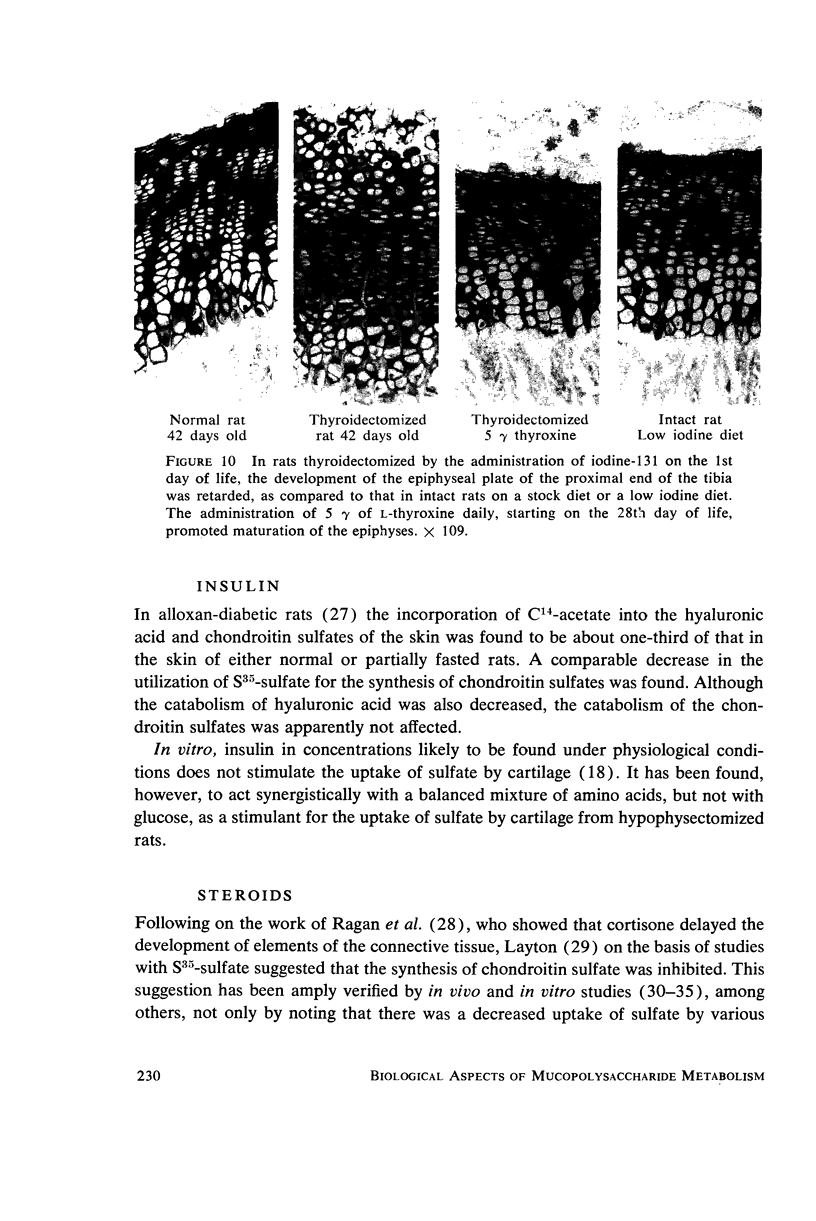
- DZIEWIATKOWSKI D. D. Synthesis of sulfomucopolysaccharides in thyroidectomized rats. J Exp Med. 1957 Jan 1;105(1):69–74. doi: 10.1084/jem.105.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS S., HUBLE J., SIMPSON M. E. Influence of hypophysectomy and growth hormone on cartilage sulfate metabolism. Proc Soc Exp Biol Med. 1953 Dec;84(3):603–605. doi: 10.3181/00379727-84-20726. [DOI] [PubMed] [Google Scholar]
- GERBER B. R., FRANKLIN E. C., SCHUBERT M. Ultracentrifugal fractionation of bovine nasal chondromucoprotein. J Biol Chem. 1960 Oct;235:2870–2875. [PubMed] [Google Scholar]
- GODMAN G. C., PORTER K. R. Chondrogenesis, studied with the electron microscope. J Biophys Biochem Cytol. 1960 Dec;8:719–760. doi: 10.1083/jcb.8.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOWALEWSKI K. Comparison of the effects of cortisone and certain anabolic-androgenic steroids on the uptake of radiosulfur in a healing fractured bone. Endocrinology. 1958 Apr;62(4):493–497. doi: 10.1210/endo-62-4-493. [DOI] [PubMed] [Google Scholar]
- LAYTON L. L. Effect of cortisone upon chondroitin sulfate synthesis by animal tissues. Proc Soc Exp Biol Med. 1951 Mar;76(3):596–598. doi: 10.3181/00379727-76-18571. [DOI] [PubMed] [Google Scholar]
- MALAWISTA I., SCHUBERT M. Chondromucoprotein: new extraction method and alkaline degradation. J Biol Chem. 1958 Jan;230(1):535–544. [PubMed] [Google Scholar]
- MURPHY W. R., DAUGHADAY W. H., HARTNETT C. The effect of hypophysectomy and growth hormone on the incorporation of labeled sulfate into tibial epiphyseal and nasal cartilage of the rat. J Lab Clin Med. 1956 May;47(5):715–722. [PubMed] [Google Scholar]
- McCLUSKEY R. T., THOMAS L. The removal of cartilage matrix in vivo by papain; prevention of recovery with cortisone, hydrocortisone and prednisoione by a direct action on cartilage. Am J Pathol. 1959 Jul-Aug;35(4):819–833. [PMC free article] [PubMed] [Google Scholar]
- PRIEST R. E., KOPLITZ R. M., BENDITT E. P. Estradiol reduces incorporation of radioactive sulfate into cartilage and aortas of rats. J Exp Med. 1960 Jul 1;112:225–236. doi: 10.1084/jem.112.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIEST R. E., KOPLITZ R. M. Inhibition of synthesis of sulfated mucopolysaccharides by estradiol. J Exp Med. 1962 Oct 1;116:565–574. doi: 10.1084/jem.116.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAY R. D., SIMPSON M. E., LI C. H., ASLING C. W., EVANS H. M. Effects of the pituitary growth hormone and thyroxin on growth and differentiation of the skeleton of the rat thyroidectomized at birth. Am J Anat. 1950 May;86(3):479–516. doi: 10.1002/aja.1000860306. [DOI] [PubMed] [Google Scholar]
- SALMON W. D., Jr, DAUGHADAY W. H. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med. 1957 Jun;49(6):825–836. [PubMed] [Google Scholar]
- SALMON W. D., Jr Importance of amino acids in the actions of insulin and serum sulfation factor to stimulate sulfate uptake by cartilage from hypophysectomized rats. J Lab Clin Med. 1960 Nov;56:673–681. [PubMed] [Google Scholar]
- SCHILLER S., DORFMAN A. The biosynthesis of mucopolysaccharides in the skin of alloxan-diabetic rats. Biochim Biophys Acta. 1955 Feb;16(2):304–305. doi: 10.1016/0006-3002(55)90229-8. [DOI] [PubMed] [Google Scholar]
- SCHILLER S., DORFMAN A. The metabolism of mucopolysaccharides in animals: the effect of cortisone and hydrocortisone on rat skin. Endocrinology. 1957 Mar;60(3):376–381. doi: 10.1210/endo-60-3-376. [DOI] [PubMed] [Google Scholar]