Abstract
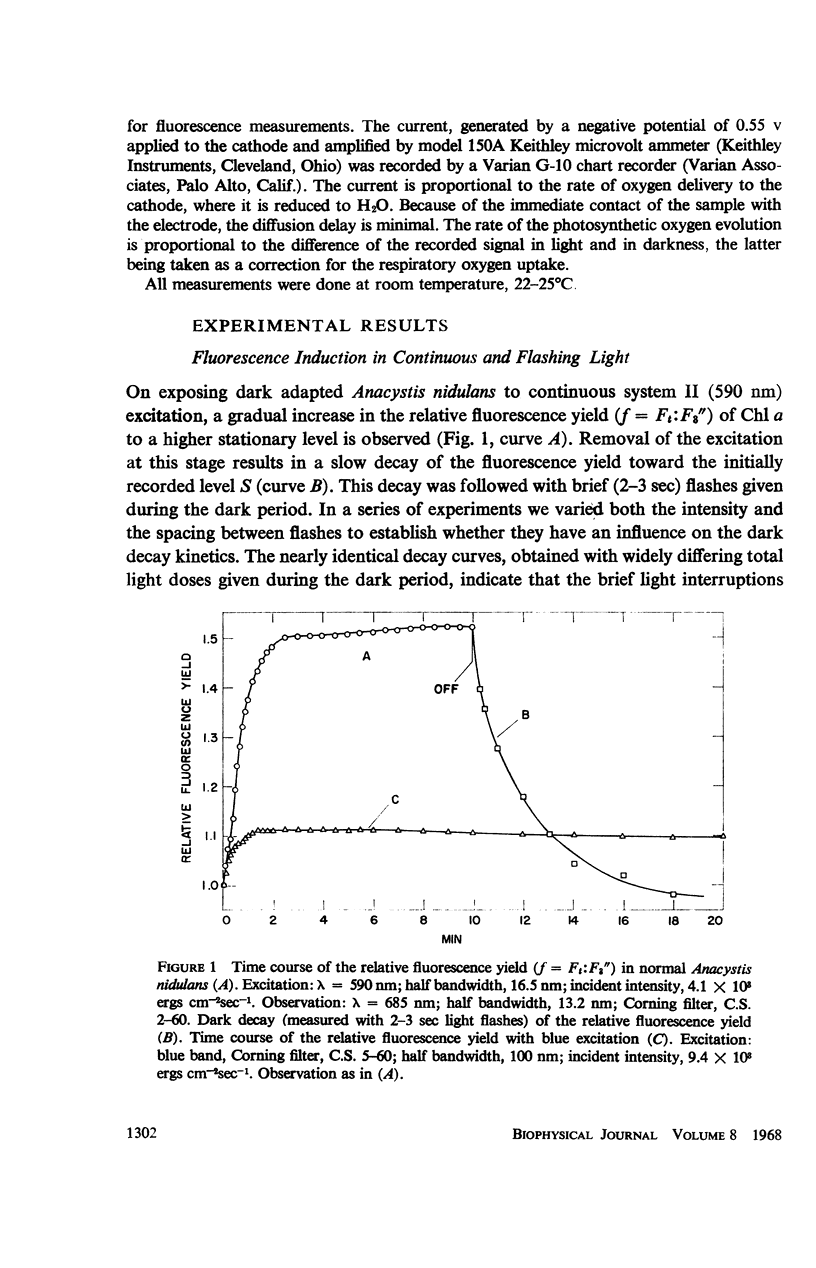
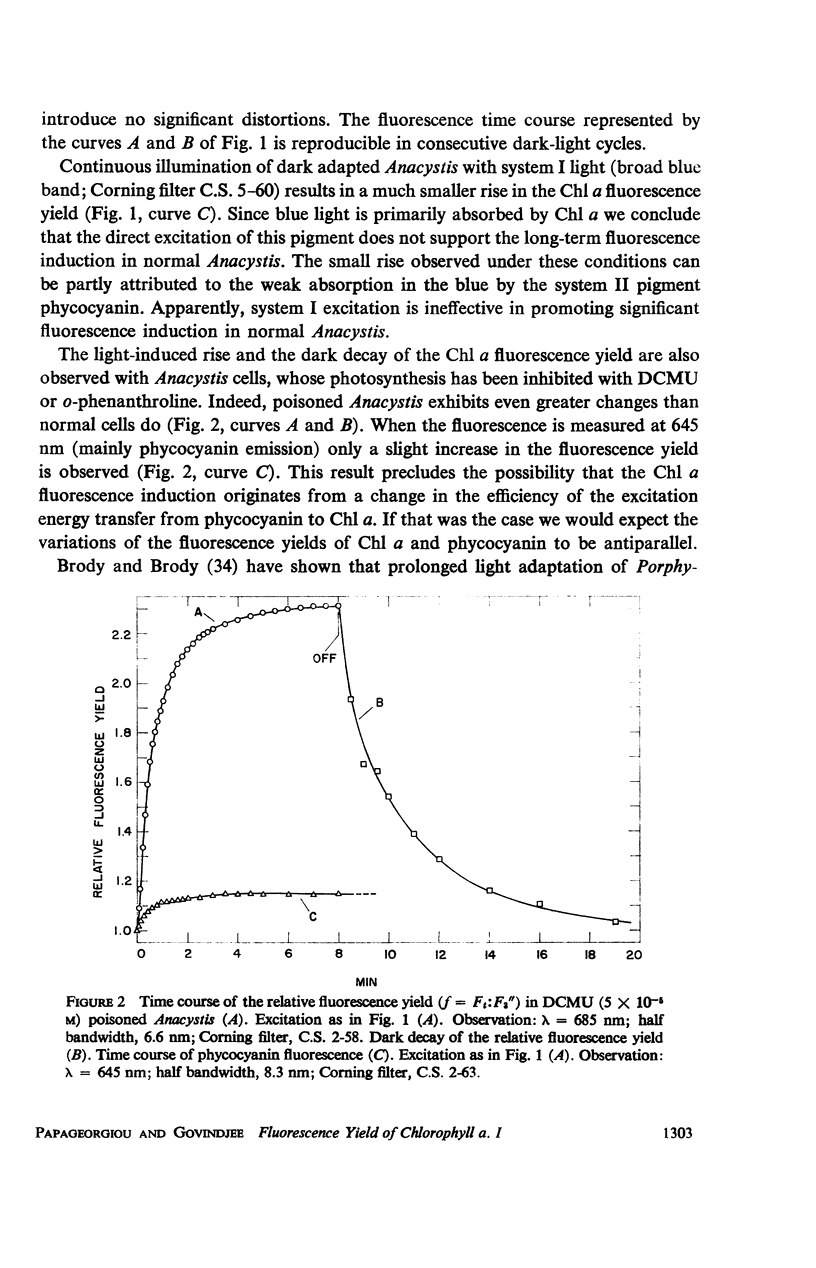
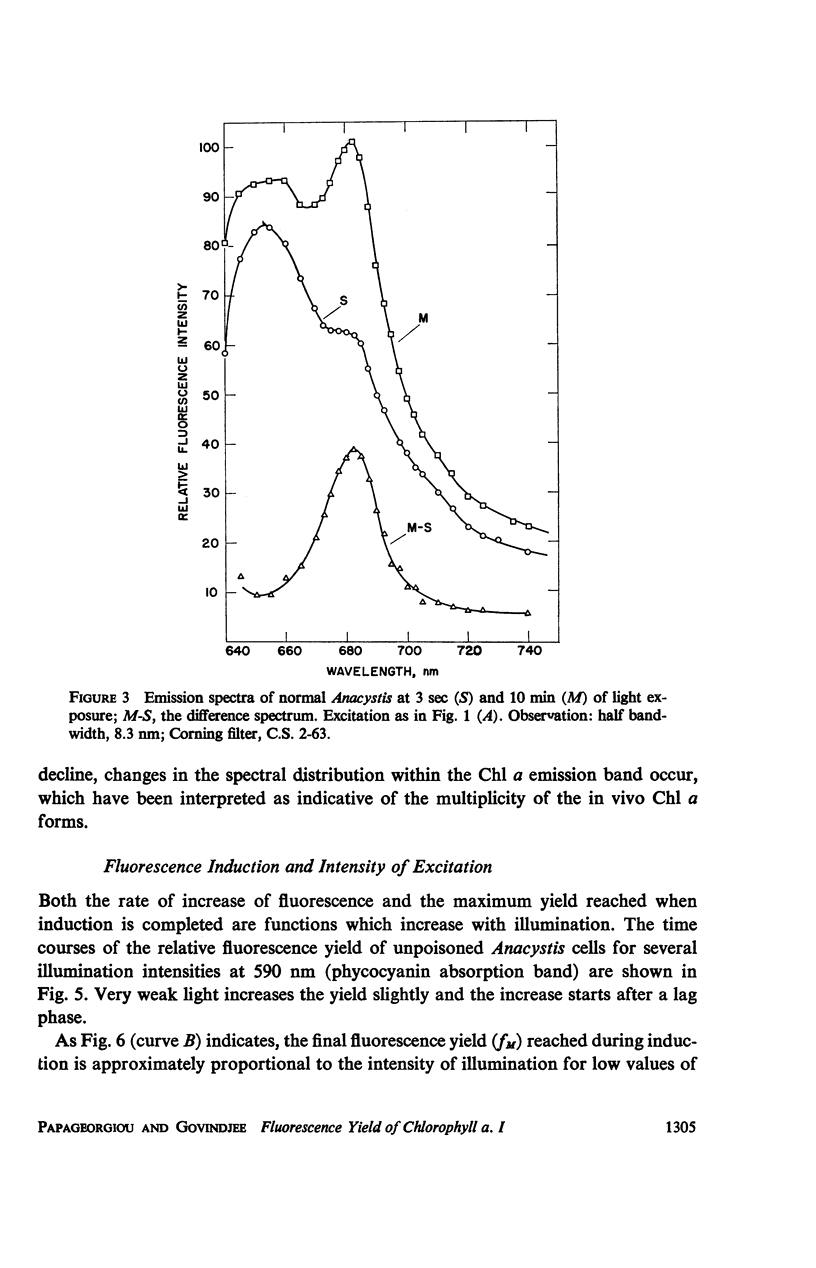
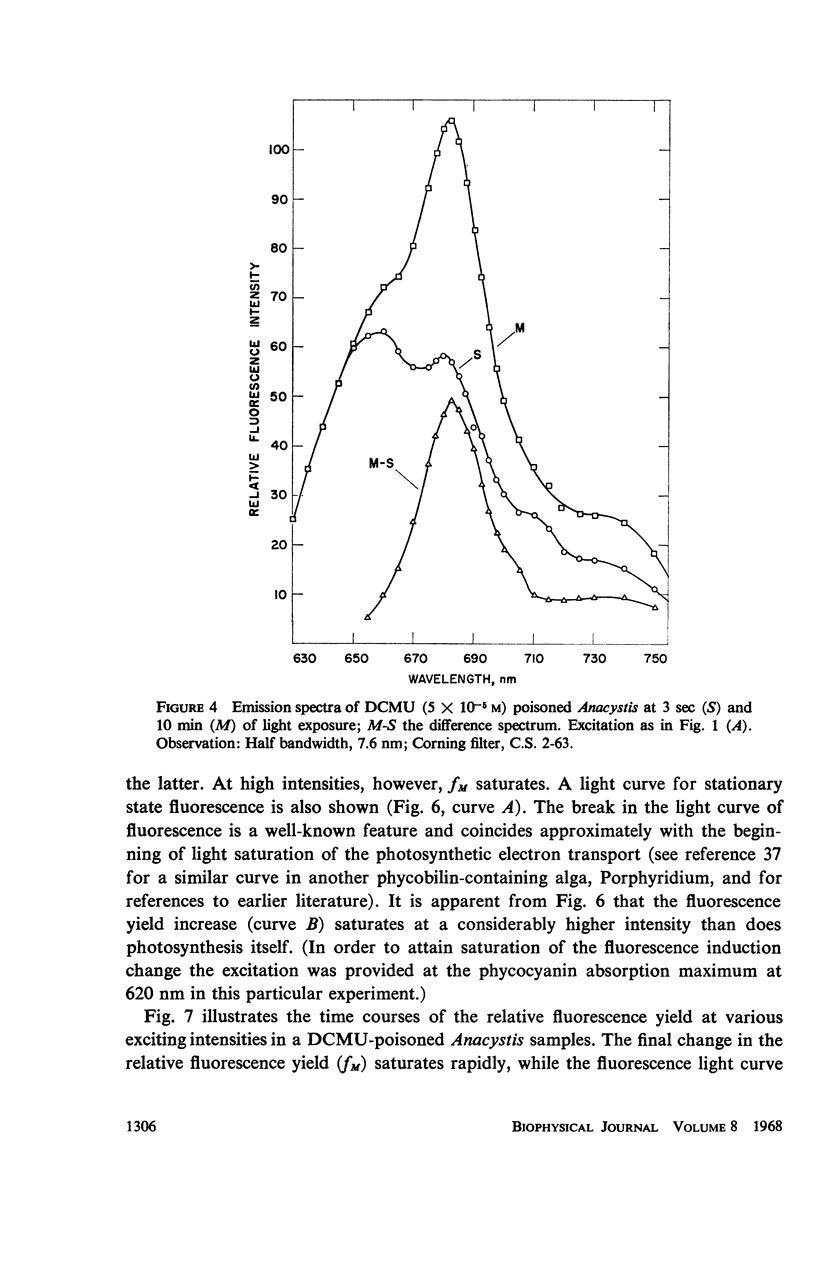
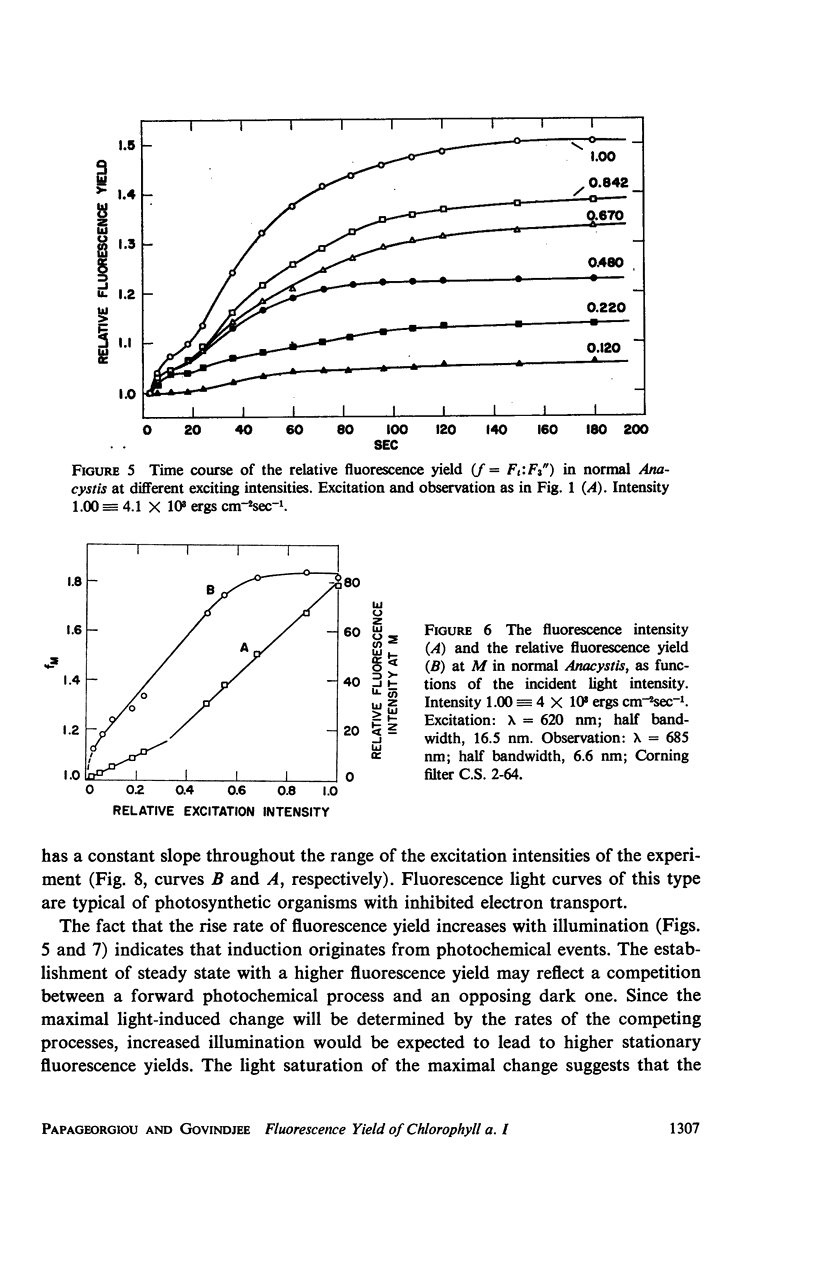
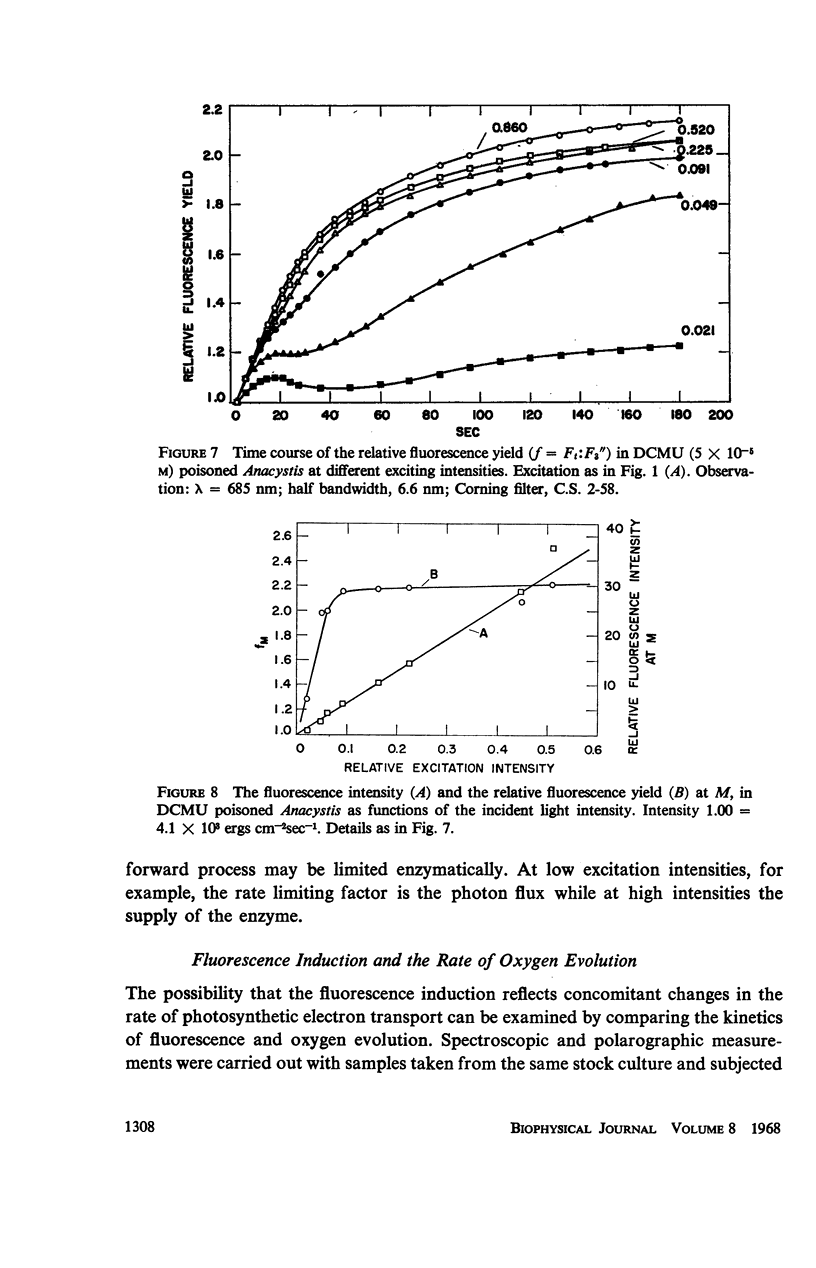
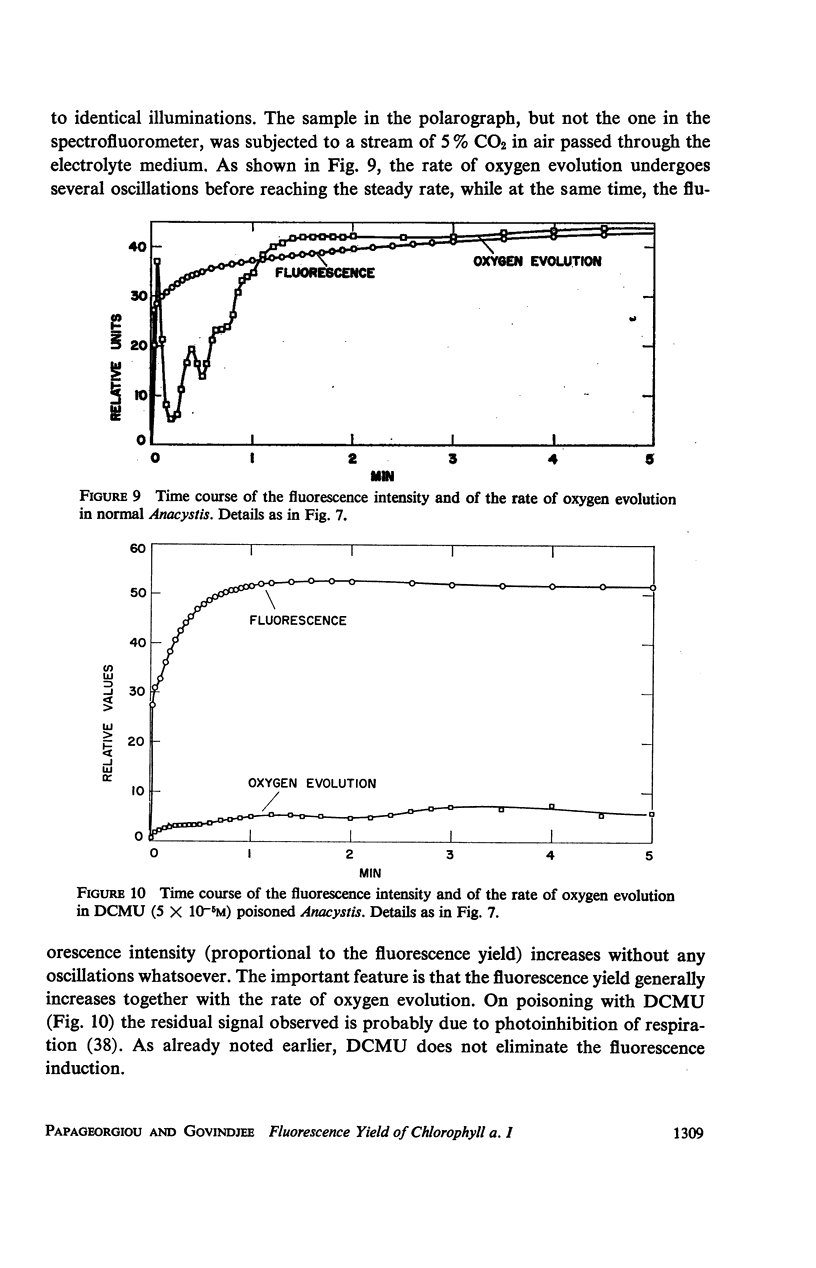
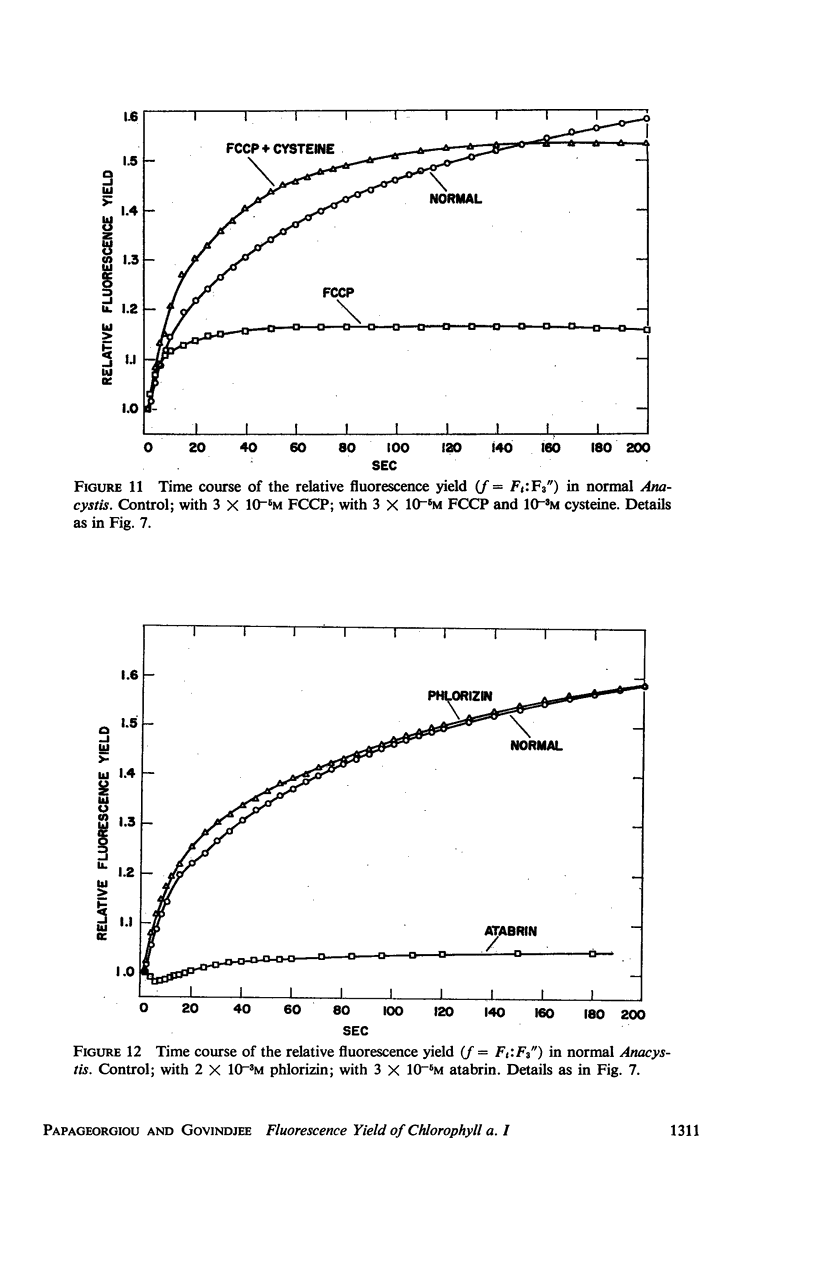
he fluorescence yield of chlorophyll a in dark adapted Anacystis nidulans undergoes a slow change with continuous illumination. After the completion of the initial fast transient, the fluorescence yield rises from the level S to a plateau M within a minute, declining only after prolonged illumination. Both normal and 1,1-dimethyl-3(3′4′-dichloro)-phenylurea (DCMU)-poisoned Anacystis are capable of these changes. In normal Anacystis, the slow increase in the fluorescence yield (S → M) requires light absorbed in system II while light absorbed in system I is ineffective. In DCMU-poisoned Anacystis, however, these changes are also promoted by light absorbed in system I. Addition of carbonyl cyanide p-trifluoromethoxy phenylhydrazone (FCCP), a photophosphorylation uncoupler acting near the photosynthetic electron transport chain, abolishes the rise from S to M in normal but has no effect in the DCMU-poisoned system. Phlorizin, a phosphorylase inhibitor, has very little effect. These results suggest that the light-induced variation in the fluorescence yield is related to the conformational changes which accompany photophosphorylation. The fluorescence yield of the auxiliary pigment phycocyanin remains constant throughout the interval of the light-induced changes in the fluorescence yield of chlorophyll a. Consequently, the fluorescence spectrum of the alga is variable on continuous illumination.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRODY S. S., BRODY M. Induced changes in the efficiency of energy transfer in Porphyridum cruentum. I. Arch Biochem Biophys. 1959 May;82(1):161–178. doi: 10.1016/0003-9861(59)90101-8. [DOI] [PubMed] [Google Scholar]
- Bannister T. T., Vrooman M. J. Enhancement of the Photosynthesis of Chlorella pyrenoidosa as a Function of Far-Red and Short-Wave Illuminations. Plant Physiol. 1964 Jul;39(4):622–629. doi: 10.1104/pp.39.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DILLEY R. A., VERNON L. P. CHANGES IN LIGHT-ABSORPTION AND LIGHT-SCATTERING PROPERTIES OF SPINACH CHLOROPLASTS UPON ILLUMINATION: RELATIONSHIP TO PHOTOPHOSPHORYLATION. Biochemistry. 1964 Jun;3:817–824. doi: 10.1021/bi00894a016. [DOI] [PubMed] [Google Scholar]
- De Kiewiet D. Y., Hall D. O., Jenner E. L. Effect of carbonylcyanide m-chlorophenylhydrazone on the photochemical reactions of isolated chloroplasts. Biochim Biophys Acta. 1965 Sep 27;109(1):284–292. doi: 10.1016/0926-6585(65)90112-3. [DOI] [PubMed] [Google Scholar]
- Delosme R. Etude de l'induction de fluorescence des algues vertes et des chloroplastes au début d'une illumination intense. Biochim Biophys Acta. 1967 Jul 5;143(1):108–128. doi: 10.1016/0005-2728(67)90115-6. [DOI] [PubMed] [Google Scholar]
- Dilley R. A., Vernon L. P. Ion and water transport processes related to the light-dependent shrinkage of spinach chloroplasts. Arch Biochem Biophys. 1965 Aug;111(2):365–375. doi: 10.1016/0003-9861(65)90198-0. [DOI] [PubMed] [Google Scholar]
- FRENCH C. S., YOUNG V. K. The fluorescence spectra of red algae and the transfer of energy from phycoerythrin to phycocyanin and chlorophyll. J Gen Physiol. 1952 Jul;35(6):873–890. doi: 10.1085/jgp.35.6.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K., Govindjee Transfer of the excitation energy in Anacystis nidulans grown to obtain different pigment ratios. Biophys J. 1966 Sep;6(5):611–619. doi: 10.1016/S0006-3495(66)86681-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEYTLER P. G. uncoupling of oxidative phosphorylation by carbonyl cyanide phenylhydrazones. I. Some characteristics of m-Cl-CCP action on mitochondria and chloroplasts. Biochemistry. 1963 Mar-Apr;2:357–361. doi: 10.1021/bi00902a031. [DOI] [PubMed] [Google Scholar]
- HIND G., JAGENDORF A. T. EFFECT OF UNCOUPLERS ON THE CONFORMATIONAL AND HIGH ENERGY STATES OF CHLOROPLASTS. J Biol Chem. 1965 Jul;240:3202–3209. [PubMed] [Google Scholar]
- Hind G., Jagendorf A. T. SEPARATION OF LIGHT AND DARK STAGES IN PHOTOPHOSPHORYLATION. Proc Natl Acad Sci U S A. 1963 May;49(5):715–722. doi: 10.1073/pnas.49.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa S., Connolly T. N., Winget G. D., Good N. E. Inhibition and uncoupling of photophosphorylation in chloroplasts. Brookhaven Symp Biol. 1966;19:169–187. [PubMed] [Google Scholar]
- Izawa S. The swelling and shrinking of chloroplasts during electron transport in the presence of phosphorylation uncouplers. Biochim Biophys Acta. 1965 Jul 22;102(2):373–378. doi: 10.1016/0926-6585(65)90127-5. [DOI] [PubMed] [Google Scholar]
- Izawa S., Winget G. D., Good N. E. Phlorizin, a specific inhibitor of photophosphorylation and phosphorylation-coupled electron transport in chloroplasts. Biochem Biophys Res Commun. 1966 Jan 24;22(2):223–226. doi: 10.1016/0006-291x(66)90436-0. [DOI] [PubMed] [Google Scholar]
- JAGENDORF A. T., NEUMANN J. EFFECT OF UNCOUPLERS ON THE LIGHT-INDUCED PH RISE WITH SPINACH CHLOROPLASTS. J Biol Chem. 1965 Jul;240:3210–3214. [PubMed] [Google Scholar]
- Joliot P. Etudes simultanées des cinétiques de fluorescence et d'émission d'oxygène photosynthétique. Biochim Biophys Acta. 1965 May 25;102(1):135–148. [PubMed] [Google Scholar]
- Joliot P. Oxygen evolution in algae illuminated by modulated light. Brookhaven Symp Biol. 1966;19:418–433. [PubMed] [Google Scholar]
- KAUTSKY H., APPEL W., AMANN H. [Chlorophyll fluorescence and carbon assimilation. Part XIII. The fluorescence and the photochemistry of plants]. Biochem Z. 1960;332:277–292. [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- NEUMANN J., JAGENDORF A. T. LIGHT-INDUCED PH CHANGES RELATED PHOSPHORYLATION BY CHLOROPLASTS. Arch Biochem Biophys. 1964 Jul;107:109–119. doi: 10.1016/0003-9861(64)90276-0. [DOI] [PubMed] [Google Scholar]
- PACKER L. STRUCTURAL CHANGES CORRELATED WITH PHOTOCHEMICAL PHOSPHORYLATION IN CHLOROPLAST MEMBRANES. Biochim Biophys Acta. 1963 Jul 23;75:12–22. doi: 10.1016/0006-3002(63)90574-2. [DOI] [PubMed] [Google Scholar]
- Papageorgiou G., Govindjee Changes in intensity and spectral distribution of fluorescence. Effect of light treatment on normal and DCMU-poisoned Anacystis nidulans. Biophys J. 1967 Jul;7(4):375–389. doi: 10.1016/S0006-3495(67)86595-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STREHLER B. L. Firefly luminescence in the study of energy transfer mechanisms. II. Adenosine triphosphate and photosynthesis. Arch Biochem Biophys. 1953 Mar;43(1):67–79. doi: 10.1016/0003-9861(53)90085-x. [DOI] [PubMed] [Google Scholar]


