Abstract
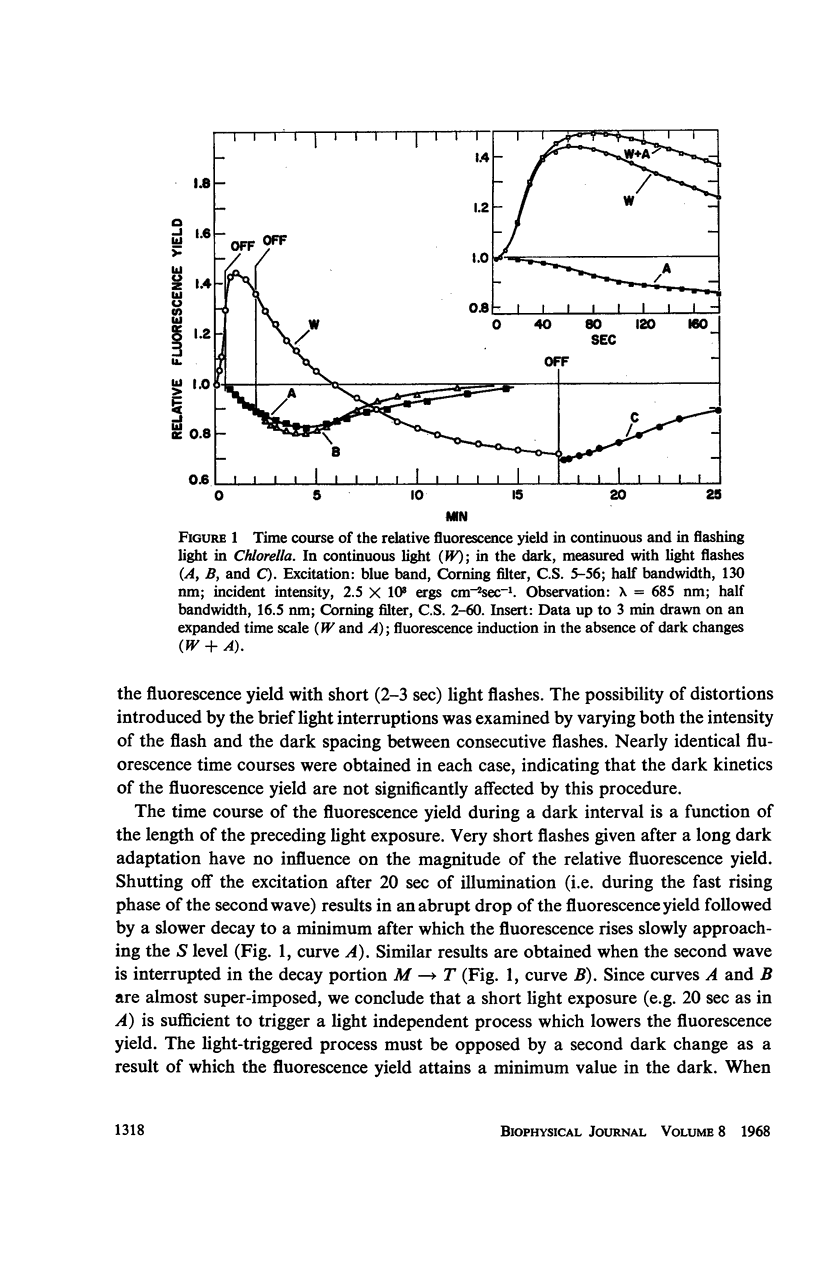
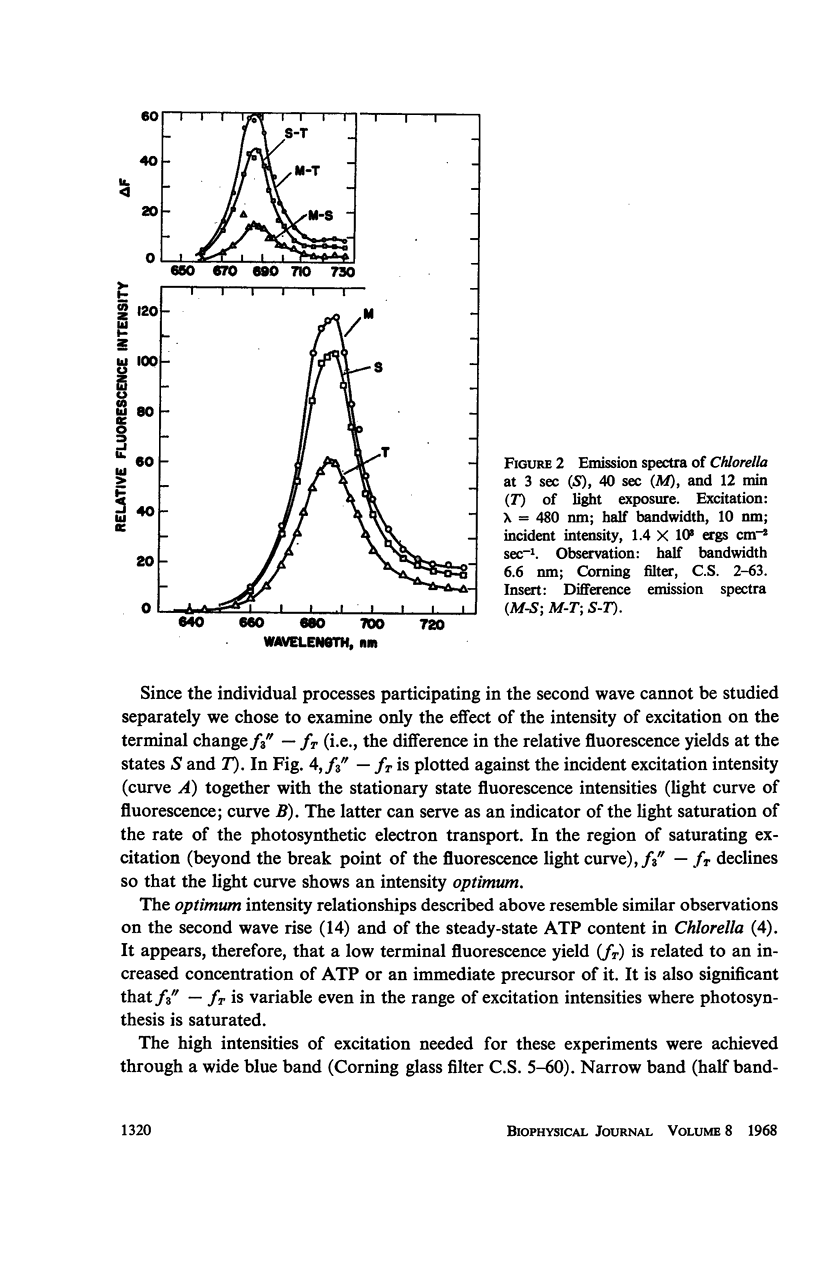
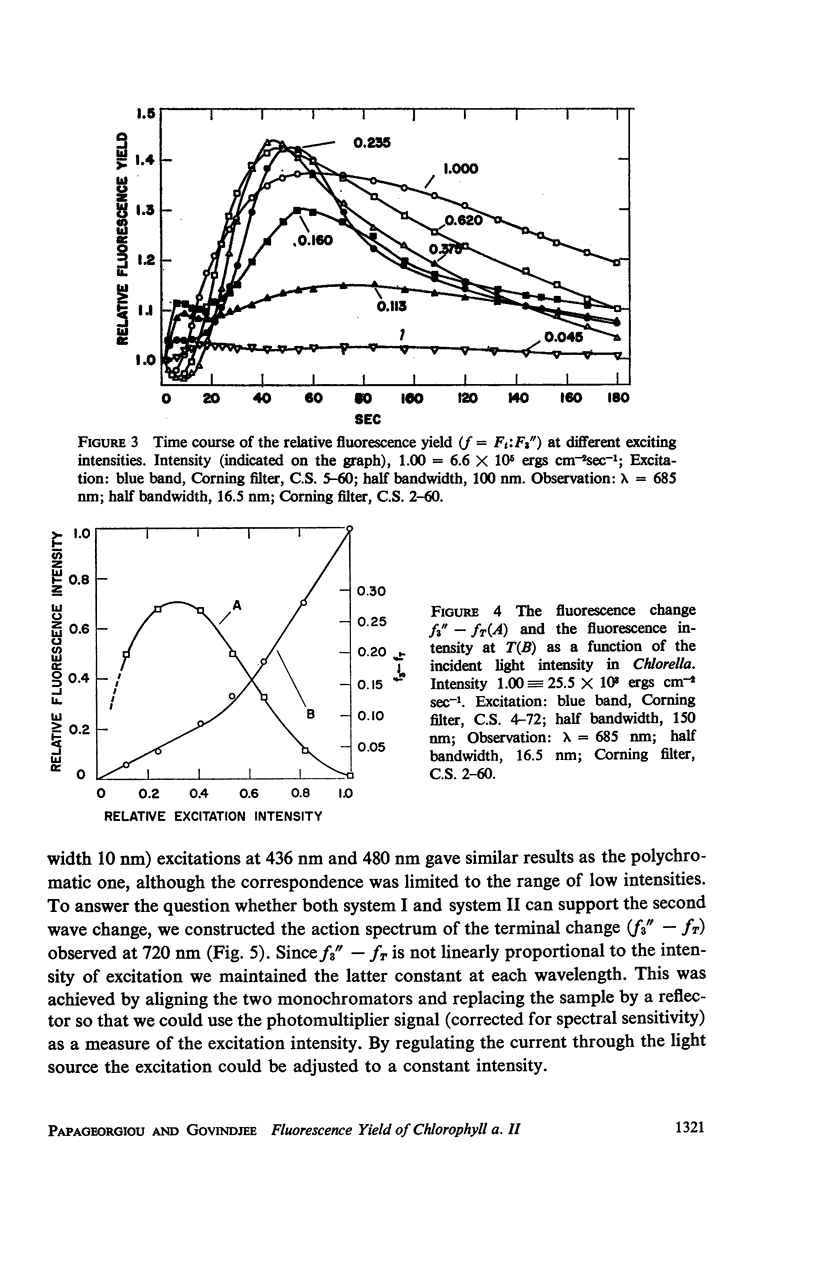
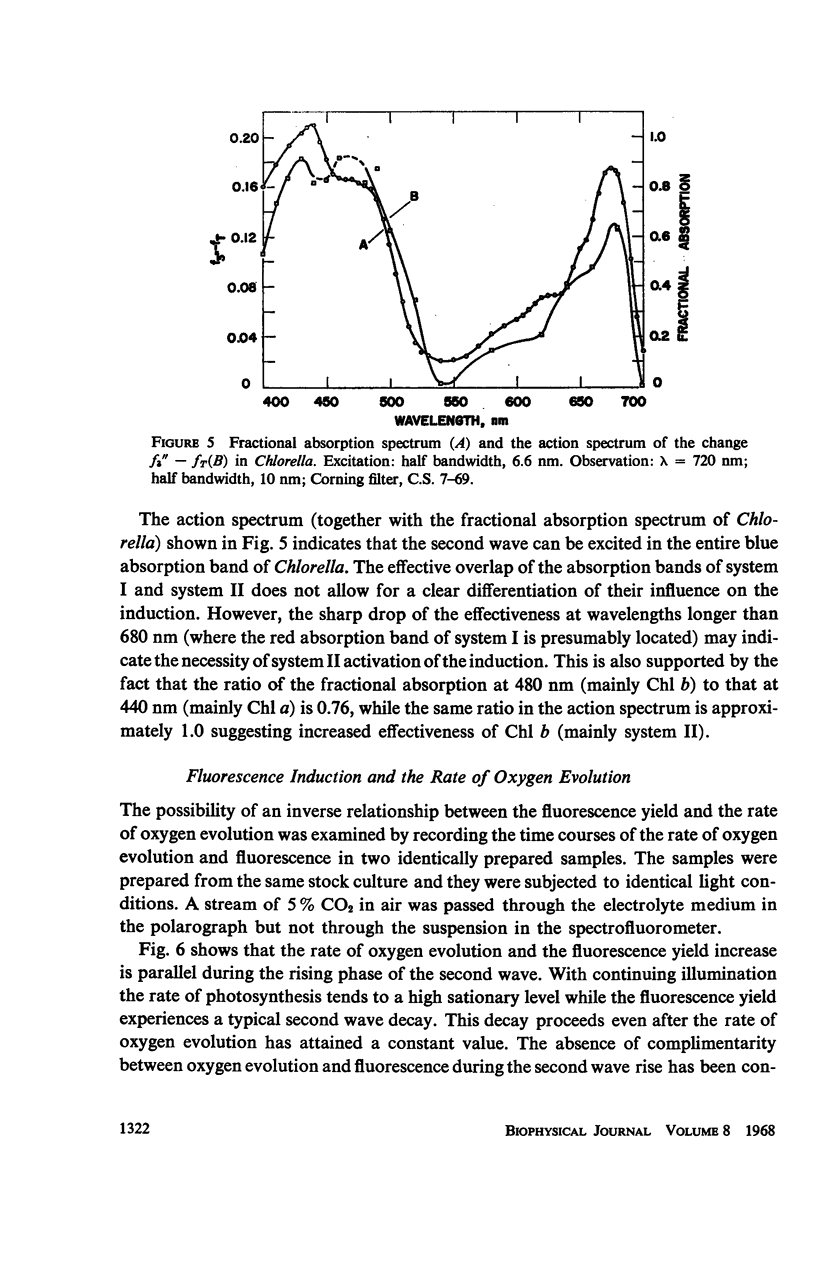
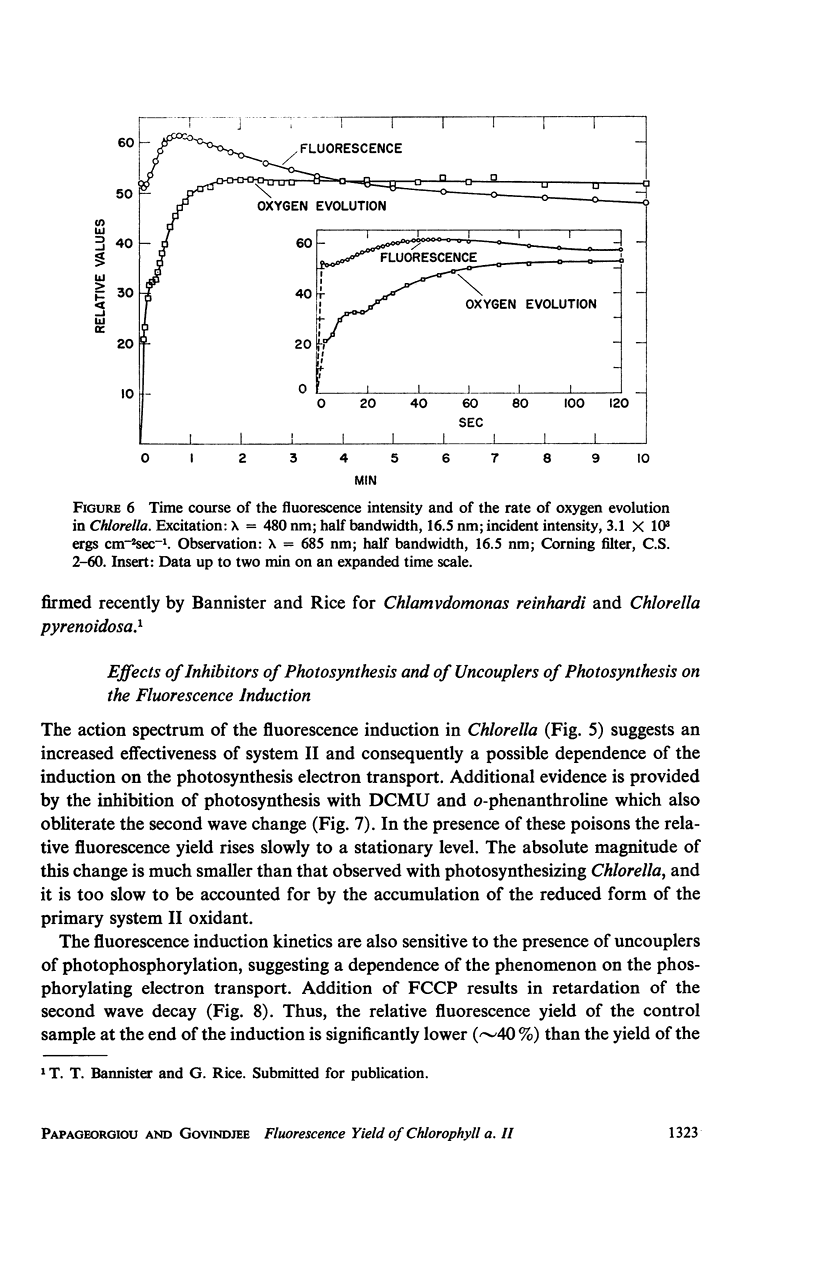
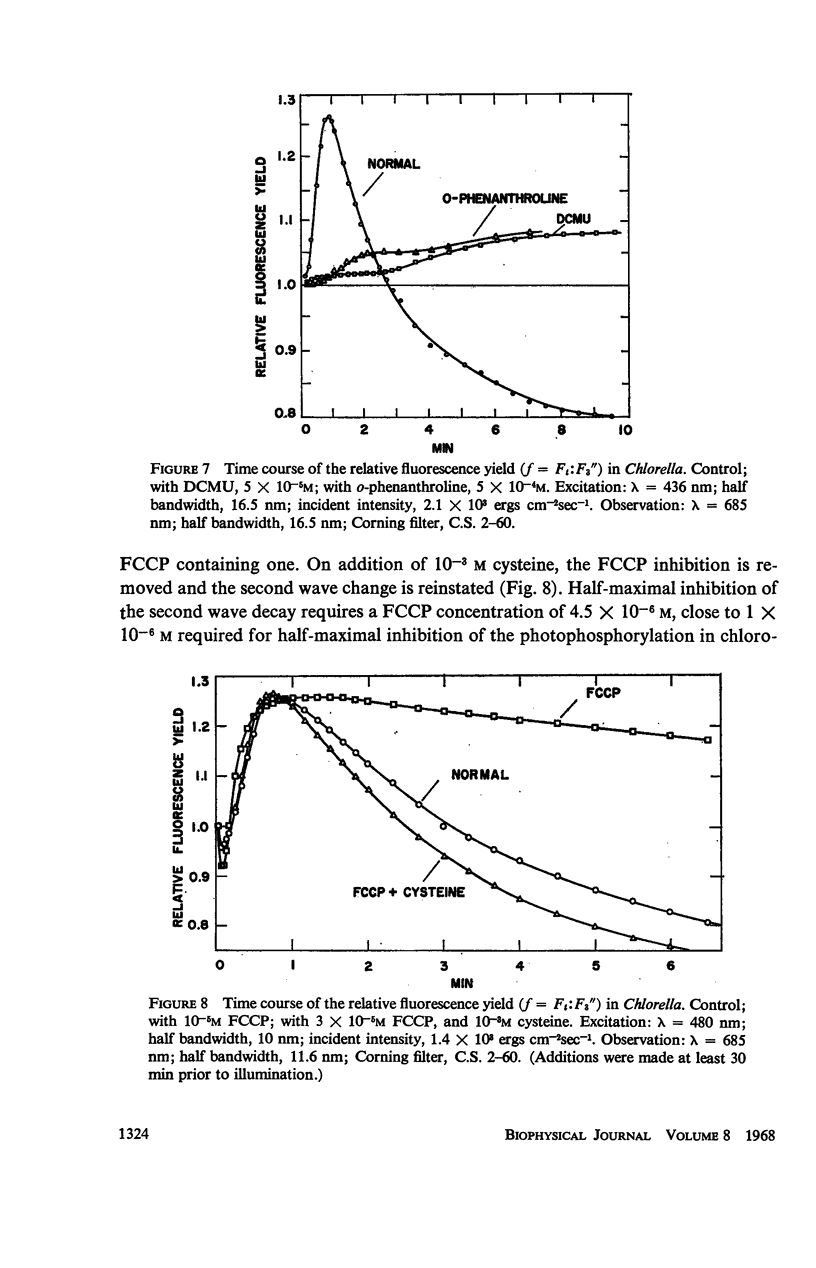
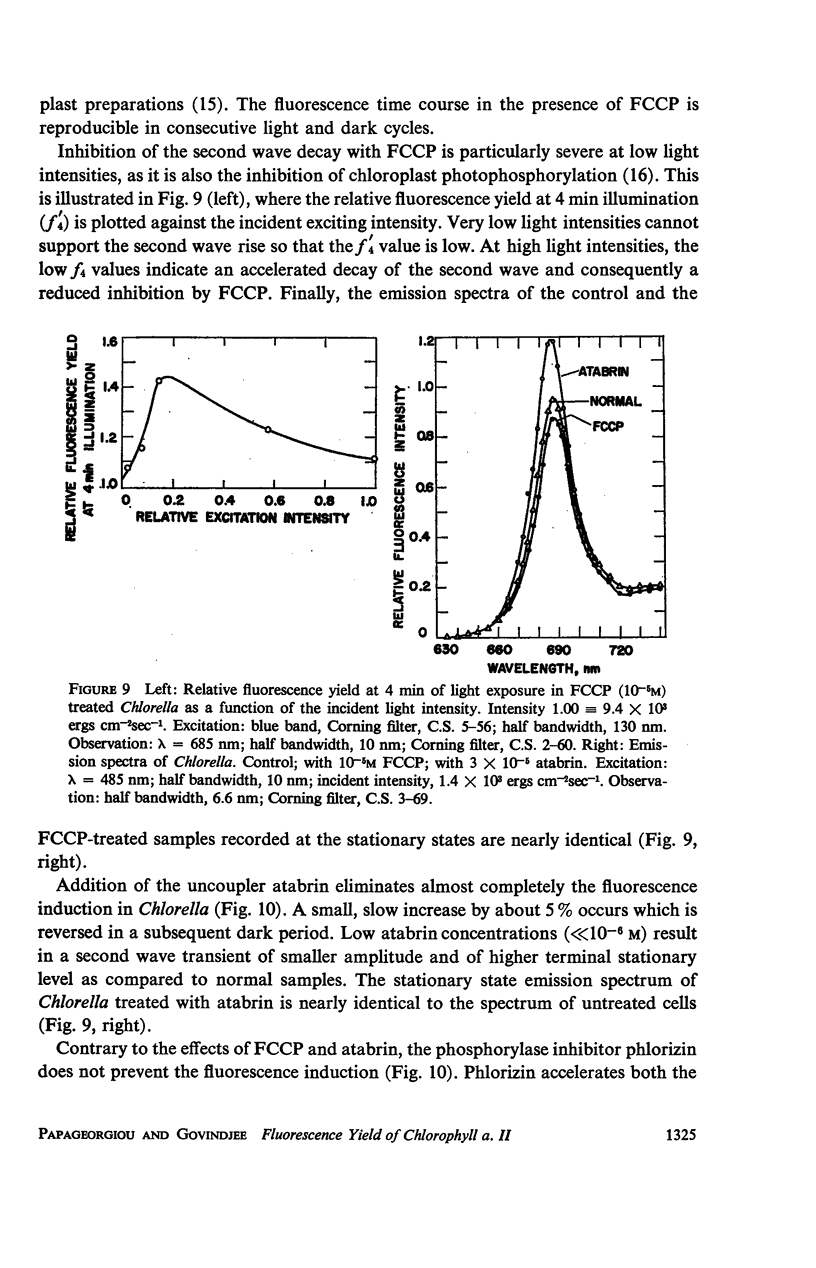
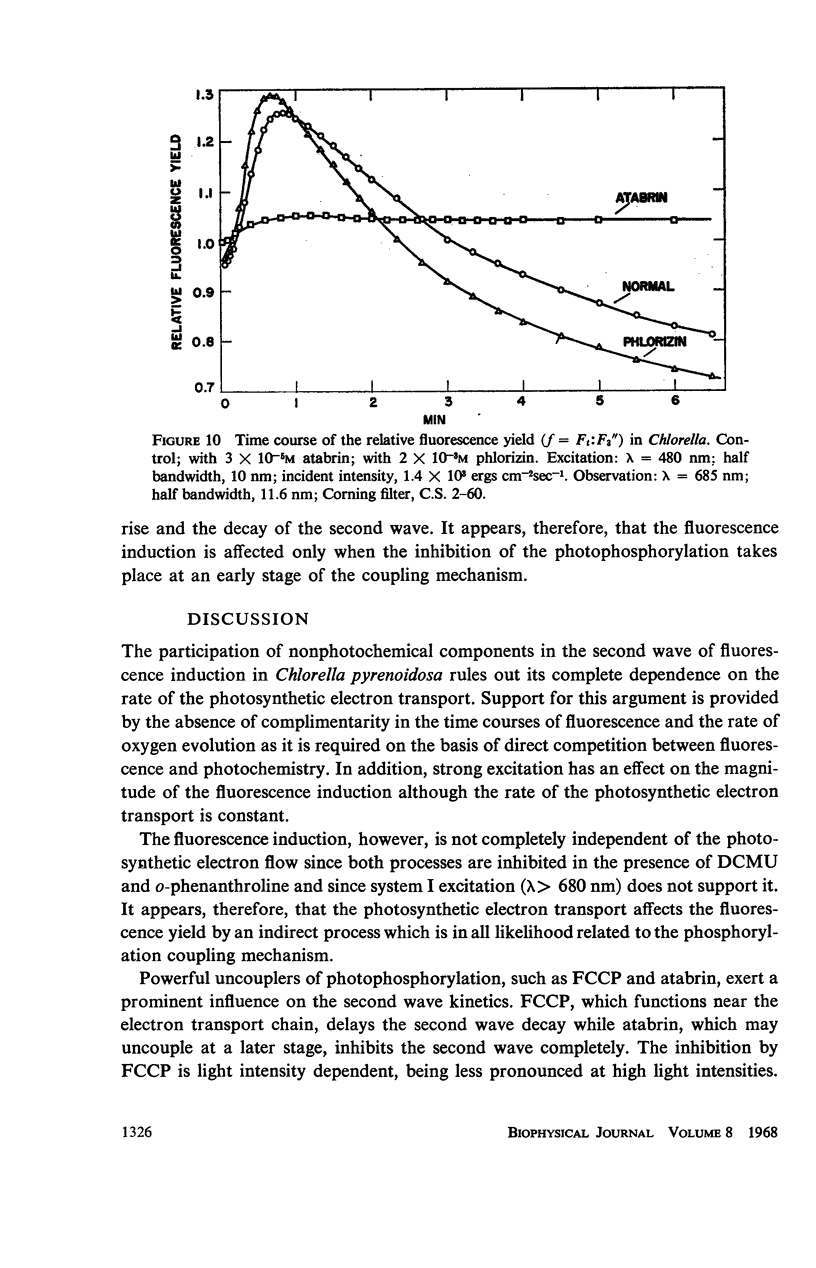
The long-term fluorescence induction in Chlorella pyrenoidosa consists of a fast rise of the fluorescence yield from the level S (of the first wave transient) to a maximum M, followed by slower decay to a terminal stationary level T. The maximum M is attained within 40 seconds from the onset of illumination while the decay to the terminal level T lasts for several minutes. The fluorescence rise (S → M) coincides with an increase in the rate of oxygen evolution, which, however, remains constant during the fluorescence decay (M → T). Poisons of photosynthesis 3, (3,4-dichlorophenyl)-1,1 dimethylurea (DCMU, o-phenathroline) inhibit the fluorescence induction, while uncouplers of photophosphorylation affect the fluorescence time course only when they function at an early stage of the coupling sequence e.g., carbonyl cyanide p-trifluoremethoxy phenylhydrazone, (FCCP, atabrin). Phosphorylation inhibitors affecting only the terminal esterification step (phlorizin) have little effect on the fluorescence kinetics. These results suggest that the fluorescence induction requires the operation of a phosphorylating electron transport and that it is possibly related to the light-induced structural changes which accompany photophosphorylation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avron M., Shavit N. Inhibitors and uncouplers of photophosphorylation. Biochim Biophys Acta. 1965 Nov 29;109(2):317–331. doi: 10.1016/0926-6585(65)90160-3. [DOI] [PubMed] [Google Scholar]
- HIND G., JAGENDORF A. T. LIGHT SCATTERING CHANGES ASSOCIATED WITH THE PRODUCTION OF A POSSIBLE INTERMEDIATE IN PHOTOPHOSPHORYLATION. J Biol Chem. 1965 Jul;240:3195–3201. [PubMed] [Google Scholar]
- Hind G., Jagendorf A. T. SEPARATION OF LIGHT AND DARK STAGES IN PHOTOPHOSPHORYLATION. Proc Natl Acad Sci U S A. 1963 May;49(5):715–722. doi: 10.1073/pnas.49.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAGENDORF A. T., NEUMANN J. EFFECT OF UNCOUPLERS ON THE LIGHT-INDUCED PH RISE WITH SPINACH CHLOROPLASTS. J Biol Chem. 1965 Jul;240:3210–3214. [PubMed] [Google Scholar]
- PACKER L. STRUCTURAL CHANGES CORRELATED WITH PHOTOCHEMICAL PHOSPHORYLATION IN CHLOROPLAST MEMBRANES. Biochim Biophys Acta. 1963 Jul 23;75:12–22. doi: 10.1016/0006-3002(63)90574-2. [DOI] [PubMed] [Google Scholar]
- Papageorgiou G., Govindjee Changes in intensity and spectral distribution of fluorescence. Effect of light treatment on normal and DCMU-poisoned Anacystis nidulans. Biophys J. 1967 Jul;7(4):375–389. doi: 10.1016/S0006-3495(67)86595-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STREHLER B. L. Firefly luminescence in the study of energy transfer mechanisms. II. Adenosine triphosphate and photosynthesis. Arch Biochem Biophys. 1953 Mar;43(1):67–79. doi: 10.1016/0003-9861(53)90085-x. [DOI] [PubMed] [Google Scholar]



