Abstract
Many viruses belonging to diverse viral families with differing structure and replication strategies induce apoptosis both in cultured cells in vitro and in tissues in vivo. Despite this fact, little is known about the specific cellular apoptotic pathways induced during viral infection. We have previously shown that reovirus-induced apoptosis of HEK cells is initiated by death receptor activation but requires augmentation by mitochondrial apoptotic pathways for its maximal expression. We now show that reovirus infection of HEK cells is associated with selective cytosolic release of the mitochondrial proapoptotic factors cytochrome c and Smac/DIABLO, but not the release of apoptosis-inducing factor. Release of these factors is not associated with loss of mitochondrial transmembrane potential and is blocked by overexpression of Bcl-2. Stable expression of caspase-9b, a dominant-negative form of caspase-9, blocks reovirus-induced caspase-9 activation but fails to significantly reduce activation of the key effector caspase, caspase-3. Smac/DIABLO enhances apoptosis through its action on cellular inhibitor of apoptosis proteins (IAPs). Reovirus infection is associated with selective down-regulation of cellular IAPs, including c-IAP1, XIAP, and survivin, effects that are blocked by Bcl-2 expression, establishing the dependence of IAP down-regulation on mitochondrial events. Taken together, these results are consistent with a model in which Smac/DIABLO-mediated inhibition of IAPs, rather than cytochrome c-mediated activation of caspase-9, is the key event responsible for mitochondrial augmentation of reovirus-induced apoptosis. These studies provide the first evidence for the association of Smac/DIABLO with virus-induced apoptosis.
The apoptotic pathways leading to cell death can be generally divided into two nonexclusive signaling cascades involving death receptors (extrinsic pathways) or mitochondria (intrinsic pathway) (2, 37). Induction of apoptosis through the death receptor pathway is initiated by the binding of ligand to the receptor, causing oligomerization of the receptor. This induces the formation of the death-induced signaling complex, leading to the activation of the initiator caspase, caspase-8 (2). Caspase-8 can then activate downstream effector caspases. Apoptosis via the mitochondrial pathway involves specific signals that allow the release of proapoptotic molecules from the inner-membrane space, including cytochrome c (28), second mitochondrion-derived activator of caspase (Smac/DIABLO) (11, 55), apoptosis-inducing factor (AIF) (50), and endonuclease G (26). Cytosolic cytochrome c, Apaf-1, and procaspase-9 form a complex termed the apoptosome (63). Formation of the apoptosome leads to the activation of caspase-9 and subsequent effector caspase activation. Relocalization of Smac/DIABLO to the cytoplasm promotes caspase activation through inhibition of the inhibitor of apoptosis (IAP) protein family (11, 55).
IAP proteins are negative regulators of apoptosis that inhibit caspase activity. IAP family members are characterized by the presence of one or more baculoviral IAP repeat domains (9). Six human IAP family members have been identified: NAIP, c-IAP1, c-IAP2, XIAP, survivin, and BRUCE (1, 12, 15, 27, 39). Four of these proteins—c-IAP1, c-IAP2, XIAP, and survivin—have been shown to directly interact with caspase-3, caspase-7, and caspase-9 and, at least in the cases of c-IAP1, c-IAP2, and XIAP, inhibit caspase activity (10, 41, 51). Additionally, reduction of c-IAP2 and XIAP protein levels is associated with apoptosis (25). The proapoptotic mitochondrial protein Smac/DIABLO has been shown to directly interact with XIAP eliminating XIAP's ability to bind to and inhibit caspases and thereby promoting apoptosis (14).
The Bcl-2 protein family is of central importance in the regulation of the mitochondrial apoptotic pathway. Members of this family may be either antiapoptotic (e.g., Bcl-2 and Bcl-xl) or proapoptotic (e.g., Bid, Bax, and Bak). Bcl-2 appears to play a role in the maintenance of mitochondrial integrity and inhibits mitochondrial release of proapoptotic factors. Conversely, Bid, Bax, and Bak appear to facilitate the release of these factors (22).
Activation of the death receptor and mitochondrion-associated death pathways are not mutually exclusive, and these pathways may interact (cross talk) at many levels. One important link between these two pathways appears to involve the caspase-8-dependent cleavage of Bid. Truncated Bid translocates to the mitochondrion, where it facilitates release of mitochondrial proteins, presumably by inducing the homo-oligomerization of Bax or Bak (58). This process may result in alteration of the mitochondrial permeability transition pore and loss of mitochondrial membrane potential (ΔΨm). However, recent evidence has shown that Bid-dependent release of mitochondrial proteins can occur without perturbing mitochondrial structure and function (20, 57).
Mammalian reoviruses are nonenveloped double-stranded RNA viruses that replicate exclusively in the cytoplasm. Reoviruses have been shown to induce apoptosis both in cultured cells in vitro (33, 38, 54) and in specific tissues, including the heart and brain, in vivo (6, 34). Apoptosis is an important mechanism of reovirus-induced tissue injury in vivo, and inhibition of apoptosis dramatically reduces the severity of disease (6).
Reovirus-induced apoptosis has been shown to involve death receptor 4 (DR4), death receptor 5 (DR5), and their cognate ligand, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Inhibition of the TRAIL/death receptor interaction with anti-TRAIL antibodies or soluble forms of DR4/DR5 inhibits cell death (5). Recently we have shown that reovirus-induced apoptosis also requires activation of the mitochondrial apoptotic pathway (21). In reovirus-infected HEK cells, there is a caspase-8-dependent cleavage of Bid leading to the subsequent release of cytochrome c and activation of caspase-9 and the effector caspase, caspase-3. Caspase-3 activation is inhibited in cells overexpressing a dominant-negative form of the adaptor protein FADD (FADD-DN) and in cells overexpressing Bcl-2, consistent with the importance of both extrinsic and intrinsic pathways in reovirus-induced apoptosis (21).
In this study we set out to better characterize the mitochondrion-dependent apoptotic processes induced following reovirus infection. Release of the mitochondrial proteins Smac/DIABLO and AIF were examined. Additionally, the fate of a number of IAP protein family members was determined. We find that Smac/DIABLO is released into the cytosol of infected HEK 293 cells, while AIF remained sequestered in the mitochondria. The release of cytochrome c and Smac/DIABLO occur without disturbing the mitochondrial membrane potential. Additionally, by utilizing a dominant-negative isoform of caspase-9, we find that while the mitochondrial pathway is required for caspase-3 activation, caspase-9 is dispensable for this process. Finally, we find that a specific subset of IAP proteins are down-regulated following reovirus infection. These results suggest that activation of Smac/DIABLO-dependent rather that caspase-9-dependent pathways represents the key mitochondrial event during reovirus-induced apoptosis and provide the first evidence for involvement of Smac/DIABLO in virus-induced apoptosis.
MATERIALS AND METHODS
Reagents.
Anti-cytochrome c (7H8.2C12) (1:1,000), anti-poly(ADP-ribose) polymerase (anti-PARP) (C2-10) (1:2,000), and anti-XIAP/hILP (1:500) antibodies were purchased from Pharmingen (San Diego, Calif.). Anti-caspase-9 (1:1,000) antibodies were purchased from Cell Signaling Technology (Beverly, Mass.). Anti-AIF, anti-c-IAP1, anti-c-IAP2, and antisurvivin antibodies were from Santa Cruz Biotechnology (Santa Cruz, Calif.). Antiactin antibodies (JLA20) (1:5,000) and anti-Smac/DIABLO (1:1,000) were from Calbiochem (Darmstadt, Germany). Anti-human cytochrome c oxidase (subunit II) antibodies (12C4-F12) (1:1,000) and the mitochondrial potential sensor JC-1 were from Molecular Probes (Eugene, Oreg.). Valinomycin was obtained from Sigma (St. Louis, Mo.). Anti-Fas antibody (CH-11) was from Upstate Biotechnology (Lake Placid, N.Y.). An ApoAlert caspase-3 fluorometric assay kit was purchased from Clontech (Palo Alto, Calif.).
Cells, virus, and DNA constructs.
HEK 293 cells (ATCC CRL1573) were grown in Dulbecco's modified Eagle's medium supplemented with of penicillin and streptomycin (100 U/ml each) and containing 10% fetal bovine serum. Jurkat cells were a gift of John Cohen and were grown in RPMI supplemented with penicillin and streptomycin (100 U/ml each) and containing 10% fetal bovine serum. HEK 293 cells stably overexpressing Bcl-2 were provided by Gary Johnson. The cell line was constructed by cloning full-length Bcl-2 into the pLXSN vector and transfecting cells via retroviral transduction. The dominant-negative caspase-9b construct was a gift of Emad Alnemri and has been previously described (47). The caspase-9b construct was transfected into HEK 293 cells using Lipofectamine (Gibco, Grand Island, N.Y.). Reovirus (type 3 Abney) is a laboratory stock, which has been plaque purified and passaged (twice) in L929 cells (ATCC CCL1) to generate working stocks (53). All experiments were performed using a multiplicity of infection of 100. High multiplicities of infection were chosen to ensure synchronized infection of all susceptible cells and to maximize the apoptotic stimulus.
Mitochondrial membrane potential measurement.
HEK 293 cells were seeded in six-well plates at 106 cells per well in a volume of 2 ml and then infected with reovirus for the indicated time periods. Control cells were treated with valinomycin at a final concentration of 100 nM for 10 min at 37°C. Cells were harvested and washed two times with phosphate-buffered saline (PBS). Cells were resuspended in 1 ml of PBS containing JC-1 (10 μg/ml) and incubated for 30 min at 37°C. Cells were washed two times in PBS and resuspended in 1 ml of PBS. Finally, cells were analyzed using a Coulter Epics XL flow cytometer (Beckman-Coulter, Hialeah, Fla.).
Western blot analysis.
Reovirus-infected cells were harvested at the indicated times, pelleted by centrifugation, washed with ice-cold phosphate-buffered saline, and lysed by sonication in 150 μl of lysis buffer (1% NP-40, 0.15 M NaCl, 5.0 mM EDTA, 0.01 M Tris [pH 8.0], 1.0 mM phenylmethylsulfonyl fluoride, leupeptin [0.02 mg/ml], trypsin inhibitor [0.02 mg/ml]). Lysates were cleared by centrifugation (20,000 × g, 2 min), mixed 1:1 with sodium dodecyl sulfate (SDS) sample buffer, boiled for 5 min, and stored at −70°C. Mitochondrion-free extracts were prepared as previously described (21) in buffer containing 220 mM mannitol, 68 mM sucrose, 50 mM PIPES-KOH (pH 7.4), 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, 1 mM dithiothreitol, and protease inhibitors (complete cocktail; Boehringer Mannheim, Indianapolis, Ind.). Proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Hybond-C Extra nitrocellulose membrane (Amersham, Little Chalfont, Buckinghamshire, England) for immunoblotting. Blots were then probed with the specified antibodies at the dilutions described above. Proteins were visualized using the ECL detection system (Amersham).
Caspase-3 activation assays.
Caspase-3 activation assays were performed using a kit obtained from Clontech. Experiments were performed using 106 cells/time point. Cells were centrifuged at 200 × g, supernatants were removed, and the cell pellets were frozen at −70°C until cells were collected at all the time points. Assays were performed as previously described (21) in 96-well plates and analyzed using a fluorescent plate reader (CytoFluor 4000; PerSeptive Biosystems, Framingham, Mass.). Results of all experiments are reported as means ± standard errors of the means.
RESULTS
Smac/DIABLO is released from the mitochondria following reovirus infection.
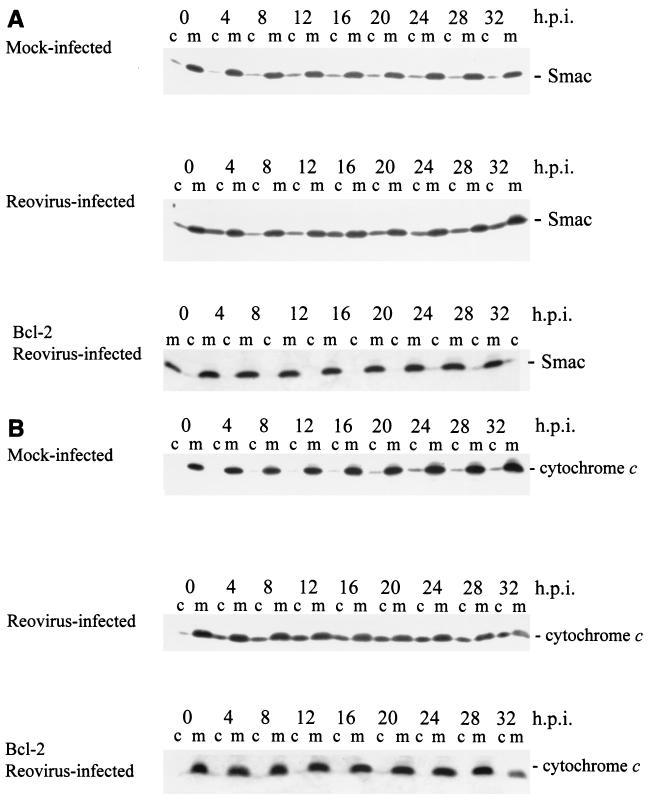
Several proteins possessing proapoptotic functions are localized to the mitochondria. These include cytochrome c (28), Smac/DIABLO (11, 55), AIF (50), and endonuclease G (26). Additionally, it has been reported that stores of the procaspase forms of several caspases are present in mitochondria (23, 29, 30, 36, 49). We have previously reported that cytochrome c is released from the mitochondria of reovirus-infected cells, leading to the activation of caspase-9 (21). We wished to determine whether additional mitochondrial molecules were also involved in reovirus-induced apoptosis. We began by examining the distribution of Smac/DIABLO following reovirus infection. Mitochondrion-free lysates were prepared from both mock- and reovirus-infected cells at the indicated time points and analyzed by Western blot for the presence of cytosolic Smac/DIABLO (Fig. 1A). Blots were also probed with anti-cytochrome c antibodies and the full time course of cytochrome c release is also shown (Fig. 1B). Antisera directed against the mitochondrial integral membrane protein cytochrome c oxidase (subunit II) were employed to ensure the samples were free of mitochondrial contamination (data not shown). Smac/DIABLO is released into the cytoplasm of reovirus-infected cells (Fig. 1A) and Smac/DIABLO and cytochrome c are detected in the cytoplasm of infected cells beginning at 4 h postinfection (Fig. 1). The Smac/DIABLO band detected at 0 h postinfection represents background levels as similar amounts of protein are also detected in mock-infected extracts (Fig. 1). Additionally, the release of both proteins is blocked in cells overexpressing Bcl-2 (Fig. 1). Interestingly, the release of Smac/DIABLO appears to exhibit a biphasic pattern which we have previously described for other events following reovirus infection, including caspase-8 activation, Bid cleavage, and caspase-3 activation (21). The early phase of Smac/DIABLO release was detectable at 4 h postinfection and then diminished. A second phase of Smac/DIABLO release was detectable at ∼16 h postinfection and was sustained over the remainder of the time course (Fig. 1A).
FIG. 1.
Smac/DIABLO and cytochrome c are present in the cytosol of reovirus-infected cells. HEK 293 lysates were prepared at the indicated time points from mock-infected cells, reovirus-infected cells, or Bcl-2-overexpressing reovirus-infected cells and resolved using SDS-PAGE. Western blot analysis was performed using anti-Smac antibodies (A) and anti-cytochrome c antibodies (B). The Western is representative of two separate experiments. Lanes c, cytoplasmic fraction; lanes m, mitochondrial fraction. h.p.i., hours postinfection.
AIF is not released from the mitochondria of reovirus-infected cells.
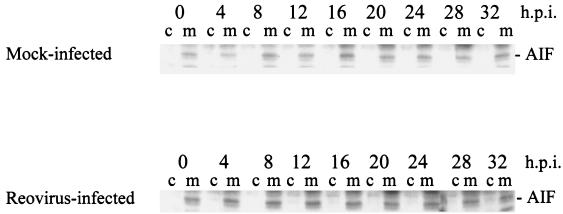
AIF is a mitochondrial protein that can translocate to the nucleus, leading to nuclear apoptosis (50). The relocalization of AIF to the nucleus induces a caspase-independent apoptotic pathway (19). It has been shown that AIF is released from the mitochondria of cells following human immunodeficiency virus (HIV) infection (13) and herpes simplex virus type 1 (HSV-1) infection (61). We examined AIF localization in reovirus-infected cells using mitochondrion-free lysates. As shown in Fig. 2, AIF is not detected in the cytoplasm following reovirus infection. We also prepared nuclear extracts from both mock- and reovirus-infected cells to ensure that AIF had not translocated the nucleus. We could not detect nuclearly localized AIF following reovirus infection (data not shown). These experiments demonstrate that the mitochondrial proapoptotic proteins Smac/DIABLO and cytochrome c, but not AIF, are released in reovirus-infected cells.
FIG. 2.
Reovirus infection does not induce the mitochondrial release of AIF. HEK 293 lysates were prepared at the indicated time points from mock-infected and reovirus-infected cells and resolved using SDS-PAGE. Blots were probed with anti-AIF antibodies and are representative of three separate experiments. Lanes c, cytoplasmic fraction; lanes m, mitochondrial fraction. h.p.i., hours postinfection.
Mitochondrial membrane potential is maintained following reovirus infection.
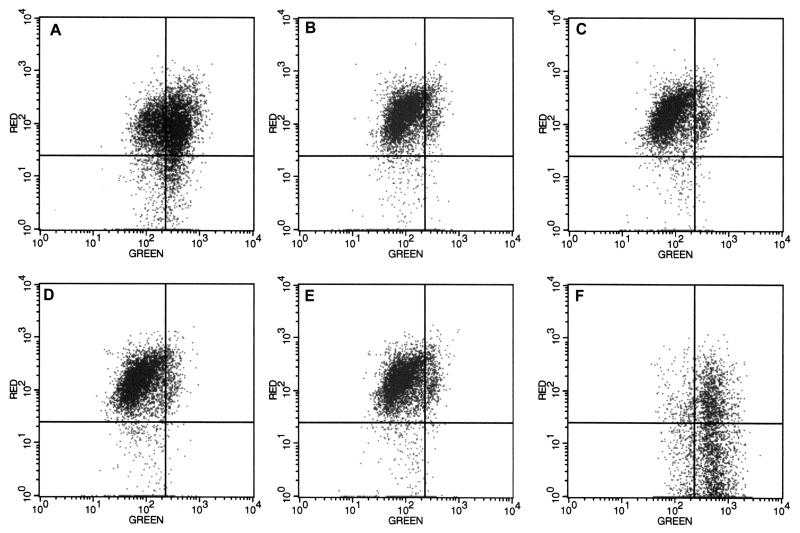
We have shown that reovirus infection results in the caspase-8-dependent cleavage of the proapoptotic protein Bid (21). It has been reported that Bid-dependent cytochrome c release can occur in the absence of mitochondrial membrane potential (ΔΨm) loss (20, 57). Previous studies suggested that reovirus infection did not alter ΔΨm in monkey kidney CV-1 cells (46). In light of our data indicating the release of both cytochrome c and Smac/DIABLO from the mitochondria of infected cells, we wanted to determine the state of the ΔΨm following infection of HEK 293 cells using the ΔΨm-sensitive dye JC-1. Using JC-1, high ΔΨm is indicated by red fluorescence and low ΔΨm is indicated by green fluorescence. As shown in Fig. 3, no ΔΨm reduction is detected over the reovirus infection time course (Fig. 3B to E) compared to mock-infected cells (Fig. 3A). As a control cells were treated with the apoptosis-inducing agent valinomycin, a K+ ionophore that induces loss of ΔΨm (Fig. 3F) to show the shift in red to green fluorescence that accompanies ΔΨm perturbation.
FIG. 3.
Mitochondrial ΔΨm is not altered in reovirus-infected cells. HEK 293 cells were mock-infected (A) or infected with reovirus for 0 h (B), 6 h (C), 12 h (D), or 24 h (E). Control cells were treated with valinomycin (panel F). Cells were incubated with the membrane potential-sensitive dye JC-1 and analyzed using flow cytometry. Red fluorescence indicates mitochondria with intact ΔΨm and green fluorescence indicates loss of ΔΨm. Results are representative of three separate experiments.
Caspase-9 activation is dispensable for effector caspase activation following reovirus infection.
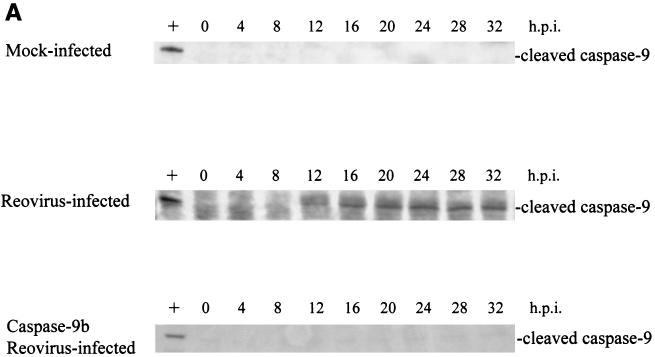
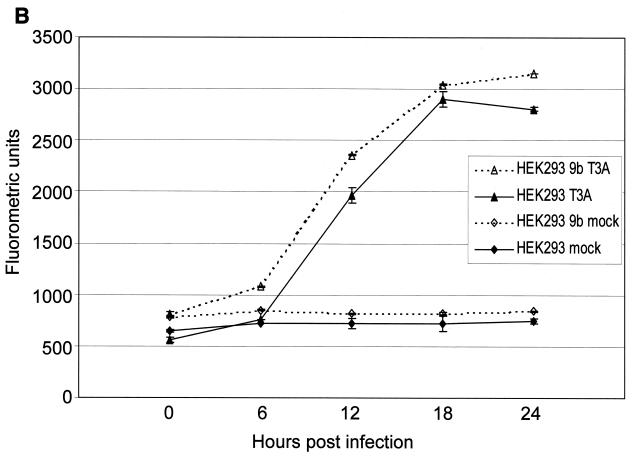
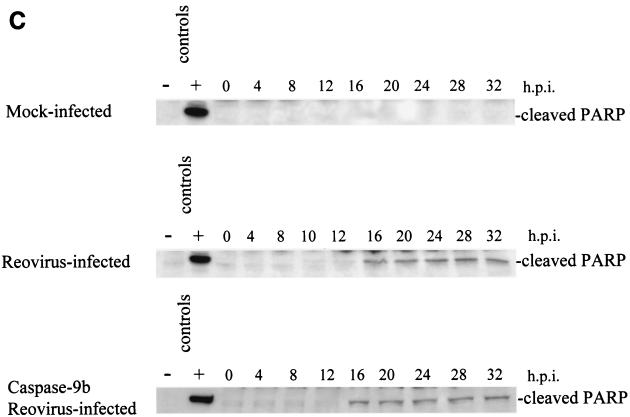
Having shown that both cytochrome c and Smac/DIABLO are released from the mitochondria of reovirus-infected cells, we wanted to determine the importance of the contribution of these proteins to reovirus-induced apoptosis. Cells were transfected with a dominant-negative form of caspase-9, caspase-9b (47). Unlike Bcl-2 overexpression, which blocks all mitochondrially mediated events, caspase-9b only interferes with the cytochrome c-dependent process of caspase-9 activation. Expression of the caspase-9b isoform was confirmed by Western blotting (data not shown). As shown in Fig. 4A, caspase-9b expression prevents the activation of endogenous caspase-9, as evidenced by the failure to detect the 37-kDa fragment of active caspase-9. We next infected cells expressing caspase-9b and utilized a caspase-3 fluorogenic substrate assay to measure the activity of caspase-3. We found that inhibition of caspase-9 activation did not prevent reovirus-induced activation of caspase-3 (Fig. 4B). This result was confirmed by examining the cleavage of the endogenous caspase-3 substrate PARP. As shown in Fig. 4C, the 85-kDa cleavage fragment of PARP is detected in both reovirus-infected cells and reovirus-infected, caspase-9b-expressing cells. These results in conjunction with our previous studies establishing the importance of the mitochondrial pathway in reovirus-induced apoptosis indicated that cytochrome c-dependent caspase-9 activation was not required for apoptosis following reovirus infection.
FIG. 4.
Inhibition of caspase-9 activation does not prevent effector caspase activation. Western blot analysis was performed using HEK 293 lysates harvested at the indicated time points from mock-infected and reovirus-infected cells and probed with anti-caspase-9 antibodies (A) or anti-PARP antibodies (C). Control lanes represent Jurkat cell lysates untreated (−) or treated (+) with activating anti-Fas antibody and harvested at 8 h posttreatment. Each Western blot is representative of two separate experiments. (B) Fluorogenic substrate assays were performed in triplicate. Error bars represent standard error of the mean. Fluorescence is expressed as arbitrary units. h.p.i., hours postinfection.
XIAP is cleaved in reovirus-infected cells.
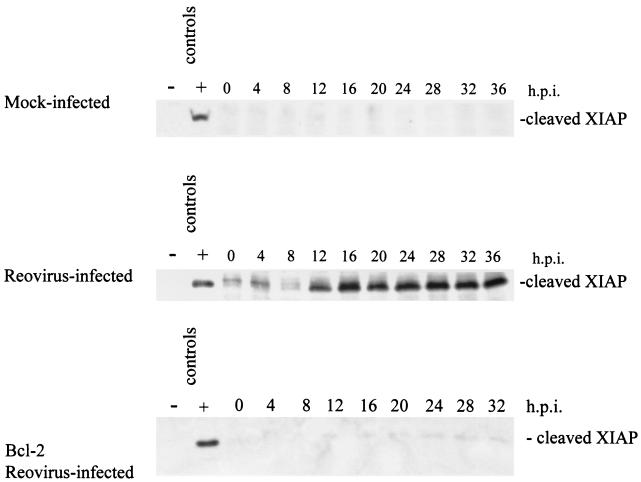
Having shown that Smac/DIABLO was released from mitochondria in reovirus-infected cells, we next wished to determine whether this was associated with alterations in cellular levels of IAPs. XIAP is a ubiquitously expressed protein (27) that is cleaved following induction of apoptosis (8, 18). Therefore, using Western blot analysis we looked for the presence of cleaved XIAP following reovirus infection. As shown in Fig. 5, the ∼30-kDa XIAP cleavage fragment is detected at 12 h postinfection and persists over the time course of the infection. This fragment is not detected in mock-infected or in reovirus-infected cells overexpressing Bcl-2 (Fig. 5), indicating that activation of the mitochondrial apoptotic pathway is required for XIAP cleavage.
FIG. 5.
Reovirus infection leads to the cleavage of XIAP. Western blot analysis was performed using HEK 293 lysates harvested at the indicated time points from mock-infected, reovirus-infected, and Bcl-2-overexpressing reovirus-infected cells and probed with anti-XIAP antibodies. Control lanes represent Jurkat cell lysates untreated (−) or treated (+) with activating anti-Fas antibody and harvested at 8 h posttreatment. The Western blot is representative of three separate experiments. h.p.i., hours postinfection.
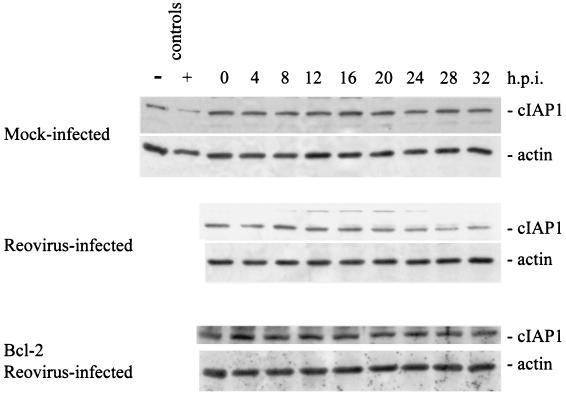
c-IAP1 and survivin are down-regulated in reovirus-infected cells.
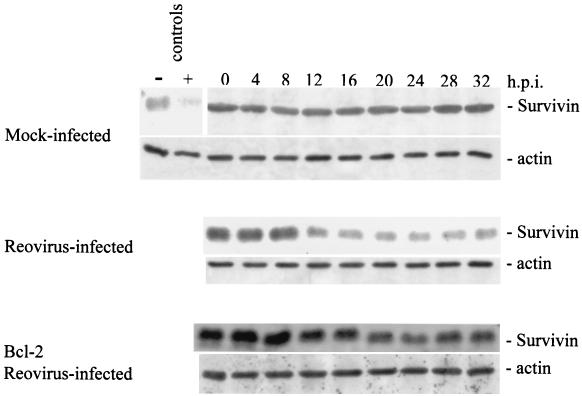
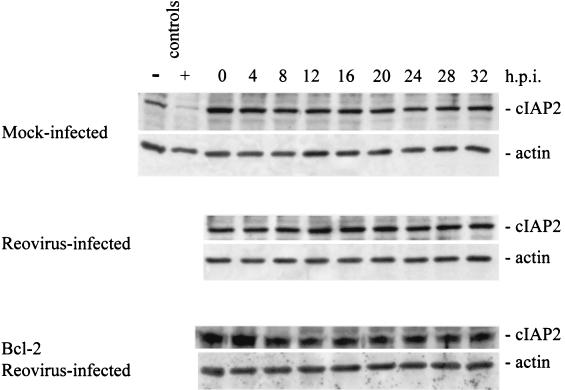
We next examined the cellular levels of several other IAP family members following reovirus infection. Survivin, like XIAP, has been shown to inhibit apoptosis. We used Western blot analysis to examine survivin protein levels following reovirus infection. Survivin protein levels were found to dramatically decrease in reovirus-infected cells beginning at ∼12 h postinfection (Fig. 6). This reduction was not seen in mock-infected cells and was strongly inhibited in reovirus-infected, Bcl-2-overexpressing cells (Fig. 6). We also examined levels of two other IAPs, c-IAP1 and c-IAP2. Both c-IAP1 and c-IAP2 have been shown to directly inhibit caspases (40). As shown in Fig. 7, c-IAP1 protein levels are reduced over the time course following reovirus infection, while c-IAP1 levels remain unchanged in both mock-infected and reovirus-infected cells overexpressing Bcl-2. The reduction of c-IAP1 protein levels appears to begin at ∼16 h postinfection (Fig. 7), which correlates well with the second phase of Smac/DIABLO release from the mitochondria (Fig. 1A). c-IAP2 levels remain relatively unchanged in both mock-infected and reovirus-infected cells (Fig. 8), indicating that c-IAP2 does not play a role in reovirus-induced apoptosis. These results indicate that reovirus infection is associated with selective reduction in cellular levels of specific IAPs.
FIG. 6.
Cellular levels of survivin are reduced following reovirus infection. Western blot analysis was performed using HEK 293 lysates harvested at the indicated time points from mock-infected, reovirus-infected, and Bcl-2-overexpressing reovirus-infected cells and probed with antisurvivin antibodies and antiactin to demonstrate equal protein loading. Control lanes represent Jurkat cell lysates untreated (−) or treated (+) with activating anti-Fas antibody and harvested at 8 h posttreatment. The Western blot is representative of two separate experiments. h.p.i., hours postinfection.
FIG. 7.
Reovirus infection induces a reduction in c-IAP1 protein level. Western blot analysis was performed using HEK 293 lysates harvested at the indicated time points from mock-infected, reovirus-infected, and Bcl-2-overexpressing reovirus-infected cells and probed with anti-c-IAP1 antibodies and antiactin to demonstrate equal protein loading. Control lanes represent Jurkat cell lysates untreated (−) or treated (+) with activating anti-Fas antibody and harvested at 8 h posttreatment. The Western blot is representative of two separate experiments. h.p.i., hours postinfection.
FIG. 8.
Cellular c-IAP2 protein levels are unaffected following reovirus infection. Western blot analysis was performed using HEK 293 lysates harvested at the indicated time points from mock-infected, reovirus-infected, and Bcl-2-overexpressing reovirus-infected cells and probed with anti-c-IAP2 antibodies and antiactin to demonstrate equal protein loading. Control lanes represent Jurkat cell lysates untreated (−) or treated (+) with activating anti-Fas antibody and harvested at 8 h posttreatment. The Western blot is representative of two separate experiments. h.p.i., hours postinfection.
DISCUSSION
Several viruses have been found to induce apoptosis in infected cells (40). Apoptosis also plays an important role in virus-induced tissue injury in vivo. However, the exact cellular pathways involved in virus-induced apoptosis are still incompletely understood. To this end a number of studies have been undertaken to elucidate the apoptotic pathways induced by viral infection both in vivo and in vitro.
Several studies have demonstrated that infection by a diverse group of viruses may induce apoptosis, at least in part, through a death-receptor-dependent mechanism. These include HIV (31, 59), measles virus (44, 56), influenza A virus (32), Sindbis virus (24), and hepatitis C virus (62) infections. The mitochondrion-dependent apoptotic pathway has also been shown to play a central role in virus-induced apoptosis. A number of viruses have been found to cause relocalization of proapoptotic mitochondrial proteins through both direct and indirect interactions. Among these are HIV (13, 16, 17), influenza A virus (4), HSV-1 (61), HSV-8 (45), hepatitis B virus (52), and West Nile virus (35). These data suggest that depending on the specific viruses and cells studied, both death receptor and mitochondrial pathways can contribute to virus-induced apoptosis.
There is considerable cross talk between death-receptor and mitochondrial apoptotic pathways. For example, death-receptor-dependent activation of caspase-8 leads to cleavage of Bid, resulting in the release of mitochondrial proapoptotic proteins, including cytochrome c and Smac/DIABLO, into the cytoplasm. Indeed, it has been suggested that cells can be grouped based upon the degree of mitochondrial involvement in death-receptor-mediated apoptosis (42, 43). The cytochrome c-dependent activation of caspase-9 has been thought to be of central importance in this process. However, several recent reports suggest that Smac/DIABLO release rather than caspase-9 activation may be the critical mitochondrial event in both TRAIL-induced (7, 60) and FasL-induced apoptosis (48).
Reovirus-induced apoptosis requires engagement of cellular receptors, including junction adhesion molecule and sialic acid residues (3), and has been shown to involve the DR4/DR5/TRAIL death receptor apoptotic pathway (5). We have shown recently that, in reovirus-infected HEK cells, although apoptosis is initiated by death receptor activation, its full expression requires the participation of mitochondrial apoptotic pathways (21). We have also previously shown that reovirus infection is associated with mitochondrial release of cytochrome c and subsequent activation of caspase-9 (21). Additionally, we demonstrated that the activation of caspase-8 and caspase-3 and the cleavage of Bid occur in a biphasic manner (21). We have postulated that the early phase of caspase activation and Bid truncation are necessary to activate the mitochondrial apoptotic pathway, which leads to the second, more intense phase of caspase activation and apoptosis (21). We now show that in addition to cytochrome c, Smac/DIABLO is also released from mitochondria of infected cells and that the release of Smac/DIABLO occurs in a biphasic manner. Another mitochondrial protein, AIF, remains sequestered, indicating that reovirus-induced release of mitochondrial proapoptotic proteins is a selective process and is not indiscriminately associated with the release of all mitochondrial proapoptotic factors. This selective release is consistent with previous results (46) and our present studies indicating that reovirus infection does not alter mitochondrial ΔΨm in infected cells and that mitochondrial ultrastructure is not significantly disrupted in the early stages of reovirus infection (K. L. Tyler, unpublished data).
We had previously demonstrated that overexpression of Bcl-2 was capable of preventing effector caspase activation following reovirus infection, indicating that mitochondrial apoptotic pathways were involved in reovirus-induced apoptosis in many cell types (21, 38). Having shown that reovirus infection induces the release of both Smac/DIABLO and cytochrome c, we next wanted to determine if we could elucidate the relative contribution of these proteins to reovirus-induced apoptosis. We utilized a dominant-negative caspase-9 construct (caspase-9b) to specifically inhibit endogenous caspase-9 activation following reovirus infection. We found that inhibition of caspase-9 activation did not significantly alter activation of the effector caspase, caspase-3. These data indicate that cytochrome c release and subsequent caspase-9 activation were not the critical mitochondrial event in reovirus-induced apoptosis.
Having shown that Smac/DIABLO was also released from mitochondria in reovirus-infected cells, we wished to determine whether this was associated with alterations of IAPs known to interact with Smac/DIABLO. We therefore examined the cellular levels of several IAP proteins following reovirus infection. We find that XIAP is cleaved following reovirus infection, beginning at ∼12 h postinfection. This cleavage is blocked in Bcl-2-overexpressing cells. Additionally, we show that survivin and c-IAP1 protein levels are reduced following reovirus infection, with the reduction first being appreciable at ∼12 h postinfection. Both of these events are inhibited in Bcl-2-overexpressing cells, indicating that they require activation of mitochondrial apoptotic pathways. Cellular levels of c-IAP2 were unaffected following reovirus infection, indicating that the effects of reovirus infection on IAPs are selective.
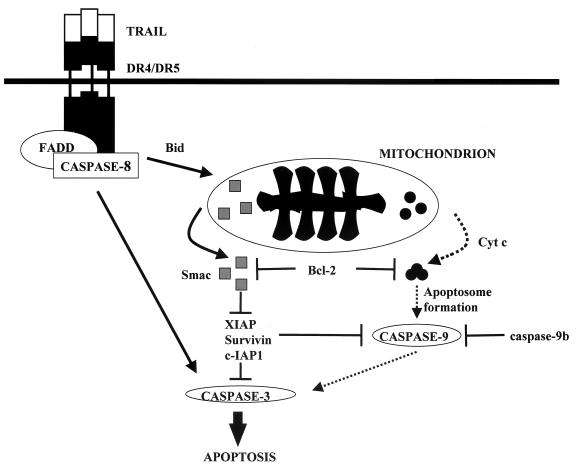
In this work we provide further evidence for the importance of the mitochondrial apoptotic pathway, and specifically for the release of Smac/DIABLO, in reovirus-induced apoptosis. Additionally, we elucidate the intracellular mechanisms of this process by showing that removal or down-regulation of certain IAP proteins appears to be an important part of cell death. Unfortunately, specific inhibitors of Smac/DIABLO release or function are not currently available, and therefore the necessity of Smac/DIABLO release cannot be directly assessed at this time. However, we believe that our data strongly implicate the release of Smac/DIABLO as the required mitochondrial event for reovirus-induced apoptosis. A possible model for the events involved in reovirus-induced apoptosis is shown in Fig. 9. Following caspase-8 activation, the proapoptotic protein Bid is cleaved. Truncated Bid either directly or indirectly induces the release of the mitochondrial proteins cytochrome c and Smac/DIABLO. Cytosolic cytochrome c induces the activation of caspase-9, and Smac/DIABLO interferes with the ability of XIAP, survivin, and c-IAP1 to prevent caspase activation. While caspase-9 may play a role in amplifying effector caspase activation, this appears to be unnecessary for reovirus-induced apoptosis. These studies provide the first direct evidence for the association of Smac/DIABLO with virus-induced apoptosis.
FIG. 9.
Model of cellular pathways involved in reovirus-induced apoptosis in HEK cells. Reovirus infection leads to the activation of caspase-8 and cleavage of the proapoptotic molecule Bid. These events promote the release of Smac/DIABLO and cytochrome c from the mitochondria of infected cells. The release of these proteins can be prevented by the overexpression of Bcl-2. Cytosolic Smac/DIABLO inhibits the antiapoptotic effect exerted by XIAP, survivin, and c-IAP1, leading to the sustained activation of both initiator caspases and the effector caspase, caspase-3. While caspase-9 is activated and undoubtedly participates in reovirus-induced apoptosis, its activation appears to be dispensable for this process.
Acknowledgments
This work was supported by a U.S. Army Medical Research and Material Command grant (DAMD179818614), merit and REAP grants from the Department of Veterans Affairs, and Public Health Service grant 1RO1AG14071 from the National Institute of Aging. K. L. Tyler is supported by the Reuler-Lewin Family Professorship of Neurology. D. J. Kominsky was supported by NIH training grant T32NS07321.
We acknowledge the UCHSC Cancer Center Media Core for supplying tissue culture medium.
REFERENCES
- 1.Ambrosini, G., C. Adida, and D. C. Altieri. 1997. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. 3:917-921. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 281:1305-1308. [DOI] [PubMed] [Google Scholar]
- 3.Barton, E. S., J. C. Forrest, J. L. Connolly, J. D. Chappell, Y. Liu, F. J. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441-451. [DOI] [PubMed] [Google Scholar]
- 4.Chen, W., P. A. Calvo, D. Malide, J. Gibbs, U. Schubert, I. Bacik, S. Basta, R. O'Neill, J. Schickli, P. Palese, P. Henklein, J. R. Bennink, and J. W. Yewdell. 2001. A novel influenza A virus mitochondrial protein that induces cell death. Nat. Med. 7:1306-1312. [DOI] [PubMed] [Google Scholar]
- 5.Clarke, P., S. M. Meintzer, S. Gibson, C. Widmann, T. P. Garrington, G. L. Johnson, and K. L. Tyler. 2000. Reovirus-induced apoptosis is mediated by TRAIL. J. Virol. 74:8135-8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeBiasi, R. L. C. L. Edelstein B. Sherry, and K. L. Tyler. 2001. Calpain inhibition protects against virus-induced apoptotic myocardial injury. J. Virol 75:351-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng, Y., Y. Lin, and X. Wu. 2002. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 16:33-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deveraux, Q. L., E. Leo, H. R. Stennicke, K. Welsh, G. S. Salvesen, and J. C. Reed. 1999. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J. 18:5242-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deveraux, Q. L., and J. C. Reed. 1999. IAP family proteins—suppressors of apoptosis. Genes Dev. 13:239-252. [DOI] [PubMed] [Google Scholar]
- 10.Deveraux, Q. L., R. Takahashi, G. S. Salvesen, and J. C. Reed. 1997. X-linked IAP is a direct inhibitor of cell-death proteases. Nature 388:300-304. [DOI] [PubMed] [Google Scholar]
- 11.Du, C., M. Fang, Y. Li, L. Li, and X. Wang. 2000. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102:33-42. [DOI] [PubMed] [Google Scholar]
- 12.Duckett, C. S., F. Li, Y. Wang, K. J. Tomaselli, C. B. Thompson, and R. C. Armstrong. 1998. Human IAP-like protein regulates programmed cell death downstream of Bcl-xL and cytochrome c. Mol. Cell. Biol. 18:608-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferri, K. F., E. Jacotot, J. Blanco, J. A. Este, N. Zamzami, S. A. Susin, Z. Xie, G. Brothers, J. C. Reed, J. M. Penninger, and G. Kroemer. 2000. Apoptosis control in syncytia induced by the HIV type 1-envelope glycoprotein complex: role of mitochondria and caspases. J. Exp. Med. 192:1081-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goyal, L. 2001. Cell death inhibition: keeping caspases in check. Cell 104:805-808. [DOI] [PubMed] [Google Scholar]
- 15.Hauser, H. P., M. Bardroff, G. Pyrowolakis, and S. Jentsch. 1998. A giant ubiquitin-conjugating enzyme related to IAP apoptosis inhibitors. J. Cell Biol. 141:1415-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacotot, E., K. F. Ferri, C. El Hamel, C. Brenner, S. Druillennec, J. Hoebeke, P. Rustin, D. Metivier, C. Lenoir, M. Geuskens, H. L. Vieira, M. Loeffler, A. S. Belzacq, J. P. Briand, N. Zamzami, L. Edelman, Z. H. Xie, J. C. Reed, B. P. Roques, and G. Kroemer. 2001. Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 viral protein rR and Bcl-2. J. Exp. Med. 193:509-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacotot, E., L. Ravagnan, M. Loeffler, K. F. Ferri, H. L. Vieira, N. Zamzami, P. Costantini, S. Druillennec, J. Hoebeke, J. P. Briand, T. Irinopoulou, E. Daugas, S. A. Susin, D. Cointe, Z. H. Xie, J. C. Reed, B. P. Roques, and G. Kroemer. 2000. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J. Exp. Med. 191:33-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, D. E., B. R. Gastman, E. Wieckowski, G. Q. Wang, A. Amoscato, S. M. Delach, and H. Rabinowich. 2000. Inhibitor of apoptosis protein hILP undergoes caspase-mediated cleavage during T lymphocyte apoptosis. Cancer Res. 60:1818-1823. [PubMed] [Google Scholar]
- 19.Joza, N., S. A. Susin, E. Daugas, W. L. Stanford, S. K. Cho, C. Y. Li, T. Sasaki, A. J. Elia, H. Y. Cheng, L. Ravagnan, K. F. Ferri, N. Zamzami, A. Wakeham, R. Hakem, H. Yoshida, Y. Y. Kong, T. W. Mak, J. C. Zuniga-Pflucker, G. Kroemer, and J. M. Penninger. 2001. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410:549-554. [DOI] [PubMed] [Google Scholar]
- 20.Kim, T. H., Y. Zhao, M. J. Barber, D. K. Kuharsky, and X. M. Yin. 2000. Bid-induced cytochrome c release is mediated by a pathway independent of mitochondrial permeability transition pore and Bax. J. Biol. Chem. 275:39474-39481. [DOI] [PubMed] [Google Scholar]
- 21.Kominsky, D. J., R. J. Bickel, and K. L. Tyler. 2002. Reovirus-induced apoptosis requires both death receptor- and mitochondrial-mediated caspase-dependent pathways of cell death. Cell Death Differ. 9:926-933. [DOI] [PubMed] [Google Scholar]
- 22.Korsmeyer, S. J., M. C. Wei, M. Saito, S. Weiler, K. J. Oh, and P. H. Schlesinger. 2000. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 7:1166-1173. [DOI] [PubMed] [Google Scholar]
- 23.Krajewski, S., M. Krajewska, L. M. Ellerby, K. Welsh, Z. Xie, Q. L. Deveraux, G. S. Salvesen, D. E. Bredesen, R. E. Rosenthal, G. Fiskum, and J. C. Reed. 1999. Release of caspase-9 from mitochondria during neuronal apoptosis and cerebral ischemia. Proc. Natl. Acad. Sci. USA 96:5752-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 25.Li, J., J. M. Kim, P. Liston, M. Li, T. Miyazaki, A. E. Mackenzie, R. G. Korneluk, and B. K. Tsang. 1998. Expression of inhibitor of apoptosis proteins (IAPs) in rat granulosa cells during ovarian follicular development and atresia. Endocrinology 139:1321-1328. [DOI] [PubMed] [Google Scholar]
- 26.Li, L. Y., X. Luo, and X. Wang. 2001. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 412:95-99. [DOI] [PubMed] [Google Scholar]
- 27.Liston, P., N. Roy, K. Tamai, C. Lefebvre, S. Baird, G. Cherton-Horvat, R. Farahani, M. McLean, J. E. Ikeda, A. MacKenzie, and R. G. Korneluk. 1996. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature 379:349-353. [DOI] [PubMed] [Google Scholar]
- 28.Liu, X., C. N. Kim, J. Yang, R. Jemmerson, and X. Wang. 1996. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147-157. [DOI] [PubMed] [Google Scholar]
- 29.Mancini, M., C. E. Machamer, S. Roy, D. W. Nicholson, N. A. Thornberry, L. A. Casciola-Rosen, and A. Rosen. 2000. Caspase-2 is localized at the Golgi complex and cleaves golgin-160 during apoptosis. J. Cell Biol. 149:603-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mancini, M., D. W. Nicholson, S. Roy, N. A. Thornberry, E. P. Peterson, L. A. Casciola-Rosen, and A. Rosen. 1998. The caspase-3 precursor has a cytosolic and mitochondrial distribution: implications for apoptotic signaling. J. Cell Biol. 140:1485-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miura, Y., N. Misawa, N. Maeda, Y. Inagaki, Y. Tanaka, M. Ito, N. Kayagaki, N. Yamamoto, H. Yagita, H. Mizusawa, and Y. Koyanagi. 2001. Critical contribution of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) to apoptosis of human CD4+ T cells in HIV-1-infected hu-PBL-NOD-SCID mice. J. Exp. Med. 193:651-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nichols, J. E., J. A. Niles, and N. J. Roberts, Jr. 2001. Human lymphocyte apoptosis after exposure to influenza A virus. J. Virol. 75:5921-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oberhaus, S. M., T. S. Dermody, and K. L. Tyler. 1998. Apoptosis and the cytopathic effects of reovirus. Curr. Top. Microbiol. Immunol. 233:23-49. [DOI] [PubMed] [Google Scholar]
- 34.Oberhaus, S. M., R. L. Smith, G. H. Clayton, T. S. Dermody, and K. L. Tyler. 1997. Reovirus infection and tissue injury in the mouse central nervous system are associated with apoptosis. J. Virol. 71:2100-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parquet, M. C., A. Kumatori, F. Hasebe, K. Morita, and A. Igarashi. 2001. West Nile virus-induced bax-dependent apoptosis. FEBS Lett. 500:17-24. [DOI] [PubMed] [Google Scholar]
- 36.Qin, Z. H., Y. Wang, K. K. Kikly, E. Sapp, K. B. Kegel, N. Aronin, and M. DiFiglia. 2001. Pro-caspase-8 is predominantly localized in mitochondria and released into cytoplasm upon apoptotic stimulation. J. Biol. Chem. 276:8079-8086. [DOI] [PubMed] [Google Scholar]
- 37.Reed, J. C. 1998. Bcl-2 family proteins. Oncogene 17:3225-3236. [DOI] [PubMed] [Google Scholar]
- 38.Rodgers, S. E., E. S. Barton, S. M. Oberhaus, B. Pike, C. A. Gibson, K. L. Tyler, and T. S. Dermody. 1997. Reovirus-induced apoptosis of MDCK cells is not linked to viral yield and is blocked by Bcl-2. J. Virol. 71:2540-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothe, M., M. G. Pan, W. J. Henzel, T. M. Ayres, and D. V. Goeddel. 1995. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell 83:1243-1252. [DOI] [PubMed] [Google Scholar]
- 40.Roulston, A., R. C. Marcellus, and P. E. Branton. 1999. Viruses and apoptosis. Annu. Rev. Microbiol. 53:577-628. [DOI] [PubMed] [Google Scholar]
- 41.Roy, N., Q. L. Deveraux, R. Takahashi, G. S. Salvesen, and J. C. Reed. 1997. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 16:6914-6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scaffidi, C., S. Fulda, A. Srinivasan, C. Friesen, F. Li, K. J. Tomaselli, K. M. Debatin, P. H. Krammer, and M. E. Peter. 1998. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scaffidi, C., I. Schmitz, J. Zha, S. J. Korsmeyer, P. H. Krammer, and M. E. Peter. 1999. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J. Biol. Chem. 274:22532-22538. [DOI] [PubMed] [Google Scholar]
- 44.Servet-Delprat, C., P. O. Vidalain, O. Azocar, F. Le Deist, A. Fischer, and C. Rabourdin-Combe. 2000. Consequences of Fas-mediated human dendritic cell apoptosis induced by measles virus. J. Virol. 74:4387-4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharp, T. V., H. W. Wang, A. Koumi, D. Hollyman, Y. Endo, H. Ye, M. Q. Du, and C. Boshoff. 2002. K15 protein of Kaposi's sarcoma-associated herpesvirus is latently expressed and binds to HAX-1, a protein with antiapoptotic function. J. Virol. 76:802-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharpe, A. H., L. B. Chen, and B. N. Fields. 1982. The interaction of mammalian reoviruses with the cytoskeleton of monkey kidney CV-1 cells. Virology 120:399-411. [DOI] [PubMed] [Google Scholar]
- 47.Srinivasula, S. M., M. Ahmad, Y. Guo, Y. Zhan, Y. Lazebnik, T. Fernandes-Alnemri, and E. S. Alnemri. 1999. Identification of an endogenous dominant-negative short isoform of caspase-9 that can regulate apoptosis. Cancer Res. 59:999-1002. [PubMed] [Google Scholar]
- 48.Sun, X. M., S. B. Bratton, M. Butterworth, M. MacFarlane, and G. M. Cohen. 2002. Bcl-2 and Bcl-xL inhibit CD95-mediated apoptosis by preventing mitochondrial release of Smac/DIABLO and subsequent inactivation of X-linked inhibitor-of-apoptosis protein. J. Biol. Chem. 277:11345-11351. [DOI] [PubMed] [Google Scholar]
- 49.Susin, S. A., H. K. Lorenzo, N. Zamzami, I. Marzo, C. Brenner, N. Larochette, M. C. Prevost, P. M. Alzari, and G. Kroemer. 1999. Mitochondrial release of caspase-2 and -9 during the apoptotic process. J. Exp. Med. 189:381-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Susin, S. A., H. K. Lorenzo, N. Zamzami, I. Marzo, B. E. Snow, G. M. Brothers, J. Mangion, E. Jacotot, P. Costantini, M. Loeffler, N. Larochette, D. R. Goodlett, R. Aebersold, D. P. Siderovski, J. M. Penninger, and G. Kroemer. 1999. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397:441-446. [DOI] [PubMed] [Google Scholar]
- 51.Tamm, I., Y. Wang, E. Sausville, D. A. Scudiero, N. Vigna, T. Oltersdorf, and J. C. Reed. 1998. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 58:5315-5320. [PubMed] [Google Scholar]
- 52.Terradillos, O., C. A. de La, T. Pollicino, C. Neuveut, D. Sitterlin, H. Lecoeur, M. L. Gougeon, A. Kahn, and M. A. Buendia. 2002. The hepatitis B virus X protein abrogates Bcl-2-mediated protection against Fas apoptosis in the liver. Oncogene 21:377-386. [DOI] [PubMed] [Google Scholar]
- 53.Tyler, K. L., M. K. Squier, A. L. Brown, B. Pike, D. Willis, S. M. Oberhaus, T. S. Dermody, and J. J. Cohen. 1996. Linkage between reovirus-induced apoptosis and inhibition of cellular DNA synthesis: role of the S1 and M2 genes. J. Virol. 70:7984-7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tyler, K. L., M. K. Squier, S. E. Rodgers, B. E. Schneider, S. M. Oberhaus, T. A. Grdina, J. J. Cohen, and T. S. Dermody. 1995. Differences in the capacity of reovirus strains to induce apoptosis are determined by the viral attachment protein sigma 1. J. Virol. 69:6972-6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verhagen, A. M., P. G. Ekert, M. Pakusch, J. Silke, L. M. Connolly, G. E. Reid, R. L. Moritz, R. J. Simpson, and D. L. Vaux. 2000. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102:43-53. [DOI] [PubMed] [Google Scholar]
- 56.Vidalain, P. O., O. Azocar, B. Lamouille, A. Astier, C. Rabourdin-Combe, and C. Servet-Delprat. 2000. Measles virus induces functional TRAIL production by human dendritic cells. J. Virol. 74:556-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.von Ahsen, O., C. Renken, G. Perkins, R. M. Kluck, E. Bossy-Wetzel, and D. D. Newmeyer. 2000. Preservation of mitochondrial structure and function after Bid- or Bax-mediated cytochrome c release. J. Cell Biol. 150:1027-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei, M. C., W. X. Zong, E. H. Cheng, T. Lindsten, V. Panoutsakopoulou, A. J. Ross, K. A. Roth, G. R. MacGregor, C. B. Thompson, and S. J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang, M., X. Li, X. Pang, L. Ding, O. Wood, K. Clouse, I. Hewlett, and A. I. Dayton. 2001. Identification of a potential HIV-induced source of bystander-mediated apoptosis in T cells: upregulation of trail in primary human macrophages by HIV-1 tat. J. Biomed. Sci. 8:290-296. [DOI] [PubMed] [Google Scholar]
- 60.Zhang, X. D., X. Y. Zhang, C. P. Gray, T. Nguyen, and P. Hersey. 2001. Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis of human melanoma is regulated by smac/DIABLO release from mitochondria. Cancer Res. 61:7339-7348. [PubMed] [Google Scholar]
- 61.Zhou, G., and B. Roizman. 2000. Wild-type herpes simplex virus 1 blocks programmed cell death and release of cytochrome c but not the translocation of mitochondrial apoptosis-inducing factor to the nuclei of human embryonic lung fibroblasts. J. Virol. 74:9048-9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu, N., C. F. Ware, and M. M. Lai. 2001. Hepatitis C virus core protein enhances FADD-mediated apoptosis and suppresses TRADD signaling of tumor necrosis factor receptor. Virology 283:178-187. [DOI] [PubMed] [Google Scholar]
- 63.Zou, H., Y. Li, X. Liu, and X. Wang. 1999. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 274:11549-11556. [DOI] [PubMed] [Google Scholar]