Abstract
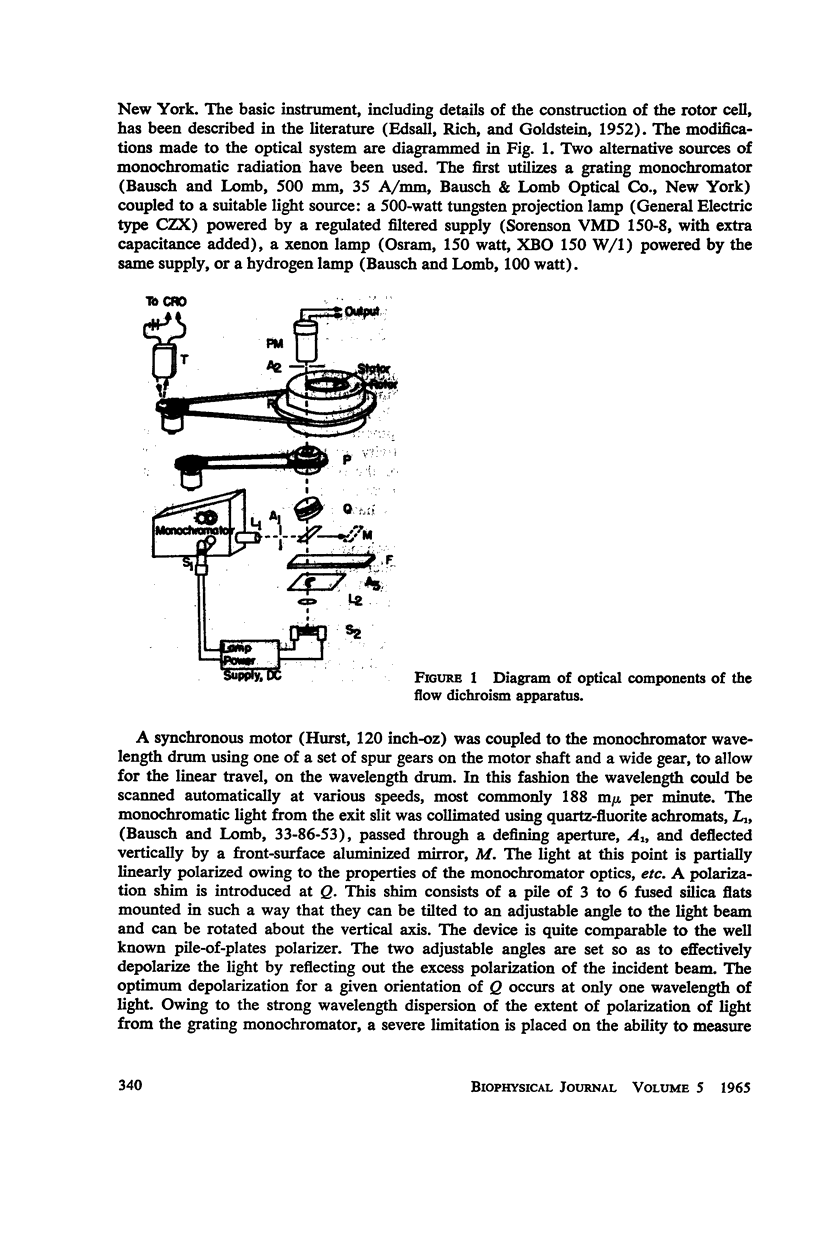
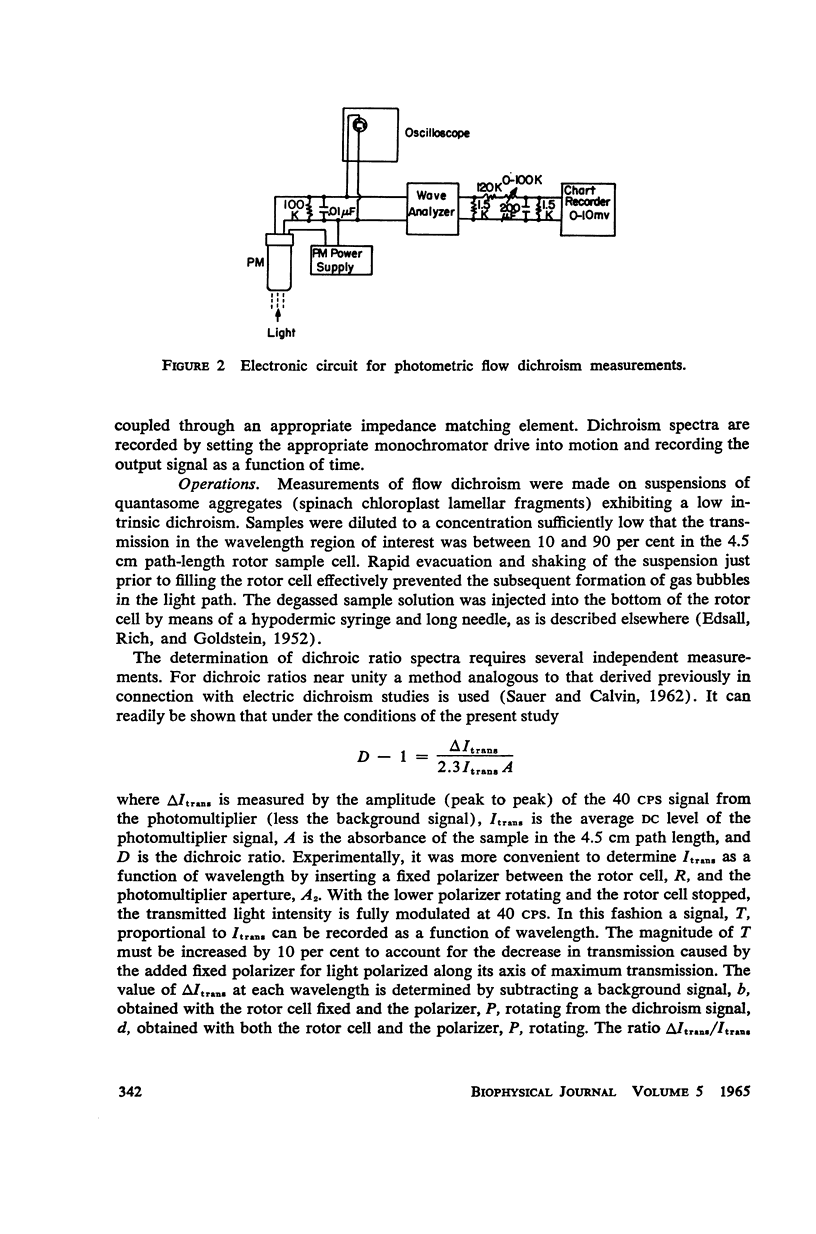
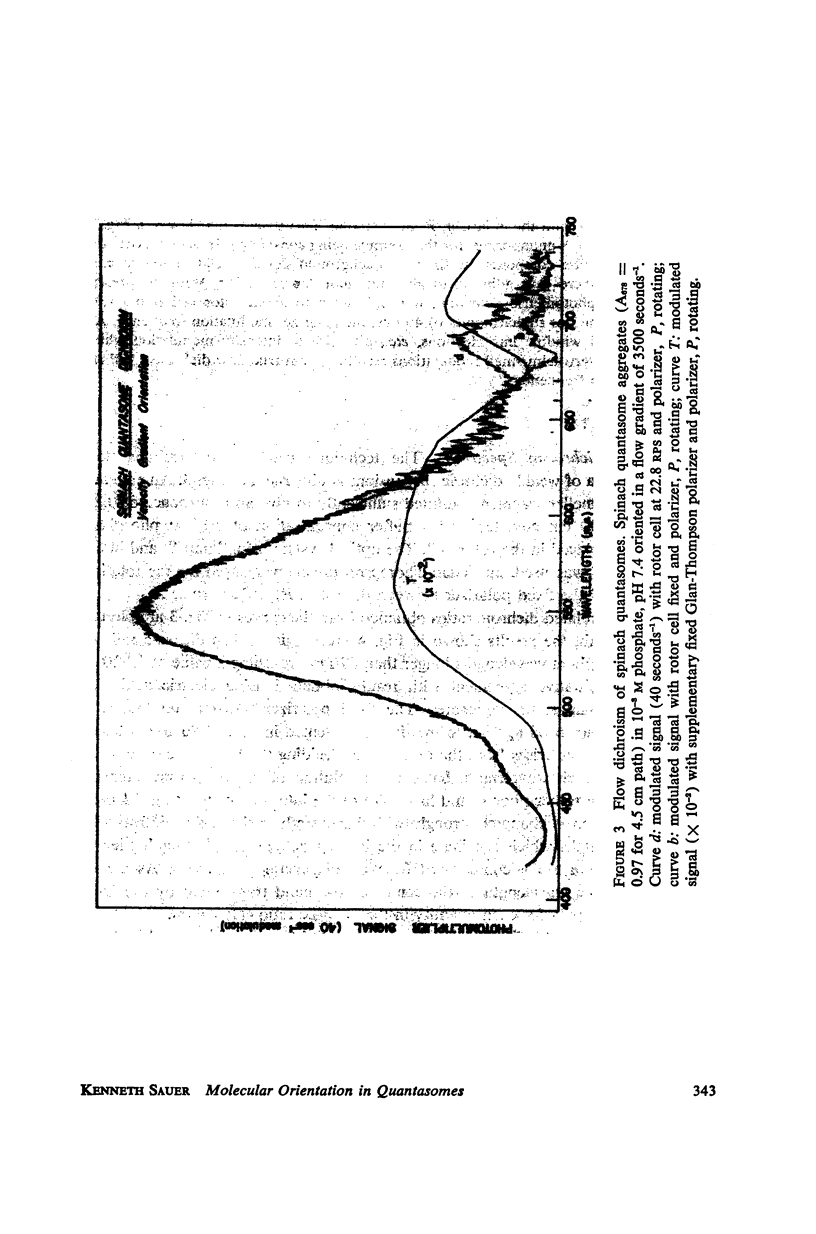
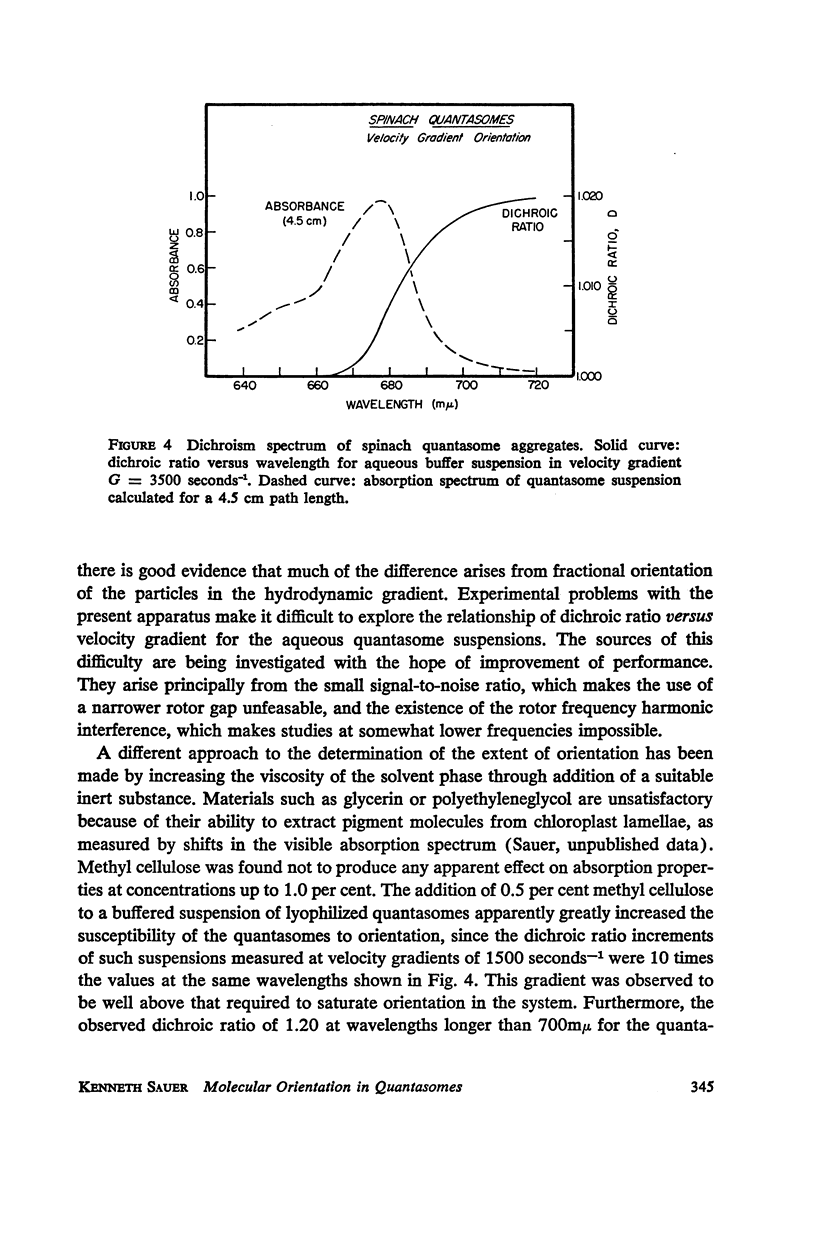
A new apparatus is described for measuring dichroism spectra with very high sensitivity for macromolecular structures oriented in a hydrodynamic gradient. The method has been used to explore the dichroism spectrum of quantasome aggregates isolated from spinach chloroplasts. The quantasome flow dichroism resembles qualitatively that observed previously using electric field orientation, in that a pigment absorbing at wavelengths longer than 680 mμ exhibits appreciably greater dichroism than those absorbing at shorter wavelengths. It is shown that the absorption oscillator for this long wavelength absorption lies parallel to the streamlines of the sheer gradient, which is assumed to be the direction in which the planes of the chloroplast lamellae are oriented.
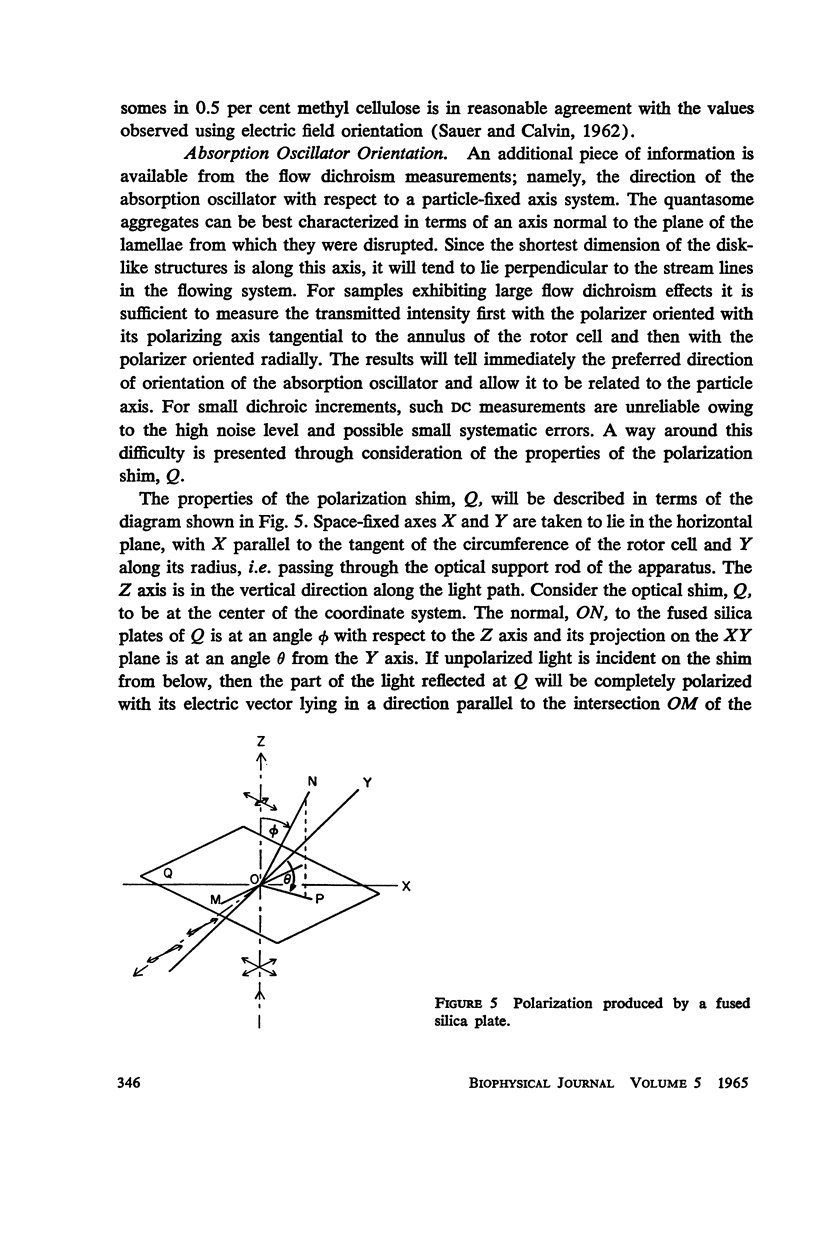
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HIGASHI S., KASAI M., OOSAWA F., WADA A. ULTRAVIOLET DICHROISM OF F-ACTIN ORIENTED BY FLOW. J Mol Biol. 1963 Oct;7:421–430. doi: 10.1016/s0022-2836(63)80034-0. [DOI] [PubMed] [Google Scholar]
- KOK B. Partial purification and determination of oxidation reduction potential of the photosynthetic chlorophyll complex absorbing at 700 millimicrons. Biochim Biophys Acta. 1961 Apr 15;48:527–533. doi: 10.1016/0006-3002(61)90050-6. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. The structure of the DNA-acridine complex. Proc Natl Acad Sci U S A. 1963 Jan 15;49:94–102. doi: 10.1073/pnas.49.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLSON R. A., BUTLER W. L., JENNINGS W. H. The orientation of chlorophyll molecules, in vivo: further evidence from dichroism. Biochim Biophys Acta. 1962 Mar 26;58:144–146. doi: 10.1016/0006-3002(62)90833-8. [DOI] [PubMed] [Google Scholar]
- PARK R. B., PON N. G. Chemical composition and the substructure of lamellae isolated from Spinacea oleracea chloroplasts. J Mol Biol. 1963 Feb;6:105–114. doi: 10.1016/s0022-2836(63)80126-6. [DOI] [PubMed] [Google Scholar]
- PARK R. B., PON N. G. Correlation of structure with function in Spinacea oleracea chloroplasts. J Mol Biol. 1961 Feb;3:1–10. doi: 10.1016/s0022-2836(61)80002-8. [DOI] [PubMed] [Google Scholar]
- Park R. B., Biggins J. Quantasome: Size and Composition. Science. 1964 May 22;144(3621):1009–1011. doi: 10.1126/science.144.3621.1009. [DOI] [PubMed] [Google Scholar]
- SAUER K., CALVIN M. Molecular orientation in quantasomes. I. Electric dichroism and electric birefringence of quantasomes from spinach chloroplasts. J Mol Biol. 1962 Jun;4:451–466. doi: 10.1016/s0022-2836(62)80102-8. [DOI] [PubMed] [Google Scholar]
- SAUER K., PARK R. B. MOLECULAR ORIENTATION IN QUANTASOMES. II. ABSORPTION SPECTRA, HILL ACTIVITY AND FLUORESCENCE YIELDS. Biochim Biophys Acta. 1964 May 25;79:476–489. doi: 10.1016/0926-6577(64)90213-x. [DOI] [PubMed] [Google Scholar]


