Abstract
Human cytomegalovirus (HCMV) US10 encodes a glycoprotein that binds to major histocompatibility complex (MHC) class I heavy chains. While expression of US10 delays the normal trafficking of MHC class I molecules out of the endoplasmic reticulum, US10 does not obviously facilitate or inhibit the action of two other HCMV-encoded MHC class I binding proteins, US2 and US11.
Human cytomegalovirus (HCMV) causes lifelong infections of its host. The ability of HCMV to replicate despite the existence of an immune response against the virus may rely on elaborate strategies of immune evasion. The unique short (US) region of the HCMV genome encodes at least four proteins (US2, US3, US6, and US11) that interfere with CD8+ T-cell recognition by suppressing major histocompatibility complex (MHC) class I expression (14). Other open reading frames in the US region of HCMV may encode similar immunoevasins. Proteins US6, -7, -8, -9, and -10 are predicted to encode membrane glycoproteins (15). An HCMV deletion mutant lacking expression of all US-encoded genes except for US10 and US12 did not downregulate MHC class I expression in infected human foreskin fibroblasts (6). Therefore, US11 was not expected to have a significant effect on MHC class I expression. However, the presence of these genes raises questions of their possible function(s). Here we provide evidence that US10 interacts with constituents of the MHC class I pathway of antigen presentation.
HCMV US10 associates with MHC class I heavy chains.
The US10 gene was amplified from a plasmid containing the US10 cDNA sequence from HCMV strain AD169 (kindly provided by Klaus Fruh) by PCR using primers that incorporated the influenza virus hemagglutinin (HA) epitope tag at the C terminus of the protein. The US10-HA PCR product was cloned into pCDNA3.1+ (Invitrogen, San Diego, Calif.), which directs expression of the US10-HA gene under the control of the CMV promoter. The US10-HA/pCDNA 3.1+ plasmid was transfected into U373 astrocytoma cells (U373), and clonal cell lines were generated following selection with Geneticin (Gibco) (12). US10-HA remained endoglycosidase H (endo H) sensitive, as assessed by pulse-chase analysis, and was localized to the endoplasmic reticulum (ER), as are several other US gene products that interfere with MHC class I maturation (data not shown). Huber et al. have also observed endo H sensitivity and ER retention of US10 expressed in HEC-1A human endometrial epithelial cells (5).
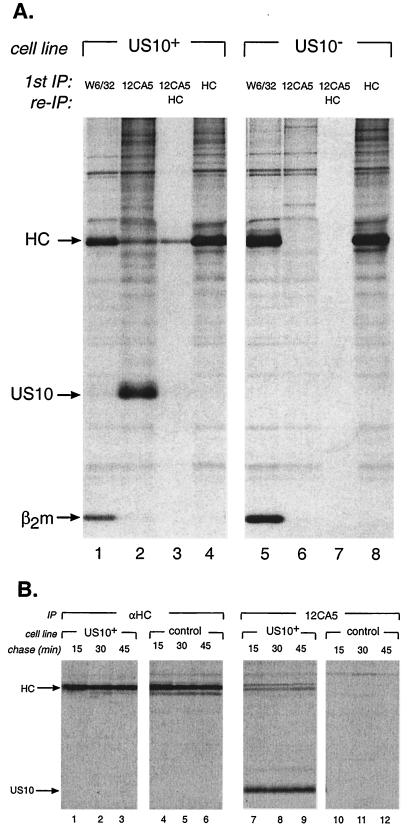
We examined possible interactions between US10 and MHC class I molecules. U373 cells or U373 cells stably expressing US10-HA (US10+) were metabolically labeled with Expres35S35S protein labeling mix (NEN) for 50 min. US10-HA, folded MHC class I complexes, and unfolded MHC class I heavy chains (HC) were recovered from labeled cell lysates, prepared in a buffer containing 0.5% NP-40, with the anti-HA monoclonal antibody 12CA5, the W6/32 monoclonal antibody, and rabbit anti-HC polyclonal antiserum, respectively (12). Immunoprecipitates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and fluorography as previously described (10). The HA-tagged US10 protein has a molecular mass of approximately 23 kDa, which is comparable to the approximately 21-kDa size predicted to result from the addition of the HA tag and one N-linked glycan. Multiple labeled polypeptides appeared in US10-HA immunoprecipitates (Fig. 1A, lane 2), a major species of which comigrated with MHC class I HC. The recovery of HC from US10 immunoprecipitates that were denatured in 1% SDS, boiled, and reimmunoprecipitated with serum that recognizes unfolded HC confirmed that the coprecipitating polypeptide was HC (Fig. 1A, lane 3), and this interaction was specific for the presence of US10 (Fig. 1A, compare lanes 3 and 6). The lack of US10 in the MHC class I immunoprecipitates from US10+ cells does not directly address the strength of interactions of these proteins. For example, US2 and US11 interact efficiently with MHC class I molecules, as judged by the rapid degradation of class I HC, yet antisera that recognize US2 and US11 in NP-40 lysates do not recover class I products (16, 17).
FIG. 1.
HCMV US10 associates with MHC class I HC. (A) US10-HA-expressing cells and control cells were metabolically labeled for 50 min. Folded MHC class I-β2m complexes, US10-HA, and free HC were recovered from cell lysates by immunoprecipitation (IP) with W6/32, 12CA5, and anti-HC antibodies (αHC), respectively. A reimmunoprecipitation(re-IP) for HC was performed from a portion of the 12CA5 immunoprecipitates that had been denatured in 1% SDS and boiled (lanes 3 and 7). The positions of prestained molecular size standards (Amersham) are shown on the right. (B) US10-HA and control cells were metabolically labeled for 10 min and chased for up to 45 min in nonradioactive medium. HC and US10-HA molecules were recovered from cell lysates with anti-HC and 12CA5 antibodies, respectively. All samples were analyzed by SDS-PAGE (12.5% polyacrylamide gels).
The kinetics of association of US10 with HC was examined by pulse-chase analysis (12). Levels of anti-HC-reactive polypeptides recovered from NP-40 lysates were constant over the chase period in both US10+ and control cells (Fig. 1B, lanes 1 to 6). Levels of HC that coprecipitated with US10-HA were stable over time (Fig. 1B, lanes 7 to 9). HC was not recovered in anti-HA immunoprecipitates from control cells (Fig. 1B, lanes 10 to 12). Therefore, HC associate with US10 in a stable fashion. Furthermore, association with US10 does not alter the stability of HC.
US10 delays the maturation of MHC class I molecules.
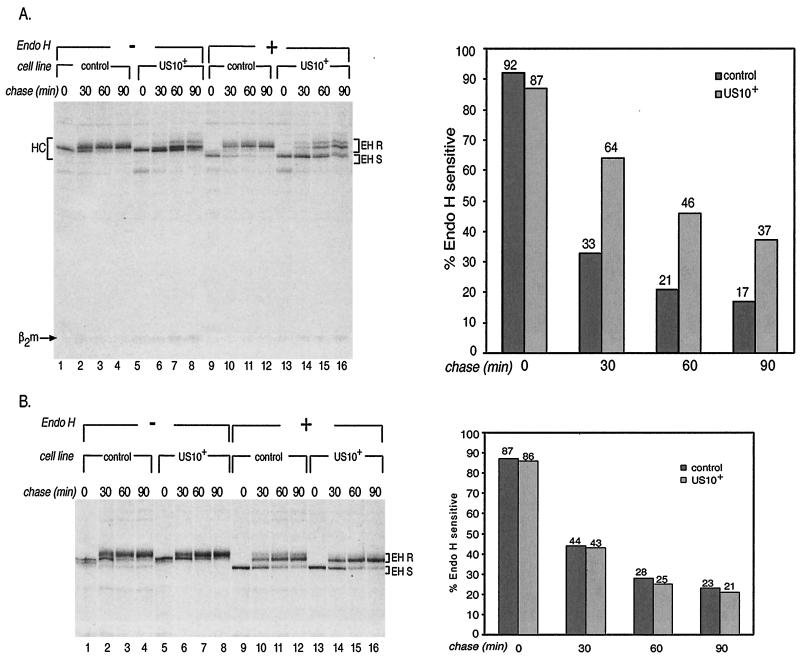
Next we investigated the maturation of MHC class I molecules in US10-HA-expressing cells by pulse-chase analysis. Recovery of folded MHC class I complexes from 35S-labeled NP-40 lysates with the W6/32 monoclonal antibody indicated that overall levels of maturing HC for control cells and US10+ cells were similar over a 90-min chase period (Fig. 2A, compare lanes 1 to 4 with lanes 5 to 8). Digestion of W6/32 immunoprecipitates with endo H showed that relative to complexes in untransfected cells, a large fraction of MHC class I complexes in US10+ cells were endo H sensitive (Fig. 2A, compare lanes 9 to 12 with lanes 13 to 16). In contrast, transferrin receptors showed similar rates of maturation in control and US10+ cells (Fig. 2B, compare lanes 9 to 12 with lanes 13 to 16). Autoradiograms were quantitated with a PhosphorImager and ImageQuant software (Molecular Dynamics). Quantitation of endo H-sensitive and -resistant fractions showed a twofold increase in endo H-sensitive material for US10+ cells compared to control cells (Fig. 2A, graph). No kinetic difference in maturation of transferrin receptors in US10+ cells and control cells was evident (Fig. 2B, graph). Thus, the expression of US10 specifically delays the egress of MHC class I molecules from the ER. While US10 delays the maturation of MHC class I HC, it does not block MHC class I maturation and cell surface expression (6).
FIG. 2.
HCMV US10 delays the egress of folded MHC class I molecules from the ER. (A) US10-HA-expressing cells and control cells were metabolically labeled for 10 min. Folded MHC class I molecules were recovered from cell lysates with monoclonal antibody W6/32. Half of the W6/32 immunoprecipitate was digested with Endo H prior to analysis (lanes 9 to 16). Samples were analyzed by SDS-PAGE (12.5% polyacrylamide gels). Fractions of Endo H-sensitive (EH S) and Endo H-resistant (EH R) MHC class I complexes were quantitated by phosphorimage analysis; the graph shows the percentages of Endo H-sensitive complexes at each time point (graph). (B) Cells were treated as described for panel A. Transferrin receptor molecules were recovered from cell lysates by using the monoclonal antibody 66Ig10. Fractions of Endo H-sensitive transferrin receptors (shown in lanes 9 to 16) were quantitated and plotted as described for panel A (graph).
US10 does not affect US2 or US11 function.
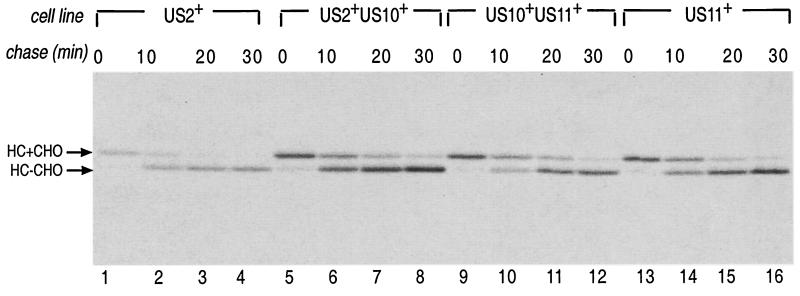
Because US10 localizes to the ER and binds MHC molecules yet does not prevent their expression, we explored whether US10 might synergize with other US gene products. Astrocytoma cells stably expressing US2 or US11 under the control of the CMV promoter and maintained in puromycin (1) were cotransfected with US10-HA in pCDNA 3.1+ (Invitrogen). Clonal cell lines expressing both US10-HA and either US2 or US11 were generated following selection with Geneticin. The half-life of MHC class I HC in cells preincubated with the proteasome inhibitor carboxybenzylleucylleucylleucine vinyl sulfone (ZL3VS) (50 μM) was examined (Fig. 3). In cells that express US2 or US11, MHC class I HC are dislocated from the ER to the cytosol and are destroyed by the proteasome. Proteasome inhibition in these cells allows the accumulation of deglycosylated HC intermediates (16, 17). Expression of US10-HA did not alter the kinetics of HC degradation in cells coexpressing US2 and US11, as shown by identical rates of conversion of glycosylated HC to deglycosylated HC (Fig. 3, lanes 5 to 12), although both US10-HA and US2 or both US10-HA and US11 were found to be abundantly coexpressed in the US2+ US10+ and US10+ US11+ cell lines, respectively, when examined by immunoprecipitation from radiolabeled lysates (data not shown). Therefore, expression of US10 does not measurably alter the activity of US2 or US11 in astrocytoma cells.
FIG. 3.
US10 does not interfere with US2 or US11 function. Human astrocytoma cells stably transfected with US2, US10-HA and US2, US10-HA and US11, or US11 only were preincubated with 50 μM ZL3VS, metabolically labeled for 5 min, and chased up to 30 min in nonradioactive medium. HC were recovered from cell lysates and analyzed by SDS-PAGE (10% polyacrylamide gels). HC+CHO and HC-CHO, glycosylated and deglycosylated forms of MHC class I HC, respectively.
The US region of the HCMV genome encodes multiple glycoproteins that interfere with MHC class I maturation. Here we identified US10 as yet another potential immunoevasin. US10 binds MHC class I HC and delays, but does not prevent, the egress of folded MHC class I complexes from the ER. What is the function of US10 in an HCMV-infected cell? US10 may retain a subset of MHC class I alleles. Indeed, the MHC class I complexes that mature in US10-expressing cells have a mobility distinct from that of complexes in control cells and may represent the maturation of a distinct subset of MHC class I alleles (Fig. 2A, compare lanes 13 to 16 with lanes 9 to 12). US2 and US11 exhibit allelic preferences for MHC class I HC. For example, HLA-G, HLA-Cw4, HLA-Cw3, and HLA-E are resistant to US2- and US11-mediated degradation (2, 13). Thus, while allelic specificity has precedents with other immunomodulatory US genes, it is not clear how retention of allelic products other than HLA-A and -B would be a favorable strategy, as these other alleles can prevent lysis by NK cells (18).
HCMV US3 also retains MHC class I molecules in the ER (1, 8). The mechanism for this retention is unclear. One report claims that US3 itself resides in the ER and retains MHC class I by association (9). Another study suggested that US3 retains MHC class I molecules while in the ER, while unbound US3 continuously traffics into lysosomes, and proposes that US3 acts by preventing forward transport of MHC class I molecules (4). US10 shares many features with US3, and both polypeptides may adopt a fold similar to that of US2 (3). US10 and US3 lack C-terminal KDEL or dilysine retention motifs (9). Both may interact with chaperones, although US3 does not require calnexin, transporter associated with antigen processing (TAP), or tapasin to interact with class I HC (9).
We were unable to observe either an effect of US10 expression on the activity of US2 and US11 (Fig. 3) or physical interactions among these US proteins (data not shown), consistent with the observations of others (5). US10 is expressed with kinetics similar to those of US11 in stable transfectants, yet the levels of transcription and US10 protein expression are lower in HCMV-infected cells (5, 7).
Finally, US10 may exert an inhibitory effect on MHC class I expression in some cell types but not others. US2 and US11 differ in their ability to attack MHC class I molecules in dendritic cells (11). It remains to be seen whether U373 cells are the best vehicle for uncovering an immunoevasive function for US10. We still do not know how the virus integrates the functions of all of the immunoevasive gene products in the course of an infection, but a more thorough understanding promises to be useful for an appreciation of the complexity of virus-host interactions.
Acknowledgments
This work was supported by NIH grant P01 AI4225705. M.H.F. is an Albert J. Ryan Fellow.
We are grateful to Klaus Fruh for the US10 cDNA and to Rebecca S. Tirabassi for a critical reading of the manuscript.
REFERENCES
- 1.Ahn, K., A. Angulo, P. Ghazal, P. A. Peterson, Y. Yang, and K. Fruh. 1996. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc. Natl. Acad. Sci. USA 93:10990-10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furman, M. H., H. L. Ploegh, and D. Tortorella. 2002. Membrane-specific, host-derived factors are required for US2- and US11-mediated degradation of major histocompatibility complex class I molecules. J. Biol. Chem. 277:3258-3267. [DOI] [PubMed] [Google Scholar]
- 3.Gewurz, B. E., R. Gaudet, D. Tortorella, E. W. Wang, H. L. Ploegh, and D. C. Wiley. 2001. Antigen presentation subverted: structure of the human cytomegalovirus protein US2 bound to the class I molecule HLA-A2. Proc. Natl. Acad. Sci. USA 98:6794-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruhler, A., P. A. Peterson, and K. Fruh. 2000. Human cytomegalovirus immediate early glycoprotein US3 retains MHC class I molecules by transient association. Traffic 1:318-325. [DOI] [PubMed] [Google Scholar]
- 5.Huber, M. T., R. Tomazin, T. Wisner, J. Boname, and D. C. Johnson. 2002. Human cytomegalovirus US7, US8, US9, and US10 are cytoplasmic glycoproteins, not found at cell surfaces, and US9 does not mediate cell-to-cell spread. J. Virol. 76:5748-5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones, T. R., L. K. Hanson, L. Sun, J. S. Slater, R. M. Stenberg, and A. E. Campbell. 1995. Multiple independent loci within the human cytomegalovirus unique short region down-regulate expression of major histocompatibility complex class I heavy chains. J. Virol. 69:4830-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones, T. R., and V. P. Muzithras. 1991. Fine mapping of transcripts expressed from the US6 gene family of human cytomegalovirus strain AD169. J. Virol. 65:2024-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones, T. R., E. J. Wiertz, L. Sun, K. N. Fish, J. A. Nelson, and H. L. Ploegh. 1996. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc. Natl. Acad. Sci. USA 93:11327-11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, S., J. Yoon, B. Park, Y. Jun, M. Jin, H. C. Sung, I. H. Kim, S. Kang, E. J. Choi, B. Y. Ahn, and K. Ahn. 2000. Structural and functional dissection of human cytomegalovirus US3 in binding major histocompatibility complex class I molecules. J. Virol. 74:11262-11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ploegh, H. L. 1995. In J. E. Coligan, B. M. Dunn, H. L. Ploegh, D. W. Speicher, and P. T. Wingfield (ed.), Current protocols in protein science, p. 10.0.1-10.1.9. John Wiley & Sons, New York, N.Y.
- 11.Rehm, A., A. Engelsberg, D. Tortorella, I. J. Korner, I. Lehmann, H. L. Ploegh, and U. E. Hopken. 2002. Human cytomegalovirus gene products US2 and US11 differ in their ability to attack major histocompatibility class I heavy chains in dendritic cells. J. Virol. 76:5043-5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rehm, A., P. Stern, H. L. Ploegh, and D. Tortorella. 2001. Signal peptide cleavage of a type I membrane protein, HCMV US11, is dependent on its membrane anchor. EMBO J. 20:1573-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schust, D. J., D. Tortorella, J. Seebach, C. Phan, and H. L. Ploegh. 1998. Trophoblast class I major histocompatibility complex (MHC) products are resistant to rapid degradation imposed by the human cytomegalovirus (HCMV) gene products US2 and US11. J. Exp. Med. 188:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tortorella, D., B. E. Gewurz, M. H. Furman, D. J. Schust, and H. L. Ploegh. 2000. Viral subversion of the immune system. Annu. Rev. Immunol. 18:861-926. [DOI] [PubMed] [Google Scholar]
- 15.Weston, K., and B. G. Barrell. 1986. Sequence of the short unique region, short repeats, and part of the long repeats of human cytomegalovirus. J. Mol. Biol. 192:177-208. [DOI] [PubMed] [Google Scholar]
- 16.Wiertz, E. J., T. R. Jones, L. Sun, M. Bogyo, H. J. Geuze, and H. L. Ploegh. 1996. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell 84:769-779. [DOI] [PubMed] [Google Scholar]
- 17.Wiertz, E. J., D. Tortorella, M. Bogyo, J. Yu, W. Mothes, T. R. Jones, T. A. Rapoport, and H. L. Ploegh. 1996. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature 384:432-438. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama, W. M. 1998. Natural killer cell receptors. Curr. Opin. Immunol. 10:298-305. [DOI] [PubMed] [Google Scholar]