Abstract
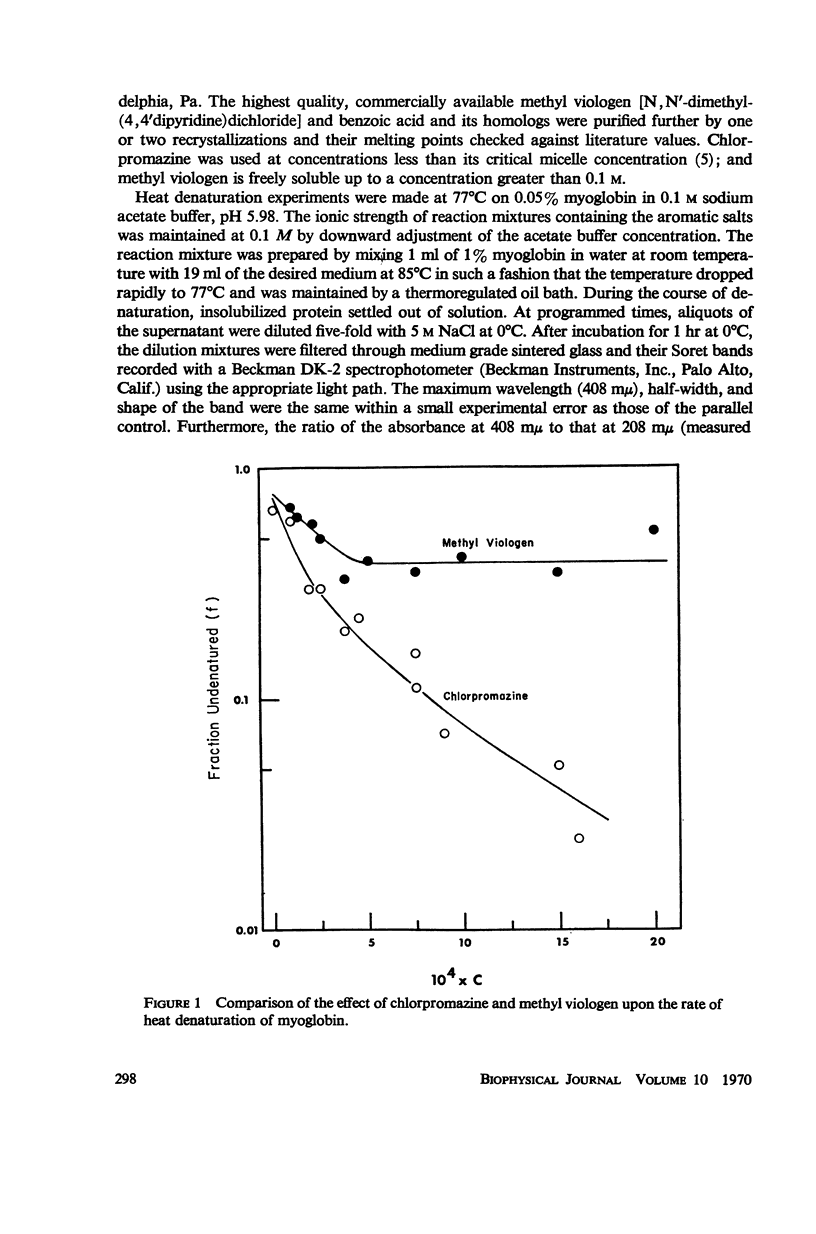
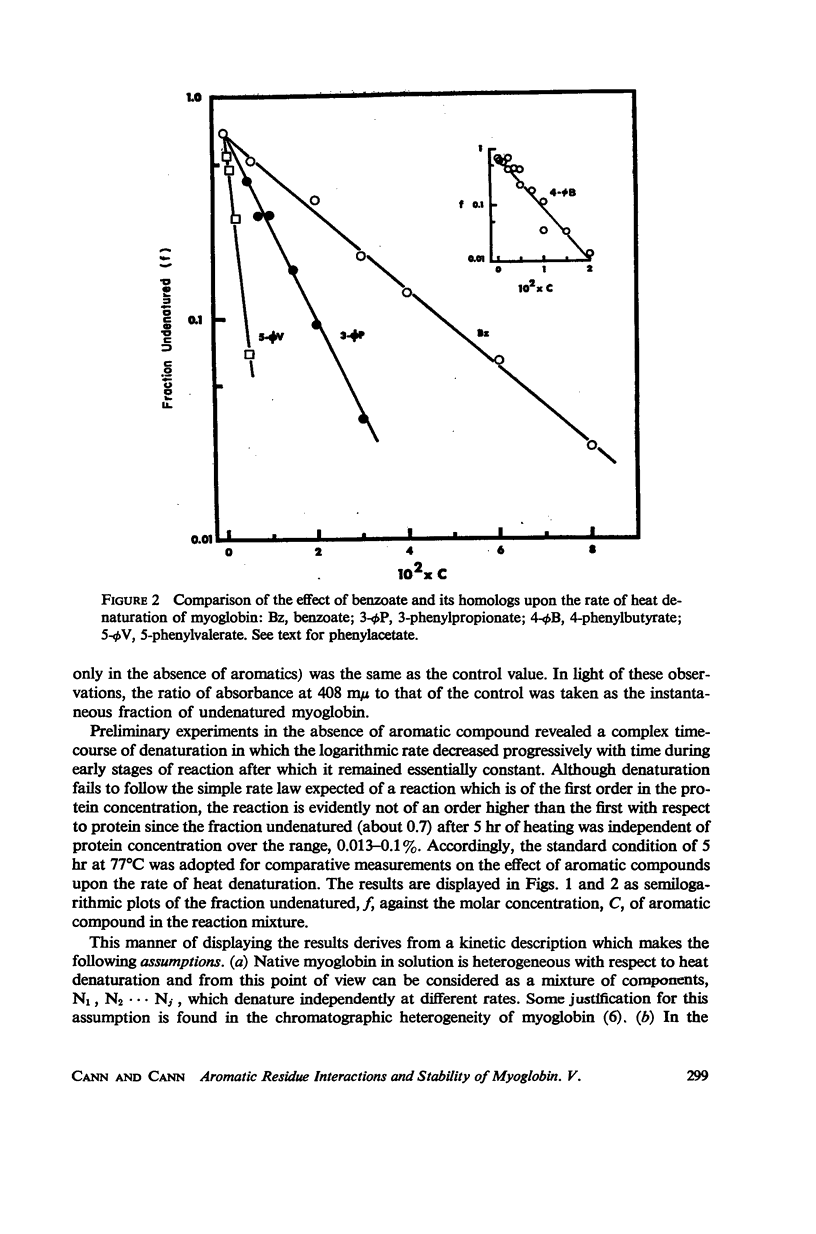
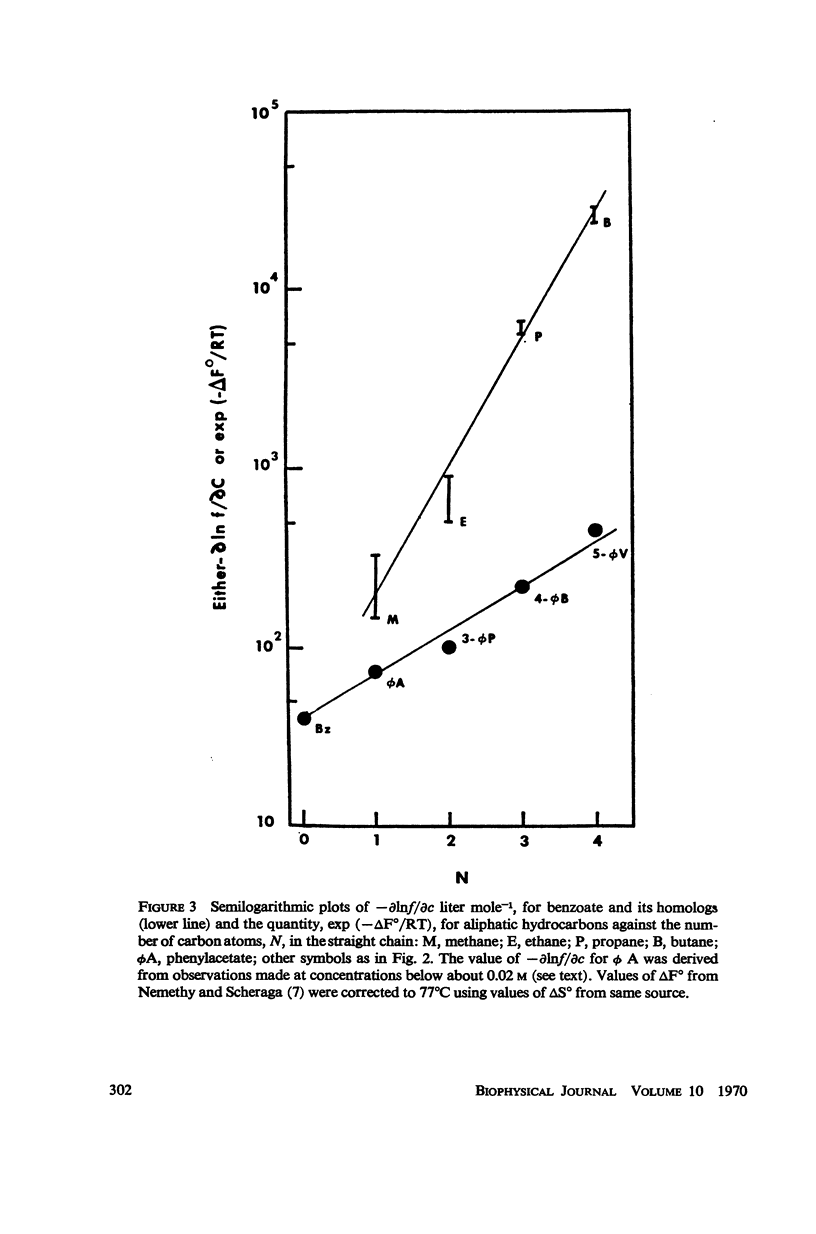
Aromatic compounds like chlorpromazine and benzoate and its homologs strongly enhance the rate of heat denaturation of myoglobin. The latter apparently exert their action by complexing with a single kind of site in the hemeprotein. Both charge-transfer and hydrophobic interactions are implicated in complex formation, most probably with the heme moiety.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cann J. R. Contribution of aromatic residue interactions to the stability of myoglobin. 3. Molecular complexes of aromatic compounds with hemin, hematoporphyrin, and myoglobin. Biochemistry. 1967 Nov;6(11):3435–3442. doi: 10.1021/bi00863a014. [DOI] [PubMed] [Google Scholar]
- Cann J. R. Contribution of aromatic residue interactions to the stability of myoglobin. II. Enhancement by aromatic compounds of the rate of urea denaturation. Biochemistry. 1967 Nov;6(11):3427–3434. doi: 10.1021/bi00863a013. [DOI] [PubMed] [Google Scholar]
- Cann J. R. Contribution of aromatic residue interactions to the stability of myoglobin. IV. Delineation of binding forces between aromatic compounds and myoglobin. Biochemistry. 1969 Oct;8(10):4036–4047. doi: 10.1021/bi00838a022. [DOI] [PubMed] [Google Scholar]
- KENDREW J. C. Side-chain interactions in myoglobin. Brookhaven Symp Biol. 1962 Dec;15:216–228. [PubMed] [Google Scholar]


