Abstract
While antiviral antibody plays a key role in resistance to acute viral infection, the contribution of antibody to the control of latent virus infection is less well understood. Gammaherpesvirus 68 (γHV68) infection of mice provides a model well suited to defining contributions of specific immune system components to the control of viral latency. B cells play a critical role in regulating γHV68 latency, but the mechanism(s) by which B cells regulate latency is not known. In the experiments reported here, we determined the effect of passively transferred antibody on established γHV68 latency in B-cell-deficient (B-cell−/−) mice. Immune antibody decreased the frequency of cells reactivating ex vivo from latency in splenocytes (>10-fold) and peritoneal cells (>100-fold) and the frequency of cells carrying latent viral genome in splenocytes (>5-fold) and peritoneal cells (>50-fold). This effect required virus-specific antibody and was observed when total and virus-specific serum antibody concentrations in recipient B-cell−/− mice were <8% of those in normal mice during latent infection. Passive transfer of antibody specific for the lytic cycle γHV68 RCA protein, but not passive transfer of antibody specific for the v-cyclin protein or the latent protein M2, decreased both the frequency of cells reactivating ex vivo from latency and the frequency of cells carrying the latent viral genome. Therefore, antibody specific for lytic cycle viral antigens can play an important role in the control of gammaherpesvirus latency in immunocompromised hosts. Based on these findings, we propose a model in which ongoing productive replication is essential for maintaining high levels of latently infected cells in immunocompromised hosts. We confirmed this model by the treatment of latently infected B-cell−/− mice with the antiviral drug cidofovir.
Infection by herpesviruses leads to the establishment of lifelong latency in different tissues (20, 25). Reactivation of herpesviruses from latency, or the expansion of latently infected cells, in immunocompromised hosts is thought to contribute to herpesvirus-induced diseases (25, 37). However, the precise mechanisms by which the host limits latent infection or reactivation from latency are incompletely understood.
Human gammaherpesviruses such as Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) establish latent infection and are associated with chronic diseases, including lymphoma in immunocompromised patients. One of the difficulties in studying the immune control of gammaherpesvirus latency and reactivation has been the lack of a suitable small animal model. Since murine gammaherpesvirus 68 (γHV68) establishes latent infection in laboratory mice and is associated with the induction of lymphomas (34; unpublished observations), infection of mice with γHV68 provides a tractable small animal model for studying mechanisms of gammaherpesvirus latency and chronic disease (6-8, 28, 32, 34, 35, 45, 47).
Acute γHV68 infection in mice lasts for about 2 weeks, after which latency is established (4, 43, 46). γHV68 establishes a latent infection in B cells, macrophages, dendritic cells, and perhaps epithelial cells (9, 33, 36, 46, 49). In addition to being a site of γHV68 latency, B cells play a number of different roles in γHV68 pathogenesis. After intraperitoneal (i.p.) inoculation, B cells are required for normal levels of splenic replication during acute infection but are not required for the establishment of splenic latency (46, 48). After intranasal inoculation of γHV68 into B-cell-deficient (B-cell−/−) mice, ex vivo reactivating virus was detectable in peritoneal cells but not in the spleen at 7 weeks postinfection (p.i.) (48). Another report also indicates that B cells are required for splenic latency when the virus is administered by the intranasal route of infection (41). In addition, B-cell−/− mice die over 200 days of infection and develop severe chronic arteritis restricted to the great elastic arteries (47). Elastic arteritis is due to the chronic productive infection of smooth muscle cells of the elastic media (6-8, 47). Additional data demonstrating the presence of ongoing productive infection in B-cell−/− mice was provided by Stewart et al., who found evidence for replicative linear forms of the γHV68 genome in the lungs of γHV68-infected B-cell−/− mice 8 weeks after infection (33). In addition to ongoing productive infection in specific anatomic sites, B-cell−/− mice exhibit abnormal regulation of latency with increases in both the number of splenocytes and peritoneal cells that reactivate ex vivo from latency and the number of peritoneal cells carrying the latent viral genome (46, 48).
While B-cell−/− mice have a number of abnormalities during γHV68 infection, the mechanism(s) responsible for these changes in pathogenesis is not known. One possible role for B cells is in the secretion of virus-specific antibody. The virus-specific antibody response to γHV68 develops slowly (30) and is long lasting, but the importance of sustained humoral immunity is not completely understood. Kim et al. have shown that antibody can limit recrudescence from latency in T-cell-depleted CD28−/− mice, showing that antibody can contribute to the control of chronic infection in immunodeficient mice (21). This observation is similar to that made in the studies of Polic et al. demonstrating an increased dissemination of murine cytomegalovirus in T-cell-depleted B-cell−/− mice compared to that in T-cell-depleted normal mice (24).
To determine whether there is a role for virus-specific antibody in controlling the establishment, maintenance, and/or ex vivo reactivation of γHV68 from latency, we used the passive transfer of antibody into B-cell−/− mice. We found that polyclonal antiviral antibody and antibody specific for a lytic cycle viral protein can play a significant role in the regulation of γHV68 latency. These data support a model in which persistent, productive viral replication contributes to the high levels of latency observed in immunocompromised hosts. Studies using the viral DNA synthesis inhibitor cidofovir further support this model.
MATERIALS AND METHODS
Viruses and tissue culture.
γHV68 clone WUMS (ATCC VR1465) was passaged and grown, and the titers of the virus in NIH 3T12 cells or BALB/c or C57BL/6 murine embryonic fibroblasts (MEFs) were determined as described previously (43). Viral stocks were generated from NIH 3T12 cells infected at a multiplicity of infection of 0.05 and harvested at a 50% cytopathic effect (CPE) (43).
Mice, infections, and organ harvests.
B-cell−/− mice backcrossed onto a C57BL/6 background (46) were bred and maintained at the Washington University School of Medicine, Saint Louis, Mo., in accordance with all federal and university policies. C57BL/6J (B6) mice were purchased from the Jackson Laboratory. Unless otherwise stated, mice were age and sex matched, used between 7 to 10 weeks of age, and infected with 106 PFU of γHV68 by i.p. injection in 0.5 ml of complete Dulbecco's modified Eagle medium (4, 43). For the data presented here, peritoneal cells and spleen tissue were harvested and processed as described previously from groups of five mice per experiment in two independent experiments (10 mice total per condition) (4, 43, 46).
Quantitation of cells harboring the γHV68 genome.
The frequency of cells harboring the γHV68 genome was determined by a limiting-dilution nested-PCR assay that amplifies γHV68 gene 50 sequences with single-copy sensitivity (48, 49). Briefly, peritoneal cells and splenocytes were frozen in 10% dimethyl sulfoxide at −80°C, thawed, counted, resuspended in isotonic buffer, and serially diluted into 96-well PCR plates. Uninfected NIH 3T12 cells were added so that each well contained 104 cells. Cells were then lysed in proteinase K, and nested PCR was performed (48, 49). Ten PCRs were analyzed for each cell dilution, with six dilutions per sample per experiment. Control reactions in each experiment included uninfected cells alone (six reactions/plate) or cells with 10, 1, or 0.1 copies of plasmid DNA containing the target sequence (six reactions/plate each). There were no false-positive reactions in the assays reported here, and all assays demonstrated approximately one-copy sensitivity for plasmid DNA, with control reactions containing 10, 1, and 0.1 copies of plasmid DNA positive in 88, 39, and 6% of all reactions, respectively.
Ex vivo reactivation from latency.
The frequency of cells reactivating from latency ex vivo was determined by using a limiting-dilution reactivation assay (46, 48). Briefly, peritoneal cells and splenocytes were harvested 42 or 65 days p.i. and plated in a serial twofold dilution (starting at 4 × 104 peritoneal cells and 105 splenocytes per well) onto MEF monolayers in 96-well plates. Twenty-four wells were plated per dilution, and 12 dilutions were plated per sample. Wells were scored microscopically 21 days later for viral CPE. When CPE was difficult to discern at high cell numbers, wells were replated onto fresh MEFs and the CPE was assessed. Preformed virus in tissues was detected by plating parallel cell samples that had been subjected to mechanical disruption. Mechanical disruption does not inactivate virus but kills >99% of cells, and thus, samples treated in this way detect preformed virus rather than virus reactivating from latency (46, 48, 49).
Serum harvest, passive transfer, and antibody ELISA.
For serum collection, peripheral blood was collected from the retro-orbital sinus of the donor (naive or day 42-γHV68-immune mice) or recipient B-cell−/− mice and serum separation was performed by using Microtainer serum separator tubes (Becton Dickinson, Franklin Lakes, N.J.) and then stored at −20°C until passive transfer into mice or determination of antibody concentration by enzyme-linked immunosorbent assay (ELISA). Rabbit polyclonal γHV68-specific antiserum was raised by injecting 6 × 106 PFU of virus (passaged in RK13, a rabbit kidney cell line with 10% rabbit serum) and harvesting serum at 4 weeks after the first boost (47). Serum collected prior to infection served as a preimmune control. In passive antibody transfer experiments, 100 μl of naive or γHV68-immune (mouse or rabbit) serum was given i.p. For the γHV68-specific immunoglobulin G (IgG) assay, plates were coated with a 1% final concentration of paraformaldehyde-fixed viral antigen or mock antigen in coating buffer (0.1 M Na2CO3, 0.2% NaN3, pH 9.6). For assay of total IgG by ELISA, plates were coated with a 1-μg/ml concentration of donkey anti-mouse IgG (Jackson Immunoresearch, West Grove, Pa.) or donkey anti-rabbit IgG (Jackson Immunoresearch) in coating buffer, incubated at 37°C for 2 h, and blocked with 3% bovine serum albumin in coating buffer. After washing the plates with ELISA wash buffer (0.15 M NaCl, 0.05% Tween 20), serum samples or mouse or rabbit IgG standards were added to each well after a serial threefold dilution in ELISA III buffer (0.15 M NaCl, 0.001 M EDTA, 0.05 M Tris-HCl, 0.05% Tween 20, 0.1% bovine serum albumin) and then incubated at 37°C for 3 h. Plates were then washed, and horseradish peroxidase-conjugated goat anti-mouse (Jackson Immunoresearch) or goat anti-rabbit (Jackson Immunoresearch) IgG was added at a 1:5,000 dilution and incubated for 2 h at 37°C. Plates were then washed, and 50 μl of ABTS substrate [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid), diamino salt (Sigma A1888)] diluted in substrate buffer (0.1 M sodium citrate, 0.016% [vol/vol] H2O2) was added. The reaction was stopped after 10 min with 0.2 N 85% phosphoric acid, and the plates were read in a microplate reader (model 550; Bio-Rad Labs, Hercules, Calif.). The concentration of antibody in the serum samples was determined by using a standard curve generated by serially diluting mouse IgG (Jackson Immunoresearch) or rabbit IgG (Jackson Immunoresearch) in the same assay.
Virus neutralization assay.
Virus neutralization assays were performed using an adaptation of a previously described assay (15, 22). Briefly, 104 PFU of virus in 80 μl was incubated with 10 μl of various dilutions of antibody at 37°C for 1 h, followed by plaque assay to quantitate the amount of virus. Wild-type γHV68 incubated with rabbit serum generated to γHV68 served as the positive control (47). For the negative control, a γHV68 RCA protein-deficient virus was incubated with rabbit antiserum generated to recombinant γHV68 regulator of complement activation (RCA) protein (18). Percent neutralization was measured based on virus titer in samples containing no antibody.
Antiviral therapy.
As described previously (6, 8), cidofovir (Vistide; Gilead Sciences, Foster City, Calif.) was diluted in low-endotoxin phosphate-buffered saline (PBS) to 6.25 mg/ml and filter sterilized. γHV68-infected B-cell−/− mice were given cidofovir or PBS subcutaneously every 3 days beginning from day 16 in the scruff of the neck at a dose of 25 mg/kg of body weight (80 to 150 μl/mouse). Individual mice were weighed prior to each injection.
Statistical methods.
All data were analyzed by using GraphPad Prism software (San Diego, Calif.). Frequencies were obtained from the cell number at which 63% of the wells scored positive for either reactivating virus or the presence of viral genome based on Poisson distribution. Data were subjected to nonlinear regression analysis to obtain the single-cell frequency for each limiting-dilution analysis. The frequencies of reactivation and genome-positive cells were statistically analyzed by paired t test. Data from virus neutralization assays were analyzed by using the Mann-Whitney test.
RESULTS
Passive transfer of antiviral antibodies into γHV68-infected B-cell−/− mice decreases the frequency of cells that reactivate from latency ex vivo.
Previous reports showed that acute infection in peritoneal cells and splenocytes is cleared and latency is established by 16 days after infection in B6 (4, 43, 46) and B-cell−/− (48) mice. This was confirmed by the lack of significant preformed infectious virus in mechanically disrupted samples throughout the experiments reported here. To address the role of virus-specific antibody in the regulation of γHV68 latency, we infected B-cell−/− mice and waited until clearance of acute infection before passively transferring immune or naive mouse serum on days 16, 23, and 30 of infection (Fig. 1, Protocol A). We delayed passive transfer until day 16 to avoid antibody effects on acute infection that might alter γHV68 latency. On day 42 of infection, we determined the frequency of latently infected cells that reactivated from latency ex vivo. B-cell−/− mice that did not receive any serum served as a control for the administration of nonimmune antiserum.
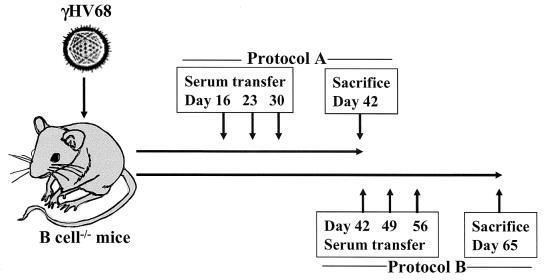
FIG. 1.
Serum transfer protocol. Groups of B-cell−/− mice were infected with 106 PFU of γHV68 i.p. and transferred i.p. with serum by following either protocol A (days 16, 23, and 30 p.i.) or protocol B (days 42, 49, and 56 p.i.). Serum recipient B-cell−/− mice and untreated B-cell−/− mice were sacrificed on day 42 or 65 for analysis of latency.
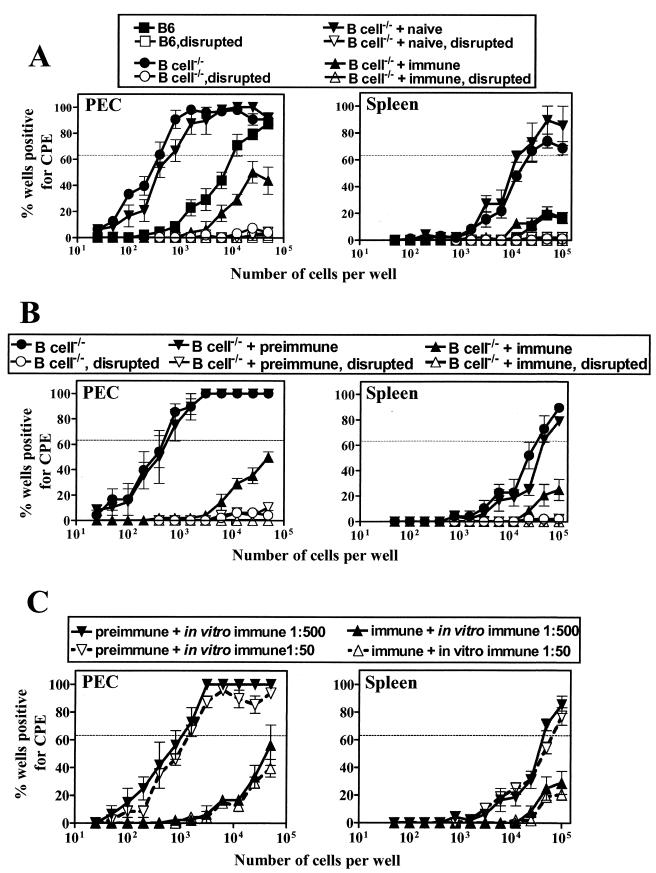
As reported previously (48), the frequency of peritoneal cells reactivating ex vivo from latency in B-cell−/− mice was about 30-fold higher in peritoneal cells (P = 0.0005; Fig. 2A, left panel) and >5-fold higher in spleen (P = 0.008; Fig. 2A, right panel) than in B6 mice. The precise increase in latently infected spleen cells in B-cell−/− mice compared to that in B6 mice could not be determined since the frequency of cells reactivating from latency in B6 splenocytes did not reach a sufficiently high level to allow the application of Poisson statistics.
FIG. 2.
γHV68-specific antibody transfer decreases the frequency of ex vivo reactivation and genome-positive cells. γHV68-infected B-cell−/− mice were transferred with mouse serum (naive or γHV68-immune) by using transfer protocol A (A) or rabbit serum (preimmune or γHV68-immune) by using transfer protocol B (B) (Fig. 1). Control B6 and B-cell−/− mice did not receive any serum. Peritoneal cells (PEC) and splenocytes (Spleen) were harvested at 42 days p.i. (panel A) or 65 days p.i. (panel B) for quantitation of the frequency of cells reactivating from latency. (C) Continuous lines with filled symbols represent reactivation cultures to which immune serum was added at a 1:500 dilution; dashed lines with open symbols represent reactivation cultures to which immune serum was added at a 1:50 dilution. Preimmune and immune refer to in vivo treatment; in vitro immune refers to serum added to the ex vivo reactivation assay. Data represent the results from two separate experiments (mean ± standard error of the mean), with each experiment containing cells pooled from five mice.
Strikingly, peritoneal cells from B-cell−/− mice given immune serum showed a >100-fold decrease in the frequency of cells reactivating from latency (Fig. 2A, left panel) compared to that of the B-cell−/− mice given naive mouse serum (P = 0.0001) or untreated B-cell−/− mice (P = 0.0001). Splenocytes from the same mice showed a >10-fold decrease in cells reactivating from latency in mice given immune serum (Fig. 2A, right panel) compared to that in the mice given naive serum (P = 0.008) or untreated B-cell−/− mice (P = 0.009).
We confirmed these results obtained with mouse antiserum by using polyclonal anti-γHV68 antiserum from rabbits (47) (Fig. 1, Protocol B, and 2B). γHV68-infected B-cell−/− mice were treated with either preimmune or γHV68-immune rabbit serum, and the frequency of peritoneal cells and splenocytes reactivating from latency was determined 65 days after infection. As shown in Fig. 2B (left panel), peritoneal cells from γHV68-immune serum recipient mice showed a >100-fold decreased reactivation in peritoneal cells compared to that found in the mice given preimmune serum (P = 0.0001) or untreated B-cell−/− mice (P = 0.0001). Splenocytes from the same mice showed a >5-fold decrease in cells reactivating from latency in mice given immune serum (Fig. 2B, right panel) compared to that from the mice given preimmune serum (P = 0.02) or untreated B-cell−/− mice (P = 0.01).
We considered the possibility that decreased reactivation from latency after the passive transfer of antiviral antibody was due to effects of antibody in the ex vivo reactivation assay rather than in vivo effects relevant to the control of viral latency. We viewed this as unlikely since cells were washed extensively prior to coculture with MEFs to detect reactivation from latency. To experimentally address this concern, we tested the effects of anti-γHV68 antibody added directly to the ex vivo reactivation assay. Direct addition of γHV68-immune serum (1:500 or 1:50 dilution) in vitro to the reactivation cultures did not alter the frequency of cells reactivating from latency compared to that of the reactivation cultures with no antiserum added (Fig. 2C).
Antiviral antibody decreases the frequency of cells that carry the viral genome in B-cell−/− mice.
Changes in the frequency of cells reactivating ex vivo from latency may be due to changes in the frequency of latently infected cells or changes in the efficiency with which cells reactivate from latency in ex vivo reactivation cultures (38). We therefore determined the effects of antiviral antibody on the frequency of cells carrying the viral genome by using a limiting-dilution PCR assay.
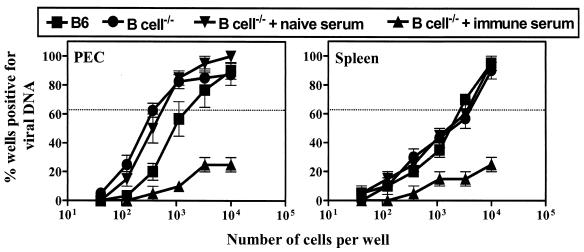
While the frequency of spleen cells carrying the latent viral genome was comparable in B6 and B-cell−/− mice (Fig. 3, right panel), peritoneal cells from B-cell−/− mice had fivefold more cells carrying the latent viral genome than the peritoneal cells from B6 mice (P = 0.054; Fig. 3, left panel). This is in agreement with a previous report on the frequency of cells carrying the viral genome in latently infected mice (48). The decrease in the frequency of cells reactivating from latency was associated with a >50-fold decrease in the frequency of peritoneal cells carrying the latent viral genome in mice given immune mouse serum (Fig. 3, left panel) compared to that in the mice given naive serum (P = 0.014) or untreated B-cell−/− mice (P = 0.006). The frequency of splenocytes carrying the viral genome was decreased >5-fold by the transfer of immune mouse serum (Fig. 3, right panel) compared to that of the transfer of naive serum (P = 0.02) or untreated B-cell−/− mice (P = 0.03). Similar to the results found with the transfer of mouse serum, transfer of immune rabbit serum decreased the frequency of genome-positive cells compared to that in either the preimmune serum-treated or untreated B-cell−/− mice (data not shown). These data show that passive transfer of immune serum dramatically decreased the frequency of cells carrying the latent viral genome.
FIG. 3.
γHV68-specific antibody transfer decreases the frequency of genome-positive cells. Groups of B-cell−/− mice were infected with 106 PFU of γHV68 and transferred with naive or γHV68-immune mouse serum by using transfer protocol A (Fig. 1). Control B6 and B-cell−/− mice did not receive any serum. Peritoneal cells (PEC) and splenocytes (Spleen) were harvested at day 42 p.i. for quantitation of the frequency of latently infected cells. Data represent the results from two separate experiments (mean ± standard error of the mean), with each experiment containing cells pooled from five mice.
Effects of antibody on latency occur at physiologically relevant antibody concentrations.
To determine whether the effects of antibody we observed occurred at physiologically relevant antibody concentrations, we analyzed donor and recipient antibody levels. Analysis of the transferred serum for total IgG and virus-specific IgG by ELISA showed that the transferred (100 μl) mouse and rabbit serum contained similar concentrations of total IgG and virus-specific IgG (Table 1). These antibody concentrations are the physiologic levels seen in mice or rabbits that have recovered from γHV68 infection. Analysis of serum from recipient B-cell−/− mice terminated at day 42 or 65 p.i. showed that both total IgG and virus-specific IgG were detectable. Comparison of antibody concentrations in the serum of normal mice after infection to concentrations in B-cell−/− recipients of immune serum showed that there was 30-fold less total IgG and 10- to 12-fold less γHV68-specific IgG in recipients of passively transferred antiserum than that observed in normal mice after infection (Table 1). These data show that the effects of the passive transfer of antiviral antibody on viral latency observed in B-cell−/− mice occur at antibody concentrations that are <10% of those observed in normal mice after infection.
TABLE 1.
Concentration of total IgG and virus-specific IgG in mouse and rabbit sera before and after transfer to B cell−/− mice
| Serum | Total IgG (μg/ml) | γHV68-specific IgG (μg/ml)a |
|---|---|---|
| Donor serum | ||
| Mouseb | ||
| Naive | 5,661 | UD |
| γHV68-immune | 6,113 | 192 |
| Rabbitc | ||
| Preimmune | 6,831 | UD |
| γHV68-immune | 8,642 | 170 |
| Recipient serumd | ||
| Mouse | ||
| Naive | 180 ± 16 | UD |
| γHV68-immune | 487 ± 110 | 15 ± 3 |
| Rabbit | ||
| Naive | 230 ± 32 | UD |
| γHV68-immune | 795 ± 157 | 25 ± 4 |
UD, undetectable.
Values represent the concentration of total IgG and γHV68-specific IgG in donor serum samples pooled from 10 individual mice.
Values represent the concentration of total IgG and γHV68-specific IgG in the serum from a single rabbit before and after infection with γHV68.
Values (mean ± standard error of the mean) represent the concentration of total IgG and γHV68-specific IgG in serum samples from five recipient B-cell−/− mice per experiment in two separate experiments.
Antibodies specific to a γHV68-lytic antigen regulate the frequency of latently infected cells in B-cell−/− mice.
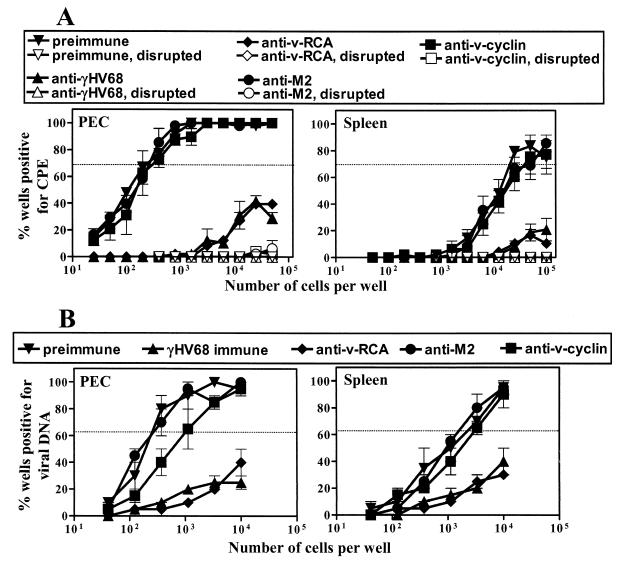
To determine the antigen specificity of the anti-γHV68 antibody that regulated latency, polyclonal rabbit sera specific for the γHV68 lytic cycle protein v-RCA (expressed on the virion surface [see below], on the infected cell surface [19], and as a soluble form [19]), the lytic cycle protein v-cyclin (expressed intracellularly) (43), and the latent γHV68 protein M2 (expressed intracellularly in latent cells) (14, 16) were used for passive-transfer experiments. Groups of γHV68-infected B-cell−/− mice were given antiserum on days 16, 23, and 30 p.i. (Fig. 1, Protocol A). B-cell−/− mice that received preimmune rabbit serum or the polyclonal γHV68-immune rabbit serum served as the control groups. Peritoneal cells and splenocytes were harvested on day 42 p.i., and the frequency of ex vivo reactivation and genome-bearing cells was determined. Interestingly, v-RCA-specific serum recipient B-cell−/− mice showed a frequency of reactivating peritoneal cells >100-fold lower (P = 0.0001; Fig. 4A, left panel) than that for mice receiving preimmune serum, M2-specific antiserum, or v-cyclin-specific antiserum. Splenocytes from the same mice showed a >5-fold decrease in cells reactivating from latency in mice given v-RCA-specific antiserum (Fig. 4A, right panel) compared to that in mice given preimmune serum (P = 0.012), M2-specific antiserum (P = 0.017), or v-cyclin-specific antiserum (P = 0.014). Also, the v-RCA-specific antiserum recipient B-cell−/− mice showed a >50-fold decreased frequency of genome-bearing peritoneal cells (P = 0.0001; Fig. 4B, left panel) compared to that for the mice that received preimmune serum, M2-specific antiserum, or v-cyclin-specific antiserum. The frequency of splenocytes carrying the viral genome was decreased >5-fold by the transfer of v-RCA-specific antiserum (Fig. 4B, right panel) compared to that by the transfer of preimmune serum (P = 0.021), M2-specific antiserum (P = 0.034), or v-cyclin-specific antiserum (P = 0.043).
FIG. 4.
Antibody to a viral lytic cycle protein regulates the frequency of genome-positive cells and ex vivo reactivation. Groups of B-cell−/− mice were infected with 106 PFU of γHV68 and given preimmune serum, γHV68-specific antiserum, or γHV68 protein-specific antiserum by following transfer protocol A (Fig. 1). Peritoneal cells (PEC) and splenocytes (Spleen) were harvested at 42 days p.i. for quantitation of the frequency of cells reactivating virus (A) and the frequency of latently infected cells (B). Data represent the results from two experiments (mean ± standard error of the mean), with each experiment containing cells pooled from five mice.
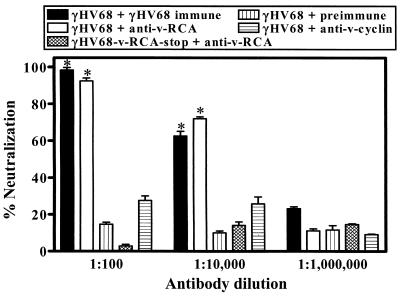
We characterized the antisera for specific viral proteins in neutralization assays to determine whether the target proteins were expressed on the virion. γHV68-specific antiserum and RCA-specific antiserum efficiently neutralized γHV68 (Fig. 5). Virus neutralization occurred at equivalent levels with γHV68-specific antiserum (P = 0.0002 at a 1:100 dilution and P = 0.0004 at a 1:10,000 dilution) and v-RCA-specific antiserum (P = 0.0003 at a 1:100 dilution and P = 0.0002 at a 1:10,000 dilution) compared to that with preimmune serum (Fig. 5). Virus neutralization by anti-RCA protein antibody was protein specific since a v-RCA protein-deficient virus (γHV68-RCA-stop) (18) was not neutralized by the anti-RCA polyclonal antiserum but rather was neutralized by polyclonal anti-γHV68 antiserum (Fig. 5). These data show that the γHV68 RCA protein is present on the surface of the virion, as well as on infected cells and in a secreted form (19).
FIG. 5.
γHV68-specific antibody and v-RCA-specific antibodies neutralize lytic virus in vitro. γHV68 virus was incubated with dilutions of antibody and plaque assayed. As a positive control, wild-type γHV68 was incubated with rabbit antiserum generated to γHV68 (47). As a negative control, a γHV68 RCA protein-deficient virus was incubated with rabbit antiserum generated to recombinant γHV68 RCA protein. Percent neutralization was measured based on virus titer in samples containing no antibody. Data (mean ± standard error of the mean) represent the results from two independent experiments.
Antiviral therapy decreases the frequency of viral genome-positive cells in B-cell−/− mice.
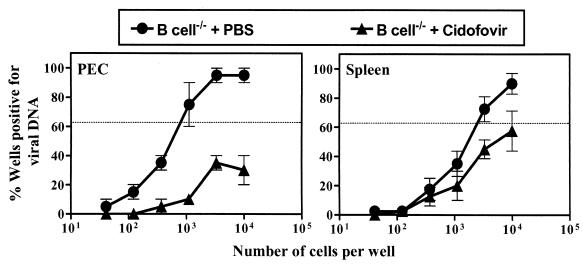
The above data show that antibody can effectively limit the frequency of latently infected cells in B-cell−/− mice and that antibody specific for a lytic cycle protein is effective in this role. Since previous work demonstrated replicative linear forms of γHV68 genome in lungs of γHV68-infected B-cell−/− mice 8 weeks after infection (33) and we have noted persistent replication in arteritic lesions in these mice (unpublished observations), these data suggest a model in which antibody to a lytic cycle protein can decrease the frequency of latently infected cells by breaking a cycle in which lytic replication contributes to the pool of latently infected cells in B-cell−/− mice. If this is the case, then interruption of lytic replication with a viral DNA synthesis inhibitor should decrease the frequency of cells carrying latent viral genome in B-cell−/− mice. Treatment with the antiviral drug 4′-S-EtdU can delay the establishment of latency (1), providing further support for this hypothesis. To test this prediction, γHV68-infected B-cell−/− mice were treated with either cidofovir or PBS by following a previously established protocol (6, 8). The dose of cidofovir selected effectively inhibits viral replication to below-detectable levels, even in SCID mice (8). Interestingly, peritoneal cells from cidofovir-treated mice (Fig. 6, left panel) showed a >10-fold decrease in the frequency of viral genome-positive cells compared to the frequency of these cells in PBS-treated mice (P = 0.014). Although the splenocytes in the same experiments (Fig. 6, right panel) showed a decrease in the frequency of viral-genome-positive cells from cidofovir-treated mice, this difference did not reach statistical significance compared to that for the PBS control (P = 0.057). These results suggest that inhibition of virus replication leads to a decrease in genome-positive cells in B-cell−/− mice.
FIG. 6.
Antiviral therapy decreases the frequency of viral genome-positive cells in B-cell−/− mice. Groups of B-cell−/− mice were infected with 106 PFU of γHV68 and treated with either cidofovir or PBS as described in Materials and Methods. Peritoneal cells (PEC) and splenocytes (Spleen) were harvested at 42 days p.i. for quantitation of the frequency of latently infected cells. Data represent the results from two experiments (mean ± standard error of the mean), with each experiment containing cells pooled from five mice.
DISCUSSION
Previous studies have demonstrated the kinetics of humoral immune response to γHV68 (30, 31) and that B cells play a critical role in regulating γHV68 latency (46, 48). However, the mechanisms by which B cells regulate latency are not known. Results from this study provide one such mechanism via demonstration that γHV68-specific antibody can significantly decrease the frequency of latently infected cells found in the spleen and peritoneum of B-cell−/− mice. This confirms the findings of Kim et al. made in CD28−/− mice by demonstrating that antibody can play a key role in the control of gammaherpesvirus latency (21). We further demonstrated that the antibody specific for a late-lytic γHV68 protein, v-RCA, decreased the frequency of latently infected cells, suggesting that antibody works by interacting with virions or productively infected cells. This in turn suggests that ongoing or sporadic productive infection plays an important part in the maintenance of high frequencies of latently infected cells in B-cell−/− mice. Consistent with this model, inhibition of productive infection with an antiviral drug also decreased the frequency of latently infected cells. These studies demonstrating a role for antibody in controlling latency under these experimental conditions provide a strong rationale for investigating the role of antibody in the control of latency and vaccination against latency.
Mechanism of antibody-mediated control of γHV68 latency.
The frequency of cells latently infected with gammaherpesviruses in vivo likely reflects a balance between viral factors and host immunity. However, the contribution of individual host factors to this balance is not yet fully understood. The γHV68-specific humoral response is slow to develop but is maintained at high levels for a long period of time (30, 31). We hypothesized that antibody might play a role in the maintenance of gammaherpesvirus latency and used the passive transfer of antibody to demonstrate that antibody can play a very significant role in limiting the number of cells that are latently infected, at least in B-cell−/− mice.
Several viral genes (4, 16, 43) and host factors (38, 40, 48) regulate γHV68 latency in infected mice. The immune system may control gammaherpesvirus infection at multiple levels, including in altering the frequency of cells carrying the viral genome during latency and/or altering the efficiency with which latently infected cells reactivate in ex vivo cultures (12, 38, 48). Alterations in both the latently infected cell number and the efficiency of reactivation from latency are observed in CD8−/− and gamma interferon−/− mice (12, 38). Since the frequencies of both ex vivo reactivation (>100-fold for peritoneal cells) and viral genome-positive cells (>50-fold for peritoneal cells) are changed with the passive transfer of immune serum in B-cell−/− mice, a significant contributor to the antibody-mediated decrease in the frequency of cells reactivating from latency is a decrease in the number of genome-positive cells rather than the change in efficiency of ex vivo reactivation.
How then does antibody control the frequency of latently infected cells? The antigen specificity of antiviral antibody that can decrease the frequency of latently infected cells is informative. Antibody specific for the v-RCA protein, a lytic cycle protein that is expressed on the surface of infected cells, in a soluble isoform, and on the surface of virions (19), was as effective as polyclonal antibody at decreasing the frequency of latently infected cells. Studies using in situ hybridization failed to detect expression of the v-RCA transcript during latency (27). Thus, the most likely explanation for the effectiveness of antibody to the v-RCA protein in controlling latency is the inhibition of lytic cycle replication in B-cell−/− mice. This hypothesis requires that ongoing virus replication contributes significantly to the high levels of latently infected cells observed in B-cell−/− mice.
It is also possible that antibody to the v-RCA protein altered latency by interacting with the soluble isoform of the v-RCA protein or by altering the nature of antiviral immunity via blocking the complement regulatory effects of the v-RCA protein. We view this as unlikely since the effects of antibody on latency are in the 100-fold range, while the effects of complement factor 3 deficiency on latency are fivefold (18). However, this led us to test the hypothesis that inhibition of lytic cycle infection decreases the frequency of latently infected cells by using an antibody-independent approach. Use of an antiviral drug for this purpose was supported by the findings of Barnes et al., which demonstrated that antiviral therapy with 4′-S-EtdU inhibits γHV68 replication in the lung and delays the establishment of latency (1). We selected the antiviral drug cidofovir for this purpose, since it has proven efficacy in limiting γHV68 replication in vivo during both acute and chronic infection (8, 23). Consistent with a critical role for ongoing productive infection in maintaining high levels of latently infected cells in B-cell−/− mice, treatment with cidofovir significantly decreased the frequency of peritoneal cells carrying viral genome in B-cell−/− mice. However, cidofovir treatment was less effective in limiting latency in the spleen, with decreases in splenic latency not reaching statistical significance. Differences in the efficacy of antibody versus cidofovir in the spleen (with antibody more effective) could be due to several factors. It may be that cidofovir is less bioavailable in the spleen than the peritoneum or that it is more effective at controlling replication in peritoneal than splenic cells.
It is notable that lytic cycle replication occurs in B-cell−/− mice for long periods after initial infection (33, 47) but that lytic replication is restricted to specific tissues once acute infection is cleared. Thus, while preformed infectious virus is not found in the spleen or peritoneum of B-cell−/− mice by using very sensitive limiting-dilution culture assays on MEF monolayers or reverse transcription-PCR assays specific for lytic cycle viral transcripts (44, 48), lytic replication is present in the media of the great vessels and in the lungs of chronically infected B-cell−/− mice (33, 47; unpublished observations). It is tempting to speculate that antibody decreases the frequency of latently infected cells in the spleen and peritoneum by limiting lytic replication elsewhere or by preventing the trafficking of virus or virus-infected cells from sites of productive replication to sites at which we measured latency.
Does antibody control latency in normal mice?
This study only addresses the role of antibody in B-cell−/− mice. We believe that these findings can be generalized for two reasons. First, the effects of passively transferred antibody on latency were detected when concentrations of both total and virus-specific antibody in recipients of immune serum were less than 10% of those observed in normal mice during latent infection. This strongly suggests that the concentration of antiviral antibody present in a normal host is sufficient to play a role in limiting the frequency of latently infected cells. Second, multiple studies show that T cells specific for lytic γHV68 antigens are continuously activated during latent γHV68 infection (2, 11, 13, 27, 29, 30). This suggests that there may be an ongoing productive infection in normal mice at a level too low to be detected by present assays. This could provide a target for the antibody-mediated control of viral latency. We would therefore predict that antibody plays at least some role in the control of γHV68 latency in normal mice. It is important to note, however, that we did not eliminate latent cells by the passive transfer of antibody to B-cell−/− mice and that latency persists in normal mice despite high levels of antibody. This suggests that there are reservoirs of latency not accessible to control by antibody. For example, since latently infected B cells are present in normal mice but absent in the B-cell−/− mice used here, it may be that B cells contribute to an antibody-resistant form of latency in normal mice.
B cells and γHV68 latent infection.
B cells are important during acute, chronic, and latent γHV68 infection (33, 41, 46-48). Does the finding that antibody can regulate latency in B-cell−/− mice explain all of the alterations in γHV68 pathogenesis and latency observed in these mice? We believe the answer is likely no. First, B cells might contribute to the pathogenesis of γHV68 infection by providing a vehicle for spread throughout the host (33), a function perhaps not duplicated by immune antibody. Second, B cells might contribute to the regulation of γHV68 latency via the presentation of antigen to T cells (17, 26). For example, B cells are critical for the induction of Vβ4-bearing CD8+ T cells during γHV68 infection (3, 5, 10, 39). While the physiologic role of these T cells is not known, this is likely a function of B cells per se rather than of secreted antibody (3). For these reasons, it is important to note that antibody is only one mechanism by which B cells contribute to the control of latent γHV68 infection. Our findings do not rule out additional antibody-independent roles of B cells in gammaherpesvirus pathogenesis and immunity.
Acknowledgments
H.W.V. was supported by grant RPG-97-134-01-MBC from the American Cancer Society and NIH grants CA96511, CA74730, and HL60090. S.H.S. was supported by NIH grants CA43143, CA52004, CA58524, and CA74730. S.G. was supported by PHS grant AI071623-23, and S.B.K. was supported by a predoctoral training grant in tumor immunology from the Cancer Research Institute.
We thank members of the laboratories of H.W.V. and S.H.S. and also David Leib and Lynda Morrison for constructive comments on this research. Helpful comments on this report were made by Scott A. Tibbetts, Rebecca L. Sparks-Thissen, Douglas Braaten, and Eric Barton.
REFERENCES
- 1.Barnes, A., H. Dyson, N. P. Sunil-Chandra, P. Collins, and A. A. Nash. 1999. 2′-Deoxy-5-ethyl-beta-4′-thiouridine inhibits replication of murine gammaherpesvirus and delays the onset of virus latency. Antivir. Chem. Chemother. 10:321-326. [DOI] [PubMed] [Google Scholar]
- 2.Belz, G. T., and P. C. Doherty. 2001. Virus-specific and bystander CD8+ T-cell proliferation in the acute and persistent phases of a gammaherpesvirus infection. J. Virol. 75:4435-4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks, J. W., A. M. Hamilton-Easton, J. P. Christensen, R. D. Cardin, C. L. Hardy, and P. C. Doherty. 1999. Requirement for CD40 ligand, CD4+ T cells, and B cells in an infectious mononucleosis-like syndrome. J. Virol. 73:9650-9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clambey, E. T., H. W. Virgin IV, and S. H. Speck. 2000. Disruption of the murine gammaherpesvirus 68 M1 open reading frame leads to enhanced reactivation from latency. J. Virol. 74:1973-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coppola, M. A., E. Flano, P. Nguyen, C. L. Hardy, R. D. Cardin, N. Shastri, D. L. Woodland, and M. A. Blackman. 1999. Apparent MHC-independent stimulation of CD8+ T cells in vivo during latent murine gammaherpesvirus infection. J. Immunol. 163:1481-1489. [PubMed] [Google Scholar]
- 6.Dal Canto, A. J., P. E. Swanson, A. K. O'Guin, S. H. Speck, and H. W. Virgin. 2001. IFN-gamma action in the media of the great elastic arteries, a novel immunoprivileged site. J. Clin. Investig. 107:R15-R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dal Canto, A. J., and H. W. Virgin IV. 2000. Animal models of infection-mediated vasculitis: implications for human disease. Int. J. Cardiol. 75(Suppl. 1):S37-S45. [DOI] [PubMed] [Google Scholar]
- 8.Dal Canto, A. J., H. W. Virgin IV, and S. H. Speck. 2000. Ongoing viral replication is required for gammaherpesvirus 68-induced vascular damage. J. Virol. 74:11304-11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flano, E., S. M. Husain, J. T. Sample, D. L. Woodland, and M. A. Blackman. 2000. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 165:1074-1081. [DOI] [PubMed] [Google Scholar]
- 10.Flano, E., D. L. Woodland, and M. A. Blackman. 1999. Requirement for CD4+ T cells in V beta 4+CD8+ T cell activation associated with latent murine gammaherpesvirus infection. J. Immunol. 163:3403-3408. [PubMed] [Google Scholar]
- 11.Flaño, E., D. L. Woodland, M. A. Blackman, and P. C. Doherty. 2001. Analysis of virus-specific CD4+ T cells during long-term gammaherpesvirus infection. J. Virol. 75:7744-7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gangappa, S., L. F. Van Dyk, T. J. Jewett, S. H. Speck, and H. W. Virgin IV. 2002. Identification of the in vivo role of a viral bcl-2. J. Exp. Med. 195:931-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton-Easton, A. M., J. P. Christensen, and P. C. Doherty. 1999. Turnover of T cells in murine gammaherpesvirus 68-infected mice. J. Virol. 73:7866-7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Husain, S. M., E. J. Usherwood, H. Dyson, C. Coleclough, M. A. Coppola, D. L. Woodland, M. A. Blackman, J. P. Stewart, and J. T. Sample. 1999. Murine gammaherpesvirus M2 gene is latency-associated and its protein a target for CD8(+) T lymphocytes. Proc. Natl. Acad. Sci. USA 96:7508-7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isaacs, S. N., G. J. Kotwal, and B. Moss. 1992. Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc. Natl. Acad. Sci. USA 89:628-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacoby, M. A., H. W. Virgin IV, and S. H. Speck. 2002. Disruption of the M2 gene of murine gammaherpesvirus 68 alters splenic latency following intranasal, but not intraperitoneal, inoculation. J. Virol. 76:1790-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janeway, C. A., Jr., J. Ron, and M. E. Katz. 1987. The B cell is the initiating antigen-presenting cell in peripheral lymph nodes. J. Immunol. 138:1051-1055. [PubMed] [Google Scholar]
- 18.Kapadia, S. B., B. Levine, S. H. Speck, and H. W. Virgin IV. 2000. Critical role of complement and viral evasion of complement in acute, persistent, and latent gamma-herpesvirus infection. Immunity 17:143-155. [DOI] [PubMed]
- 19.Kapadia, S. B., H. Molina, B. van Berkel, V., S. H. Speck, and H. W. Virgin IV. 1999. Murine gammaherpesvirus 68 encodes a functional regulator of complement activation. J. Virol. 73:7658-7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khanna, R., and S. R. Burrows. 2000. Role of cytotoxic T lymphocytes in Epstein-Barr virus-associated diseases. Annu. Rev. Microbiol. 54:19-48. [DOI] [PubMed] [Google Scholar]
- 21.Kim, I. J., E. Flano, D. L. Woodland, and M. A. Blackman. 2002. Antibody-mediated control of persistent γ-herpesvirus infection. J. Immunol. 168:3958-3964. [DOI] [PubMed] [Google Scholar]
- 22.McNearney, T. A., C. Odell, V. M. Holers, P. G. Spear, and J. P. Atkinson. 1987. Herpes simplex virus glycoproteins gC-1 and gC-2 bind to the third component of complement and provide protection against complement-mediated neutralization of viral infectivity. J. Exp. Med. 166:1525-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neyts, J., and C. E. De. 1998. In vitro and in vivo inhibition of murine gamma herpesvirus 68 replication by selected antiviral agents. Antimicrob. Agents Chemother. 42:170-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polic, B., H. Hengel, A. Krmpotic, J. Trgovcich, I. Pavic, P. Luccaronin, S. Jonjic, and U. H. Koszinowski. 1998. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J. Exp. Med. 188:1047-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roizman, B. 1996. Herpesviridae, p. 2221-2230. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott-Raven Publishers, Philadelphia, Pa.
- 26.Ron, Y., and J. Sprent. 1987. T cell priming in vivo: a major role for B cells in presenting antigen to T cells in lymph nodes. J. Immunol. 138:2848-2856. [PubMed] [Google Scholar]
- 27.Simas, J. P., D. Swann, R. Bowden, and S. Efstathiou. 1999. Analysis of murine gammaherpesvirus-68 transcription during lytic and latent infection. J. Gen. Virol. 80:75-82. [DOI] [PubMed] [Google Scholar]
- 28.Speck, S. H., and H. W. Virgin. 1999. Host and viral genetics of chronic infection: a mouse model of gamma-herpesvirus pathogenesis. Curr. Opin. Microbiol. 2:403-409. [DOI] [PubMed] [Google Scholar]
- 29.Stevenson, P. G., G. T. Belz, J. D. Altman, and P. C. Doherty. 1999. Changing patterns of dominance in the CD8+ T cell response during acute and persistent murine gamma-herpesvirus infection. Eur. J. Immunol. 29:1059-1067. [DOI] [PubMed] [Google Scholar]
- 30.Stevenson, P. G., and P. C. Doherty. 1998. Kinetic analysis of the specific host response to a murine gammaherpesvirus. J. Virol. 72:943-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevenson, P. G., and P. C. Doherty. 1999. Non-antigen-specific B-cell activation following murine gammaherpesvirus infection is CD4 independent in vitro but CD4 dependent in vivo. J. Virol. 73:1075-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart, J. P. 1999. Of mice and men: murine gammaherpesvirus 68 as a model. Epstein-Barr Virus Rep. 6:31-35. [Google Scholar]
- 33.Stewart, J. P., E. J. Usherwood, A. Ross, H. Dyson, and T. Nash. 1998. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J. Exp. Med. 187:1941-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sunil-Chandra, N. P., J. Arno, J. Fazakerley, and A. A. Nash. 1994. Lymphoproliferative disease in mice infected with murine gammaherpesvirus 68. Am. J. Pathol. 145:818-826. [PMC free article] [PubMed] [Google Scholar]
- 35.Sunil-Chandra, N. P., S. Efstathiou, J. Arno, and A. A. Nash. 1992. Virological and pathological features of mice infected with murine gammaherpesvirus 68. J. Gen. Virol. 73:2347-2356. [DOI] [PubMed] [Google Scholar]
- 36.Sunil-Chandra, N. P., S. Efstathiou, and A. A. Nash. 1992. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J. Gen. Virol. 73:3275-3279. [DOI] [PubMed] [Google Scholar]
- 37.Taylor, T. J., M. A. Brockman, E. E. McNamee, and D. M. Knipe. 2002. Herpes simplex virus. Front. Biosci. 7:D752-D764. [DOI] [PubMed] [Google Scholar]
- 38.Tibbetts, S. A., L. F. van Dyk, S. H. Speck, and H. W. Virgin IV. 2002. Immune control of the number and reactivation phenotype of cells latently infected with a gammaherpesvirus. J. Virol. 76:7125-7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tripp, R. A., A. M. Hamilton-Easton, R. D. Cardin, P. Nguyen, F. G. Behm, D. L. Woodland, P. C. Doherty, and M. A. Blackman. 1997. Pathogenesis of an infectious mononucleosis-like disease induced by a murine gamma-herpesvirus: role for a viral superantigen? J. Exp. Med. 185:1641-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Usherwood, E. J., D. J. Roy, K. Ward, S. L. Surman, B. M. Dutia, M. A. Blackman, J. P. Stewart, and D. L. Woodland. 2000. Control of gammaherpesvirus latency by latent antigen-specific CD8(+) T cells. J. Exp. Med. 192:943-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Usherwood, E. J., J. P. Stewart, K. Robertson, D. J. Allen, and A. A. Nash. 1996. Absence of splenic latency in murine gammaherpesvirus 68-infected B cell-deficient mice. J. Gen. Virol. 77:2819-2825. [DOI] [PubMed] [Google Scholar]
- 42.van Dyk, L. F., J. L. Hess, J. D. Katz, M. Jacoby, S. H. Speck, and H. W. Virgin IV. 1999. The murine gammaherpesvirus 68 v-cyclin is an oncogene that promotes cell cycle progression in primary lymphocytes. J. Virol. 73:5110-5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Dyk, L. F., H. W. Virgin IV, and S. H. Speck. 2000. The murine gammaherpesvirus 68 v-cyclin is a critical regulator of reactivation from latency. J. Virol. 74:7451-7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Virgin, H. W., IV, R. M. Presti, X.-Y. Li, C. Liu, and S. H. Speck. 1999. Three distinct regions of the murine gammaherpesvirus 68 genome are transcriptionally active in latently infected mice. J. Virol. 73:2321-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Virgin, H. W., and S. H. Speck. 1999. Unraveling immunity to gamma-herpesviruses: a new model for understanding the role of immunity in chronic virus infection. Curr. Opin. Immunol. 11:371-379. [DOI] [PubMed] [Google Scholar]
- 46.Weck, K. E., M. L. Barkon, L. I. Yoo, S. H. Speck, and H. W. Virgin IV. 1996. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J. Virol. 70:6775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weck, K. E., A. J. Dal Canto, J. D. Gould, A. K. O'Guin, K. A. Roth, J. E. Saffitz, S. H. Speck, and H. W. Virgin. 1997. Murine gamma-herpesvirus 68 causes severe large-vessel arteritis in mice lacking interferon-gamma responsiveness: a new model for virus-induced vascular disease. Nat. Med. 3:1346-1353. [DOI] [PubMed] [Google Scholar]
- 48.Weck, K. E., S. S. Kim, H. W. Virgin IV, and S. H. Speck. 1999. B cells regulate murine gammaherpesvirus 68 latency. J. Virol. 73:4651-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weck, K. E., S. S. Kim, H. W. Virgin IV, and S. H. Speck. 1999. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J. Virol. 73:3273-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]