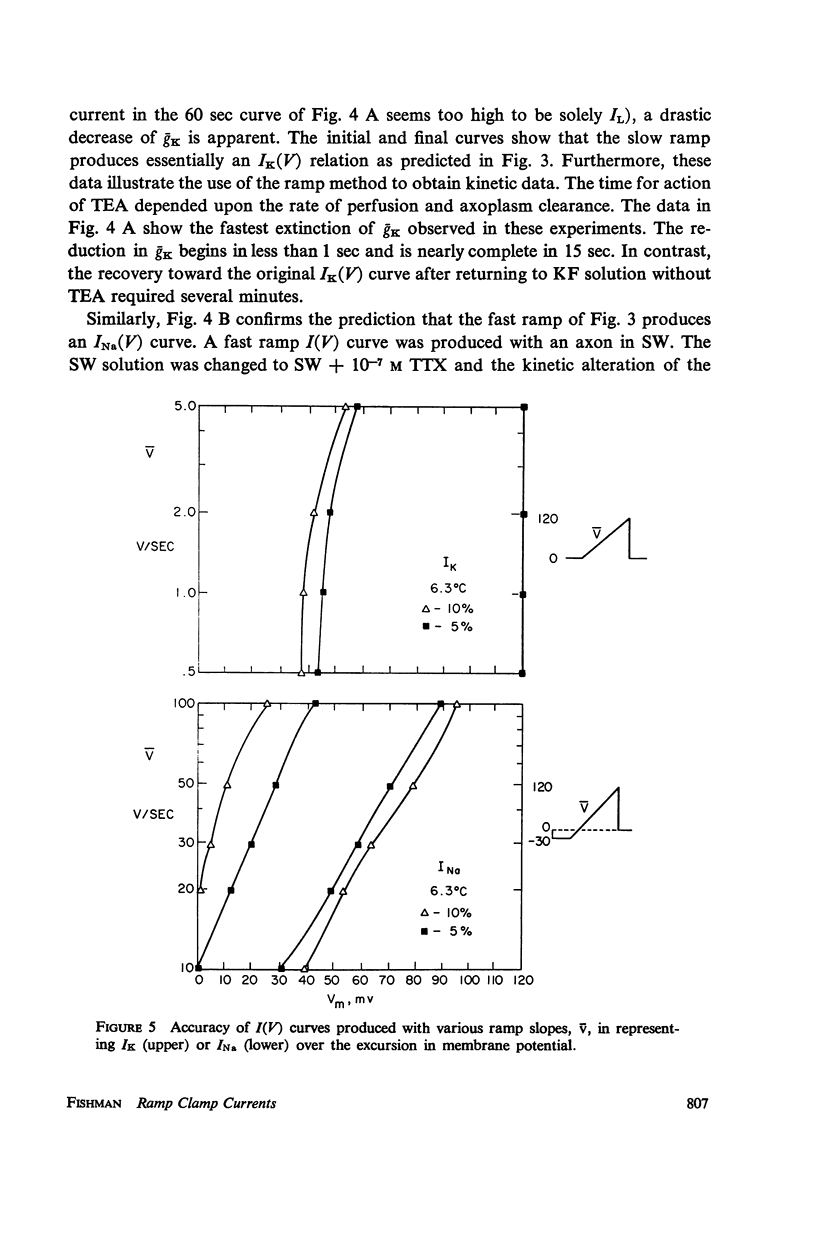
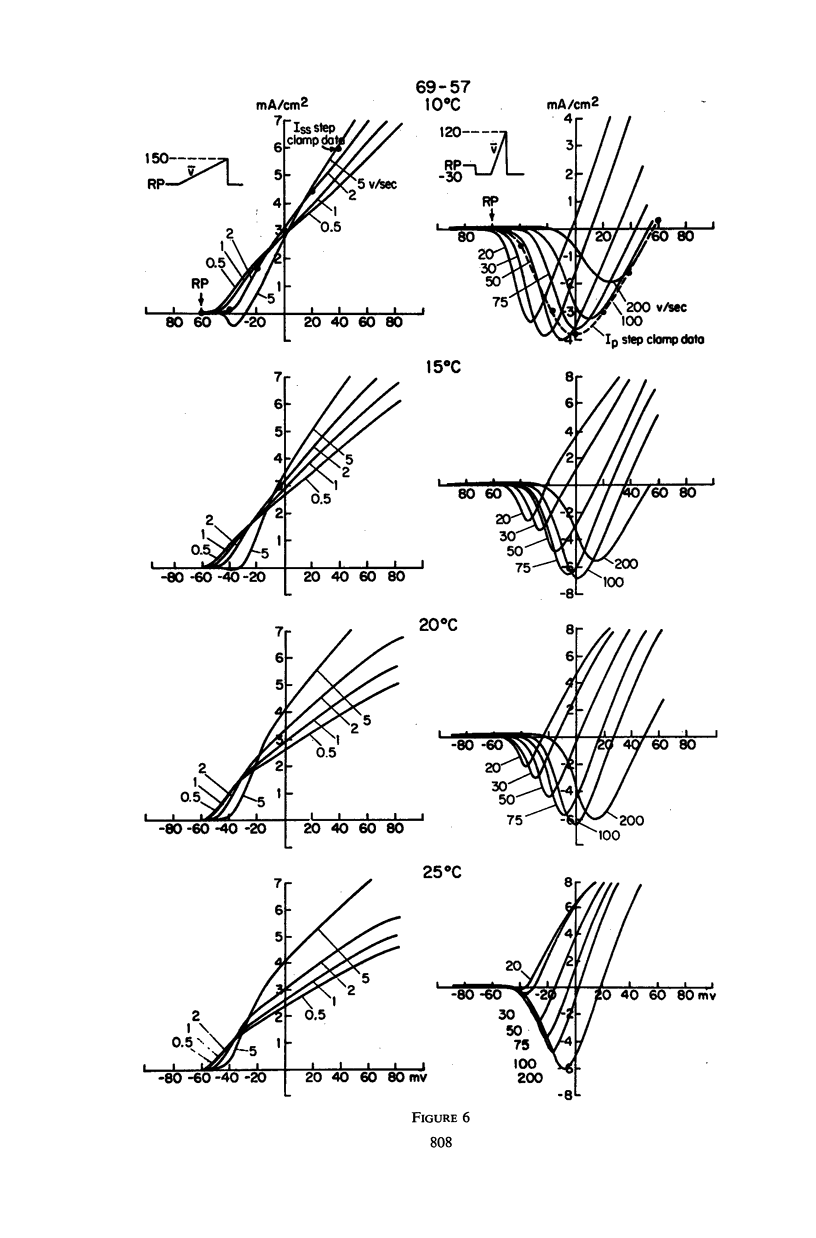
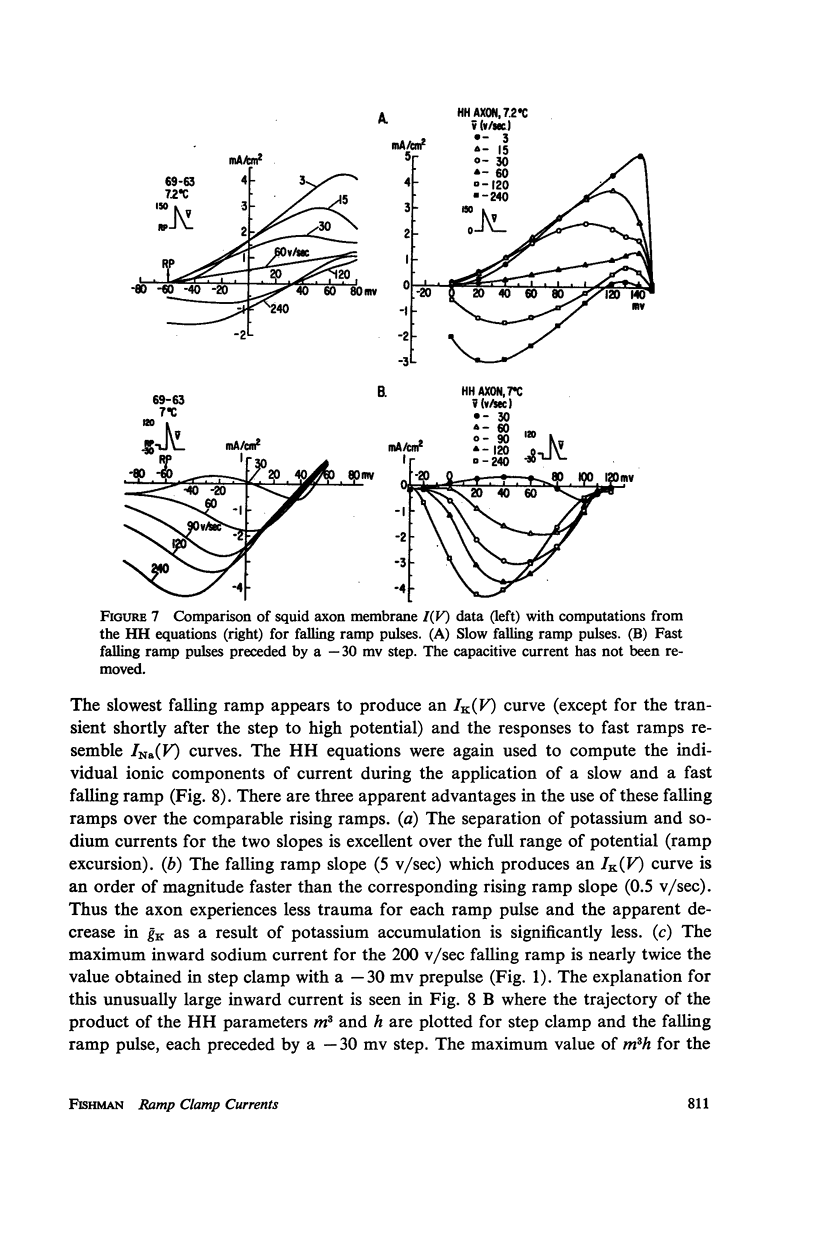
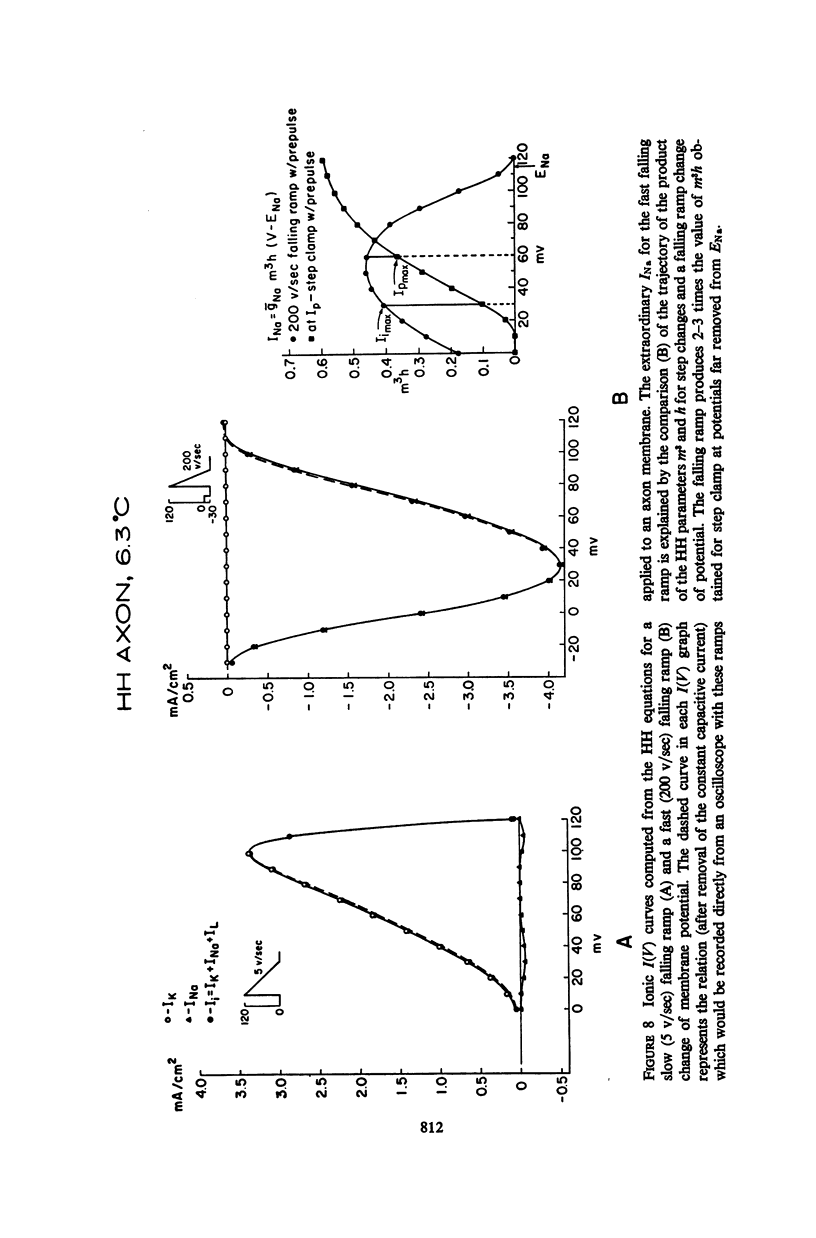
Abstract
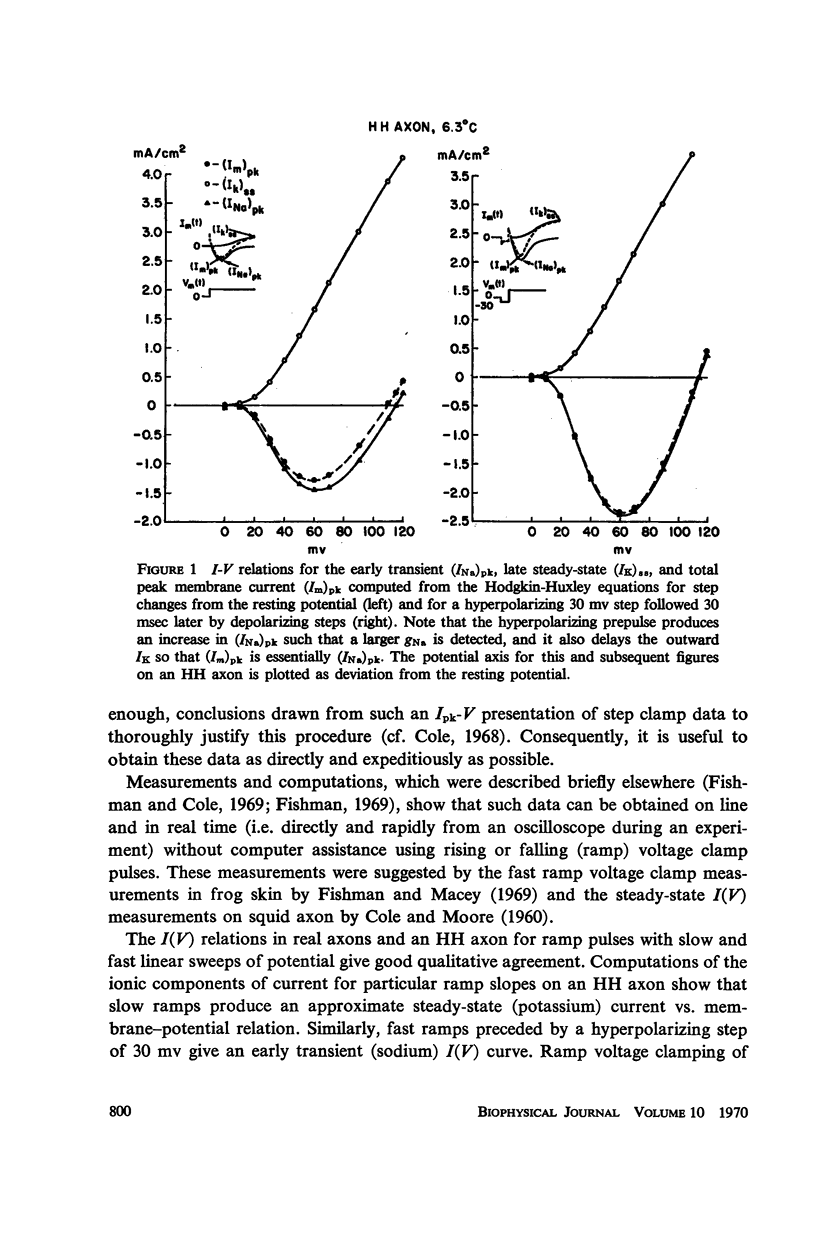
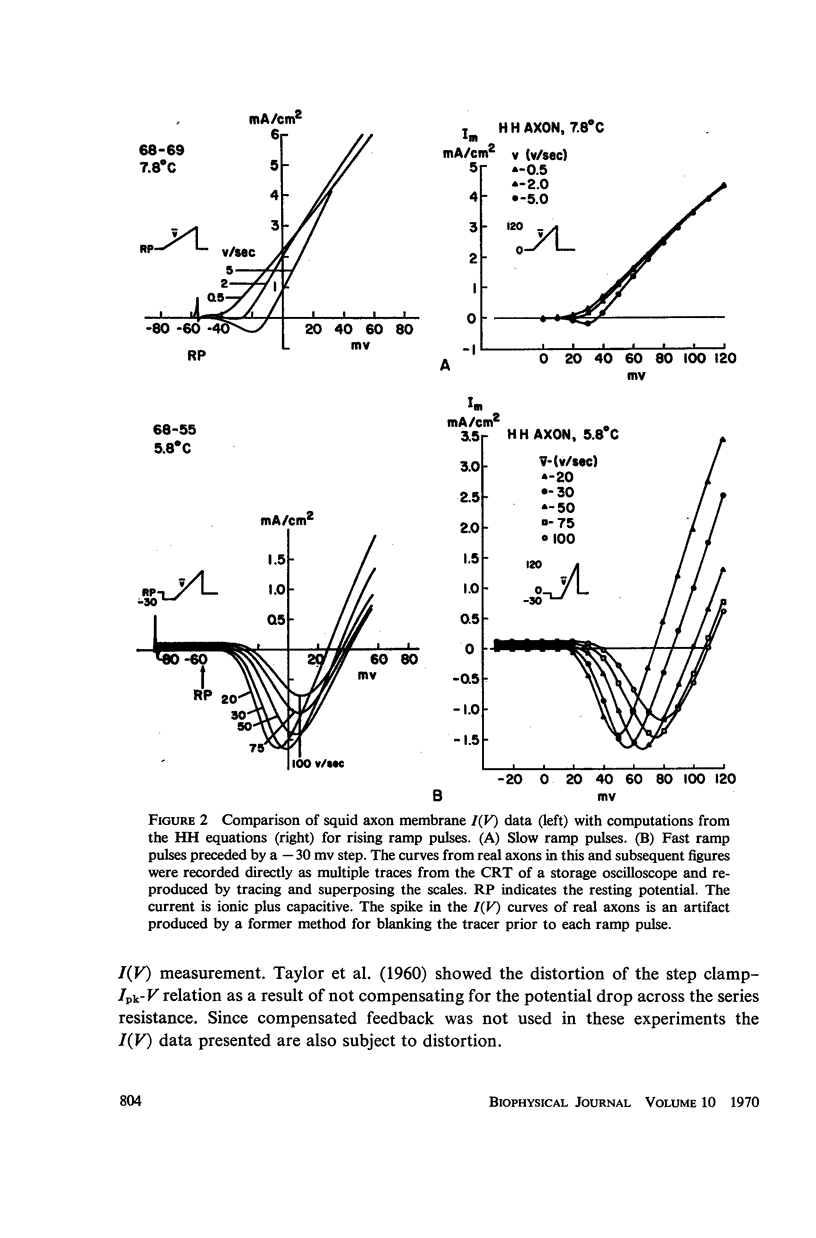
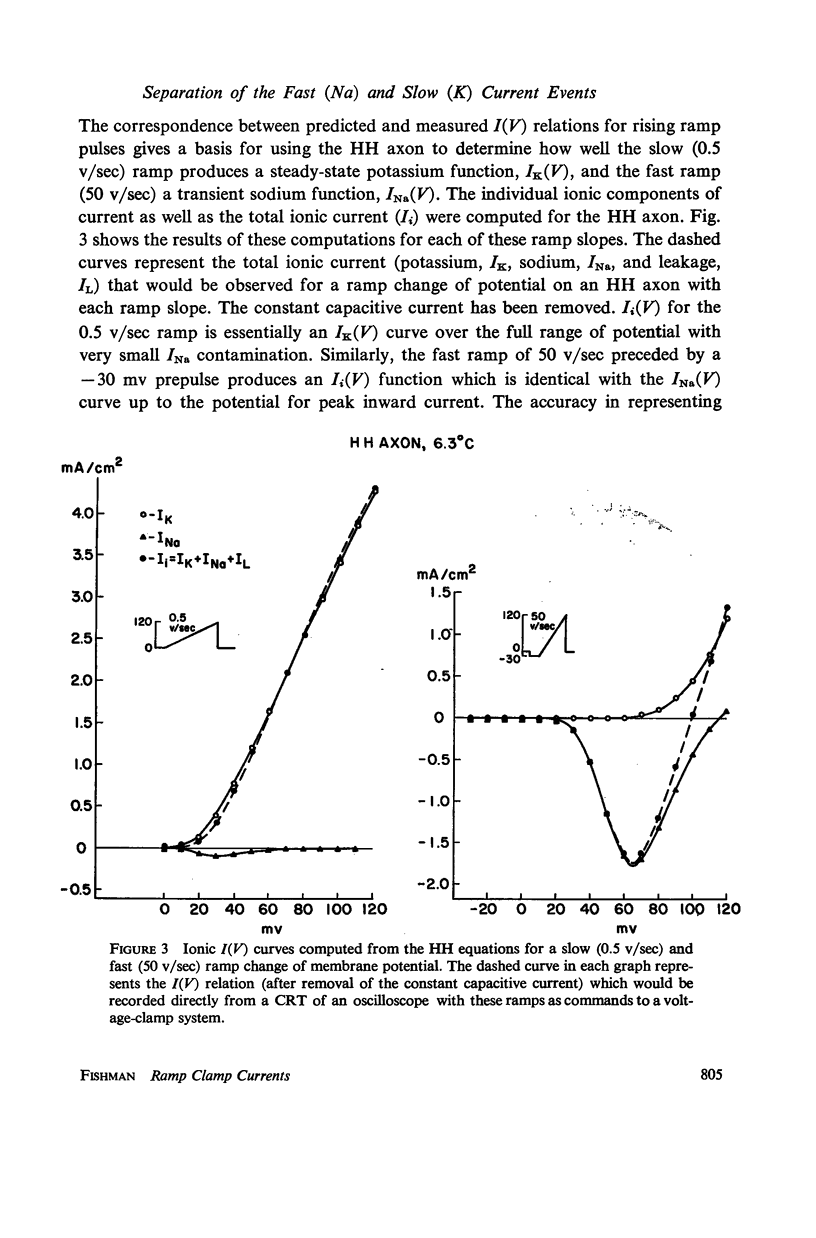
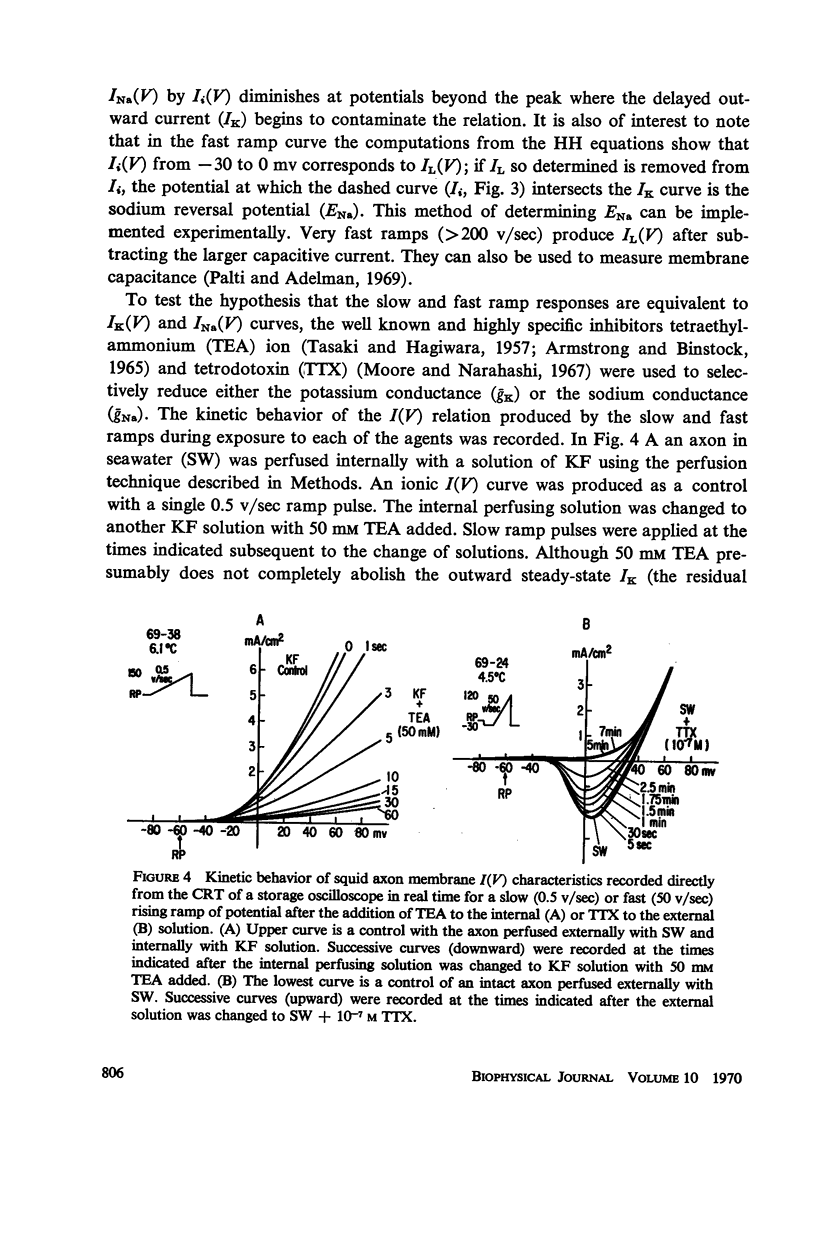
Computations based upon the Hodgkin-Huxley equations and experimental data from squid axons show that ramp functions can be used as commands to a voltage clamp system to selectively observe either the fast (sodium) or slow (potassium) process in axon membranes without chemical separation techniques or computer assistance. Each process is characterized directly (on line) and rapidly (real time) by generating a current-potential curve on an oscilloscope for fast or slow rates of change of membrane potential (ramps). The speed and directness of this method of characterizing each of the essential axonal events permit quantitative measurement of the kinetics of rapid effects on these processes due to various pharmacological agents such as tetrodotoxin and tetraethylammonium ion or other experimental changes in the membrane environment.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwater I., Bezanilla F., Rojas E. Sodium influxes in internally perfused squid giant axon during voltage clamp. J Physiol. 1969 May;201(3):657–664. doi: 10.1113/jphysiol.1969.sp008778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Grundfest H. Analysis of depolarizing and hyperpolarizing inactivation responses in gymnotid electroplaques. J Gen Physiol. 1966 Sep;50(1):141–169. doi: 10.1085/jgp.50.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLE K. S., MOORE J. W. Ionic current measurements in the squid giant axon membrane. J Gen Physiol. 1960 Sep;44:123–167. doi: 10.1085/jgp.44.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Voltage clamp experiments on internally perfused giant axons. J Physiol. 1965 Oct;180(4):788–820. doi: 10.1113/jphysiol.1965.sp007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole K. S., Curtis H. J. ELECTRIC IMPEDANCE OF THE SQUID GIANT AXON DURING ACTIVITY. J Gen Physiol. 1939 May 20;22(5):649–670. doi: 10.1085/jgp.22.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman H. M. Direct recording of K and Na current-potential characteristics of squid axon membrane. Nature. 1969 Dec 13;224(5224):1116–1118. doi: 10.1038/2241116a0. [DOI] [PubMed] [Google Scholar]
- Fishman H. M., Macey R. I. The N-shaped current-potential characteristic in frog skin. 3. Ionic dependence. Biophys J. 1969 Feb;9(2):151–162. doi: 10.1016/s0006-3495(69)86376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman H. M., Macey R. I. The N-shaped current-potential characteristic in frog skin. I. Time development during step voltage clamp. Biophys J. 1969 Feb;9(2):127–139. doi: 10.1016/S0006-3495(69)86374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman H. M., Macey R. I. The N-shaped current-potential characteristic in frog skin. II. Kinetic behavior during ramp voltage clamp. Biophys J. 1969 Feb;9(2):140–150. doi: 10.1016/S0006-3495(69)86375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzhugh R. Theoretical effect of temperature on threshold in the Hodgkin-Huxley nerve model. J Gen Physiol. 1966 May;49(5):989–1005. doi: 10.1085/jgp.49.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W., Narahashi T. Tetrodotoxin's highly selective blockage of an ionic channel. Fed Proc. 1967 Nov-Dec;26(6):1655–1663. [PubMed] [Google Scholar]
- TAYLOR R. E., MOORE J. W., COLE K. S. Analysis of certain errors in squid axon voltage clamp measurements. Biophys J. 1960 Nov;1:161–202. doi: 10.1016/s0006-3495(60)86882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


