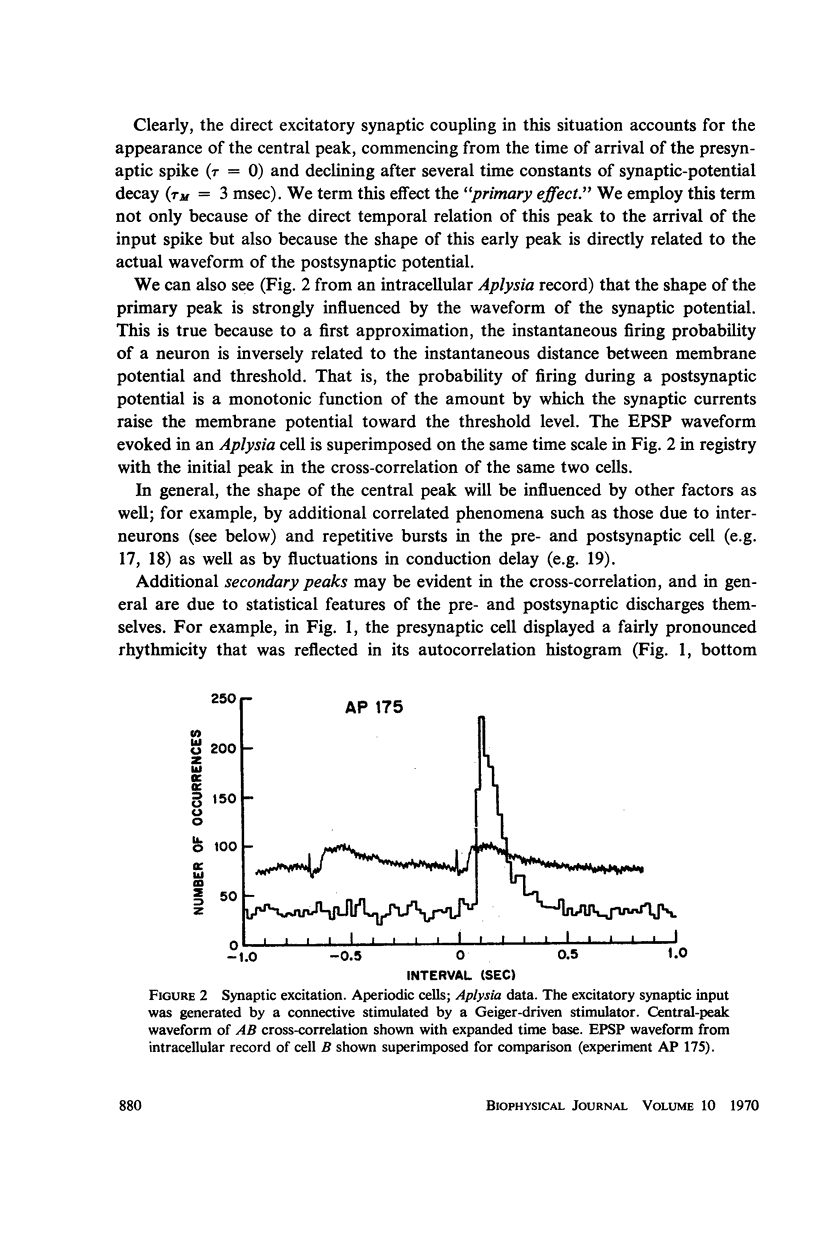
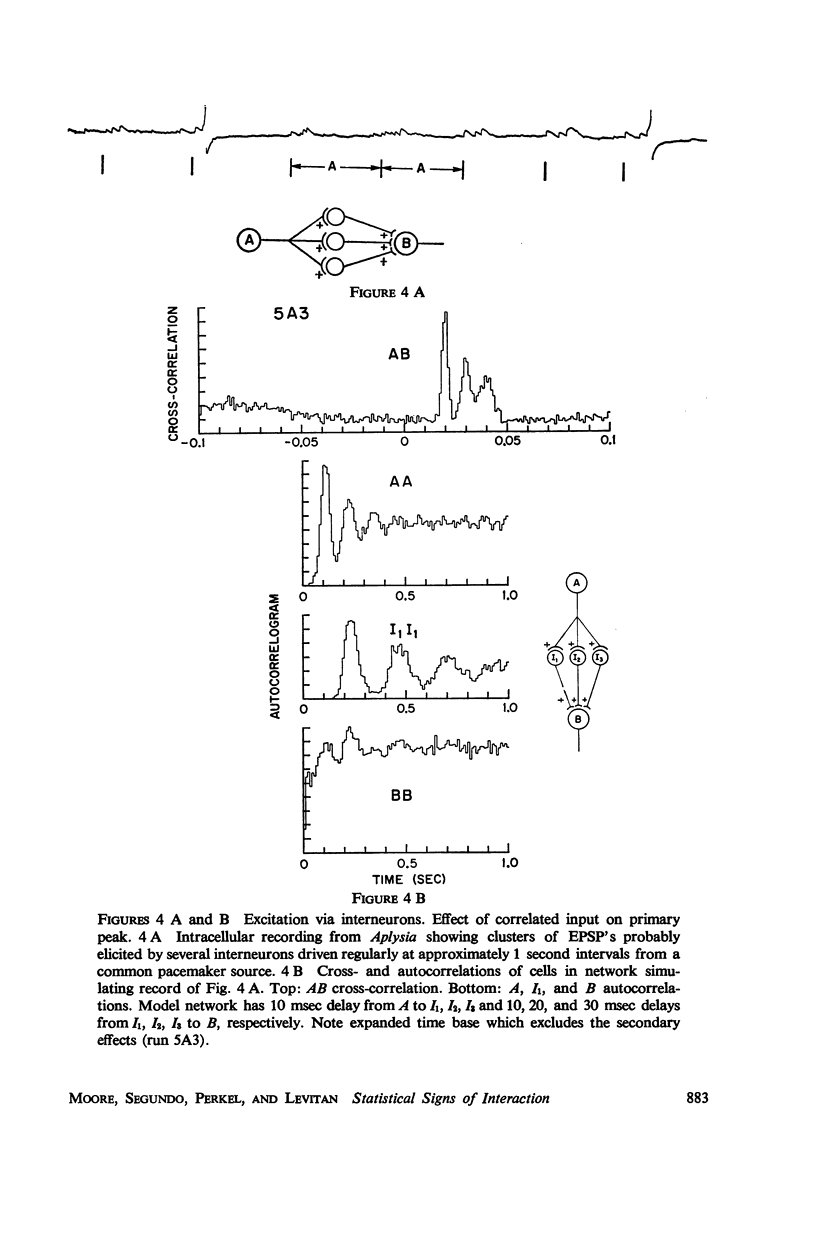
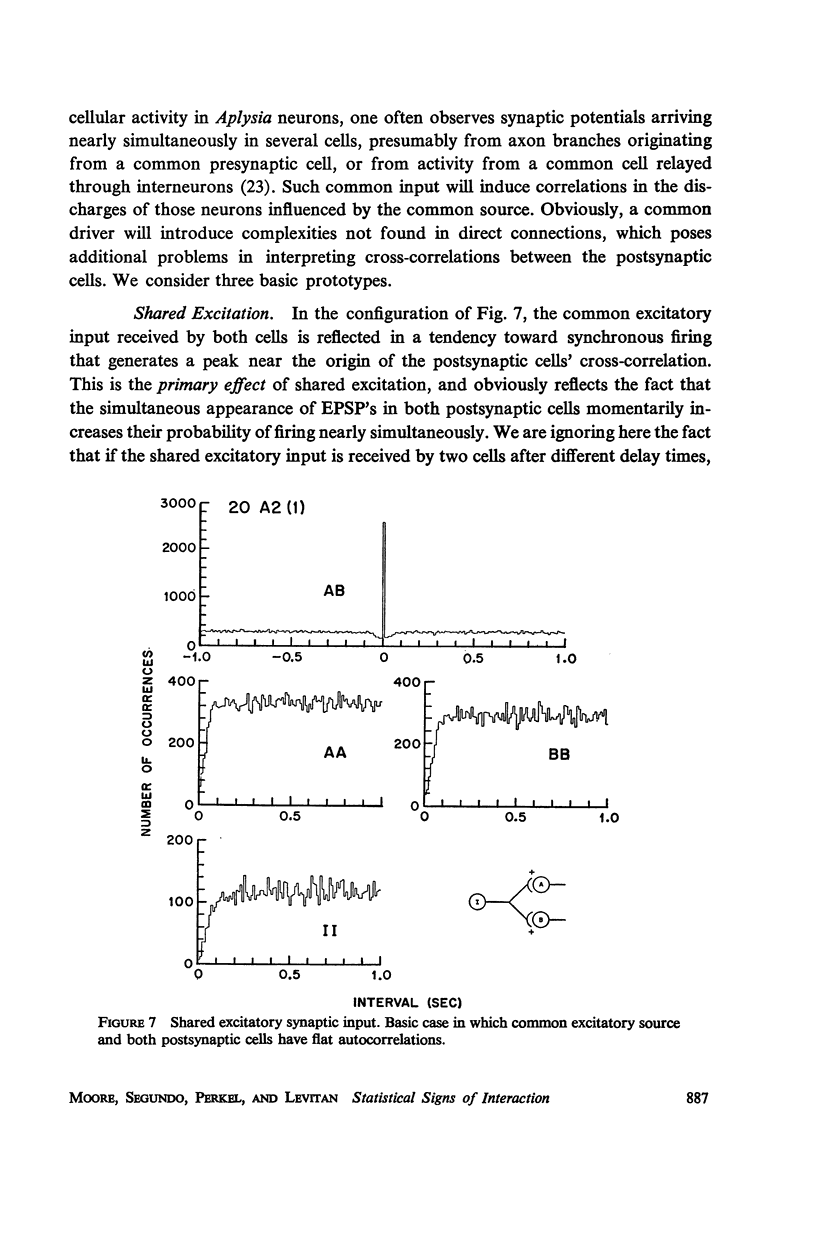
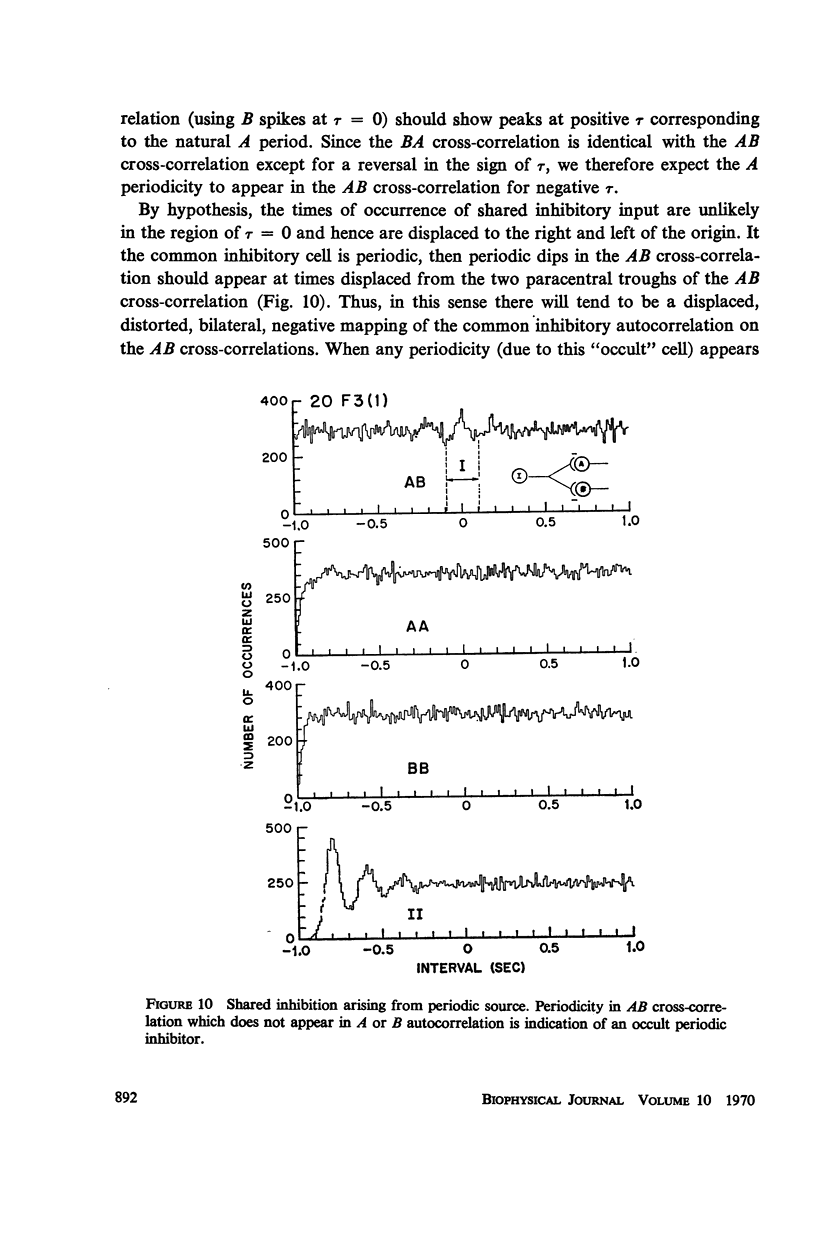
Abstract
The influence of basic open-loop synaptic connections on the firing of simultaneously recorded neurons has been investigated with auto- and cross-correlation histograms, using experimental records and computer simulations. The basic connections examined were direct synaptic excitation, direct synaptic inhibition, and shared synaptic input. Each type of synaptic connection produces certain characteristic features in the cross-correlogram depending on the properties of the synapse and statistical features in the firing pattern of each neuron. Thus, empirically derived cross-correlation measures can be interpreted in terms of the underlying physiological mechanisms. Their potential uses and limitations in the detection and identification of synaptic connections between neurons whose extracellularly recorded spike trains are available are discussed.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell C. C., Grimm R. J. Discharge properties of Purkinje cells recorded on single and double microelectrodes. J Neurophysiol. 1969 Nov;32(6):1044–1055. doi: 10.1152/jn.1969.32.6.1044. [DOI] [PubMed] [Google Scholar]
- Fox S. S., Norman R. J. Functional congruence: an index of neural homogeneity and a new measure of brain activity. Science. 1968 Mar 15;159(3820):1257–1259. doi: 10.1126/science.159.3820.1257. [DOI] [PubMed] [Google Scholar]
- Fox S. S., O'brien J. H. Duplication of Evoked Potential Waveform by Curve of Probability of Firing of a Single Cell. Science. 1965 Feb 19;147(3660):888–890. doi: 10.1126/science.147.3660.888. [DOI] [PubMed] [Google Scholar]
- Frost J. D., Jr EEG-intracellular potential relationships in isolated cerebral cortex. Electroencephalogr Clin Neurophysiol. 1968 May;24(5):434–443. doi: 10.1016/0013-4694(68)90103-x. [DOI] [PubMed] [Google Scholar]
- GERSTEIN G. L., CLARK W. A. SIMULTANEOUS STUDIES OF FIRING PATTERNS IN SEVERAL NEURONS. Science. 1964 Mar 20;143(3612):1325–1327. [PubMed] [Google Scholar]
- GERSTEIN G. L., KIANG N. Y. An approach to the quantitative analysis of electrophysiological data from single neurons. Biophys J. 1960 Sep;1:15–28. doi: 10.1016/s0006-3495(60)86872-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein G. L., Perkel D. H. Simultaneously recorded trains of action potentials: analysis and functional interpretation. Science. 1969 May 16;164(3881):828–830. doi: 10.1126/science.164.3881.828. [DOI] [PubMed] [Google Scholar]
- Holmes O., Houchin J. Analysis of the activity of one type of spontaneously discharging unit in the cortex cerebri of the anaesthetized rat. J Physiol. 1967 Nov;193(1):173–186. doi: 10.1113/jphysiol.1967.sp008350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes O., Houchin J. Units in the cerebral cortex of the anaesthetized rat and the correlations between their discharges. J Physiol. 1966 Dec;187(3):651–671. doi: 10.1113/jphysiol.1966.sp008116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge D., Moore G. P. Interspike interval fluctuations in aplysia pacemaker neurons. Biophys J. 2008 Dec 31;6(4):411–434. doi: 10.1016/S0006-3495(66)86667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keder-Stepanova I. A., Ponomarev V. A., Chetaev A. N. O zavisimosti v rabote dykhatel'nykh neironov prodolgovatogo mozga. Biofizika. 1966;11(1):123–128. [PubMed] [Google Scholar]
- MCCANN G. D. TOWARD A TRUE UNDERSTANDING OF THE NERVOUS SYSTEM. Ann Intern Med. 1965 Apr;62:823–837. doi: 10.7326/0003-4819-62-4-823. [DOI] [PubMed] [Google Scholar]
- Michael C. R. Receptive fields of single optic nerve fibers in a mammal with an all-cone retina. II: directionally selective units. J Neurophysiol. 1968 Mar;31(2):257–267. doi: 10.1152/jn.1968.31.2.257. [DOI] [PubMed] [Google Scholar]
- Moore G. P., Perkel D. H., Segundo J. P. Statistical analysis and functional interpretation of neuronal spike data. Annu Rev Physiol. 1966;28:493–522. doi: 10.1146/annurev.ph.28.030166.002425. [DOI] [PubMed] [Google Scholar]
- Noda H., Manohar S., Adey W. R. Correlated firing of hippocampal neuron pairs in sleep and wakefulness. Exp Neurol. 1969 Jun;24(2):232–247. doi: 10.1016/0014-4886(69)90017-x. [DOI] [PubMed] [Google Scholar]
- OIKAWA T., KOSHI T., FUJITANI Y., UEDA I., KAWAHARA N. SYNCHRONISM BETWEEN TWO TRAINS OF SPONTANEOUS SPIKE ACTIVITY IN THE CAT'S VISUAL CORTEX. Yonago Acta Med. 1965 Feb;18:44–55. [PubMed] [Google Scholar]
- PERKEL D. H., MOORE G. P., SEGUNDO J. P. CONTINUOUS-TIME SIMULATION OF GANGLION NERVE CELLS IN APLYSIA. Biomed Sci Instrum. 1963;1:347–357. [PubMed] [Google Scholar]
- PERL E. R. Observations on the discharge of flexor motoneurones. J Physiol. 1962 Dec;164:450–464. doi: 10.1113/jphysiol.1962.sp007031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel D. H., Gerstein G. L., Moore G. P. Neuronal spike trains and stochastic point processes. I. The single spike train. Biophys J. 1967 Jul;7(4):391–418. doi: 10.1016/S0006-3495(67)86596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel D. H., Gerstein G. L., Moore G. P. Neuronal spike trains and stochastic point processes. II. Simultaneous spike trains. Biophys J. 1967 Jul;7(4):419–440. doi: 10.1016/S0006-3495(67)86597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODIECK R. W., KIANG N. Y., GERSTEIN G. L. Some quantitative methods for the study of spontaneous activity of single neurons. Biophys J. 1962 Jul;2:351–368. doi: 10.1016/s0006-3495(62)86860-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck R. W. Maintained activity of cat retinal ganglion cells. J Neurophysiol. 1967 Sep;30(5):1043–1071. doi: 10.1152/jn.1967.30.5.1043. [DOI] [PubMed] [Google Scholar]
- SIMON W. THE REAL-TIME SORTING OF NEURO-ELECTRIC ACTION POTENTIALS IN MULTIPLE UNIT STUDIES. Electroencephalogr Clin Neurophysiol. 1965 Feb;18:192–195. doi: 10.1016/0013-4694(65)90029-5. [DOI] [PubMed] [Google Scholar]
- Segundo J. P., Perkel D. H., Moore G. P. Spike probability in neurones: influence of temporal structure in the train of synaptic events. Kybernetik. 1966 May;3(2):67–82. doi: 10.1007/BF00299899. [DOI] [PubMed] [Google Scholar]
- Segundo J. P., Perkel D. H., Wyman H., Hegstad H., Moore G. P. Input-output relations in computer-simulated nerve cells. Influence of the statistical properties, strength, number and inter-dependence of excitatory pre-synaptic terminals. Kybernetik. 1968 May;4(5):157–171. doi: 10.1007/BF00289038. [DOI] [PubMed] [Google Scholar]
- Tapper D. N., Mann M. D. Single presynaptic impulse evokes postsynaptic discharge. Brain Res. 1968 Dec;11(3):688–690. doi: 10.1016/0006-8993(68)90158-3. [DOI] [PubMed] [Google Scholar]
- VERZEANO M., NEGISHI K. Neuronal activity in cortical and thalamic networks. J Gen Physiol. 1960 Jul;43(6):177–195. doi: 10.1085/jgp.43.6.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel H., Kandel E. R. A direct synaptic connection mediating both excitation and inhibition. Science. 1967 Dec 1;158(3805):1206–1208. doi: 10.1126/science.158.3805.1206. [DOI] [PubMed] [Google Scholar]
- Waldron I. Mechanisms for the production of the motor output pattern in flying locusts. J Exp Biol. 1967 Oct;47(2):201–212. doi: 10.1242/jeb.47.2.201. [DOI] [PubMed] [Google Scholar]
- Wyman R. J. Lateral inhibition in a motor output system. I. Reciprocal inhibition in Dipteran flight motor system. J Neurophysiol. 1969 May;32(3):297–306. doi: 10.1152/jn.1969.32.3.297. [DOI] [PubMed] [Google Scholar]


