Abstract
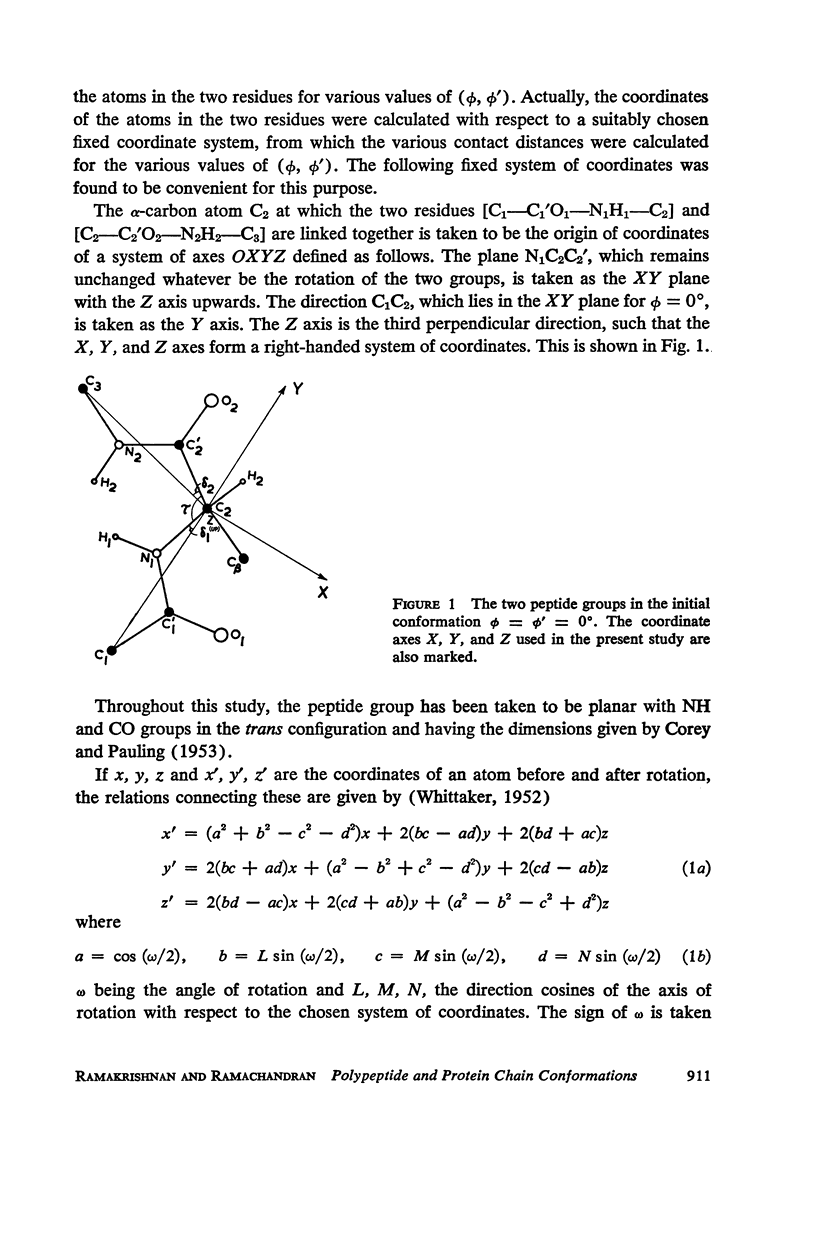
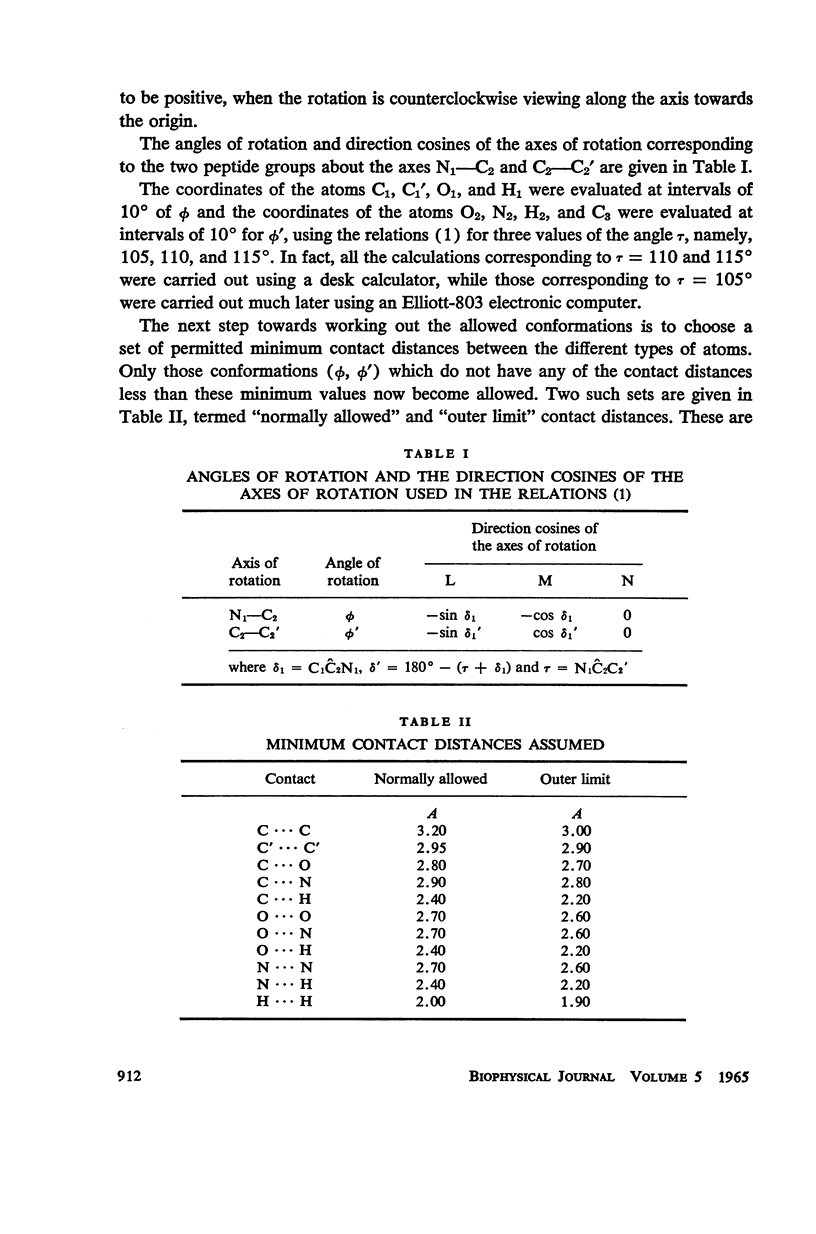
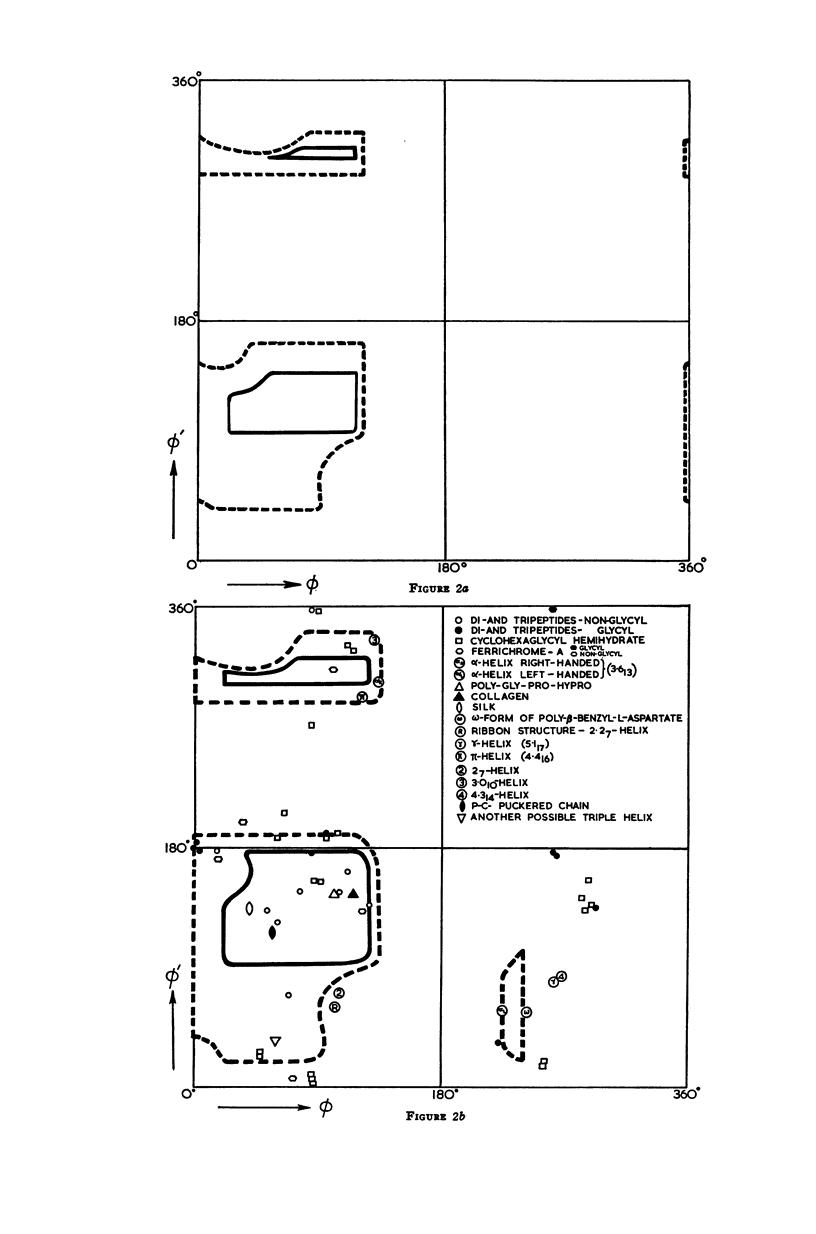
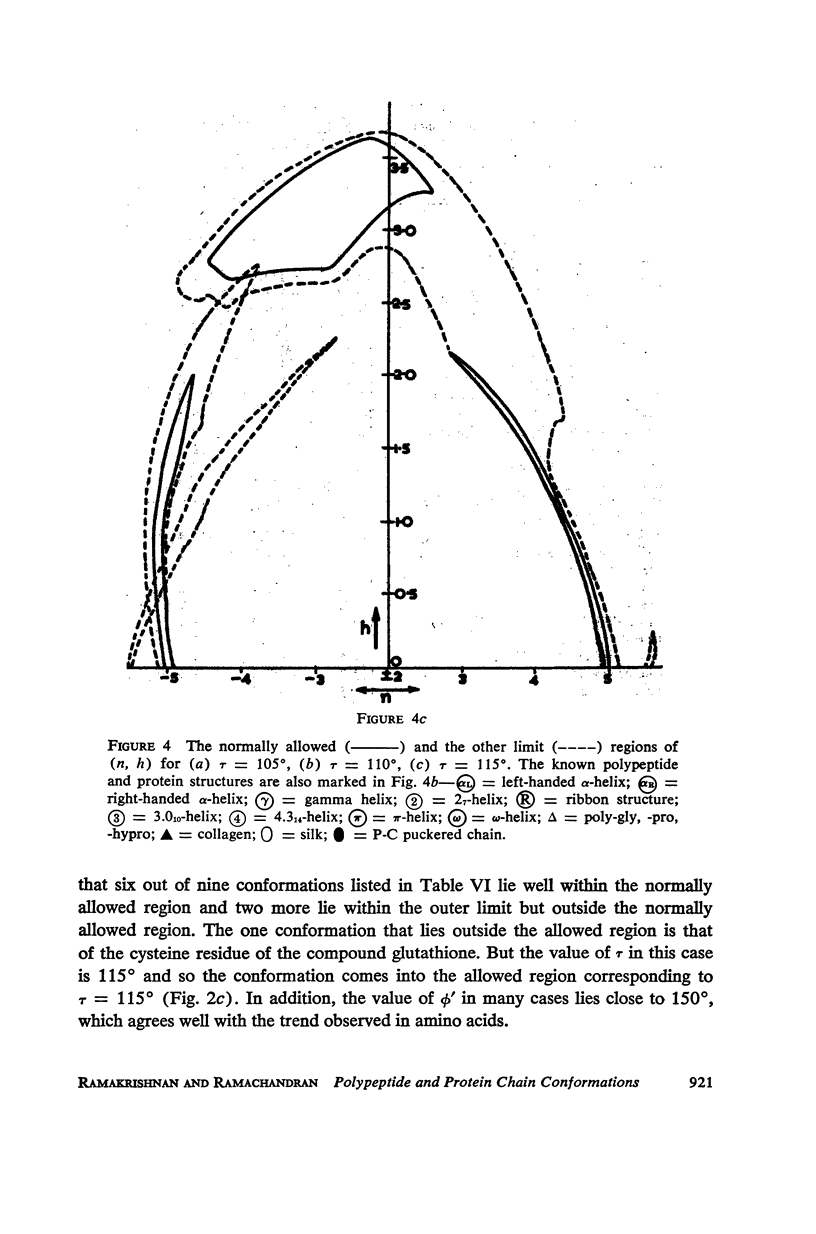
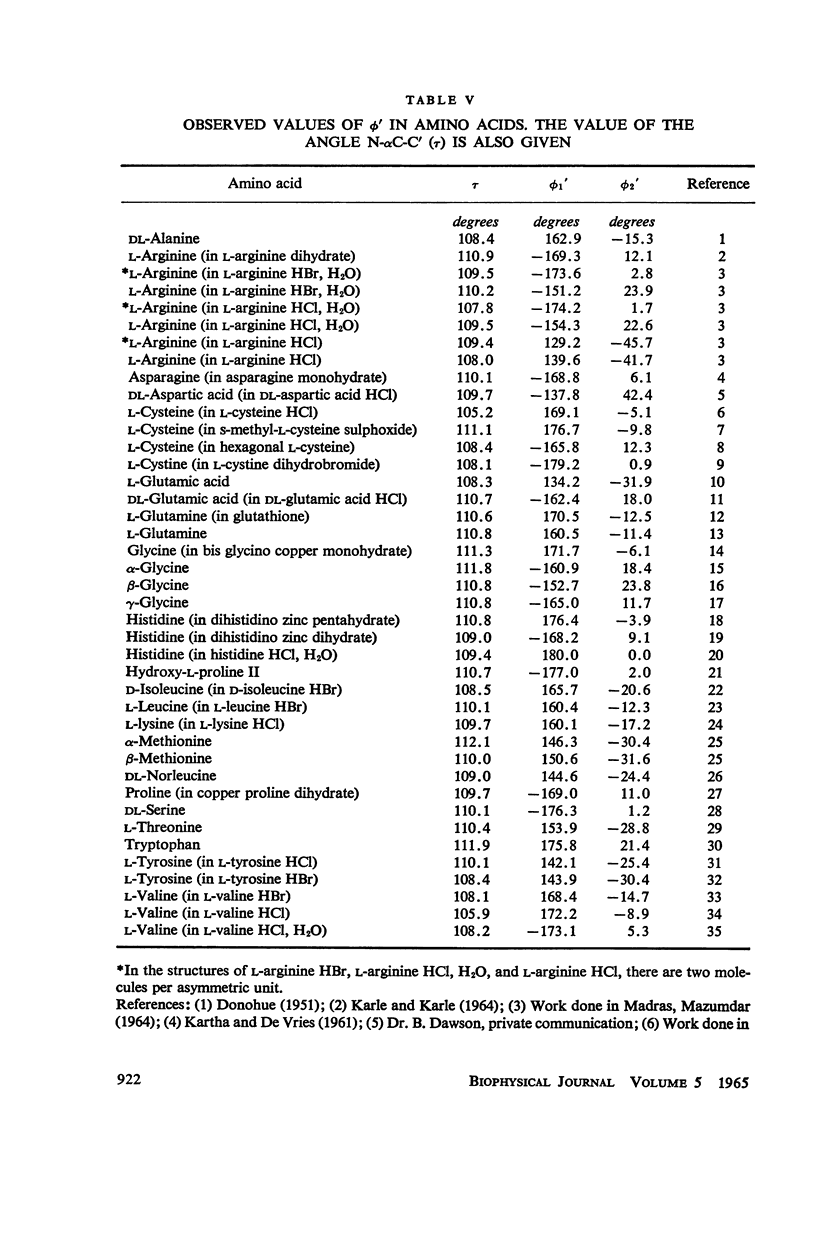
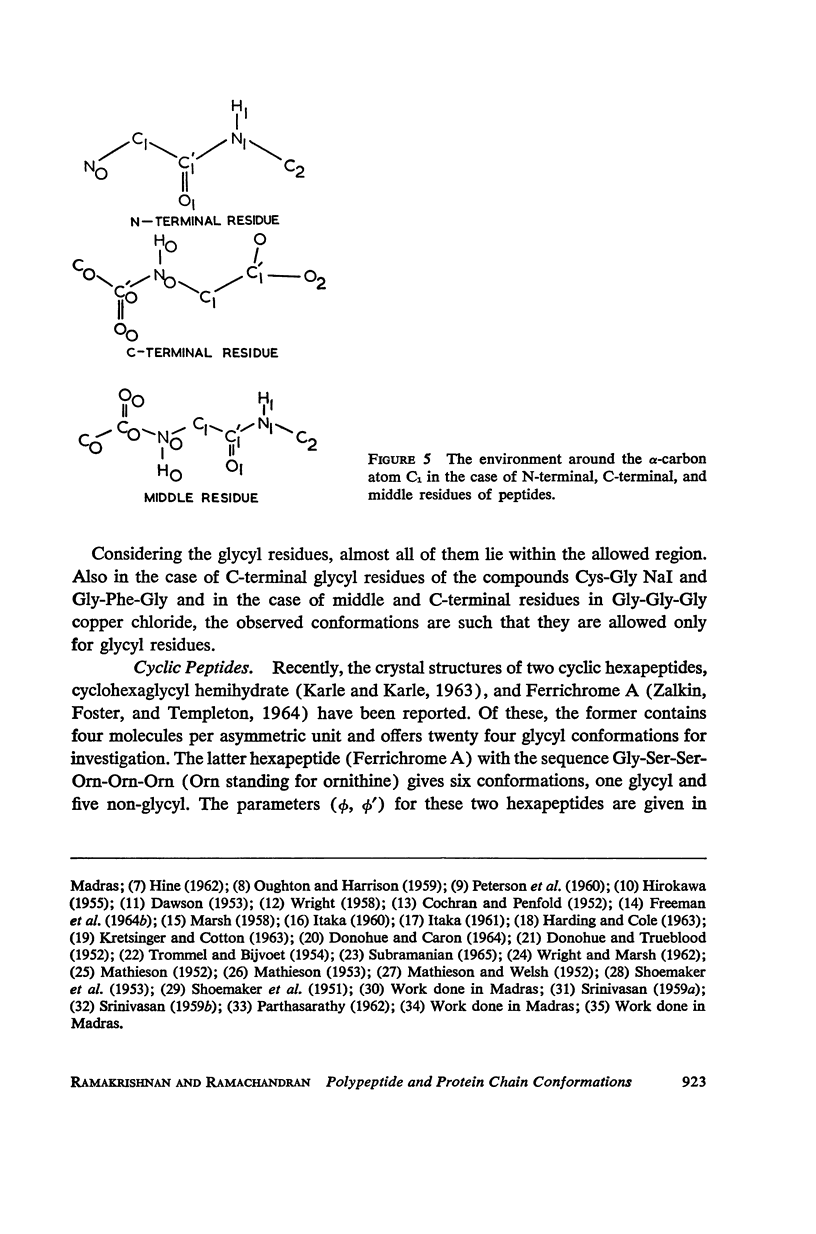
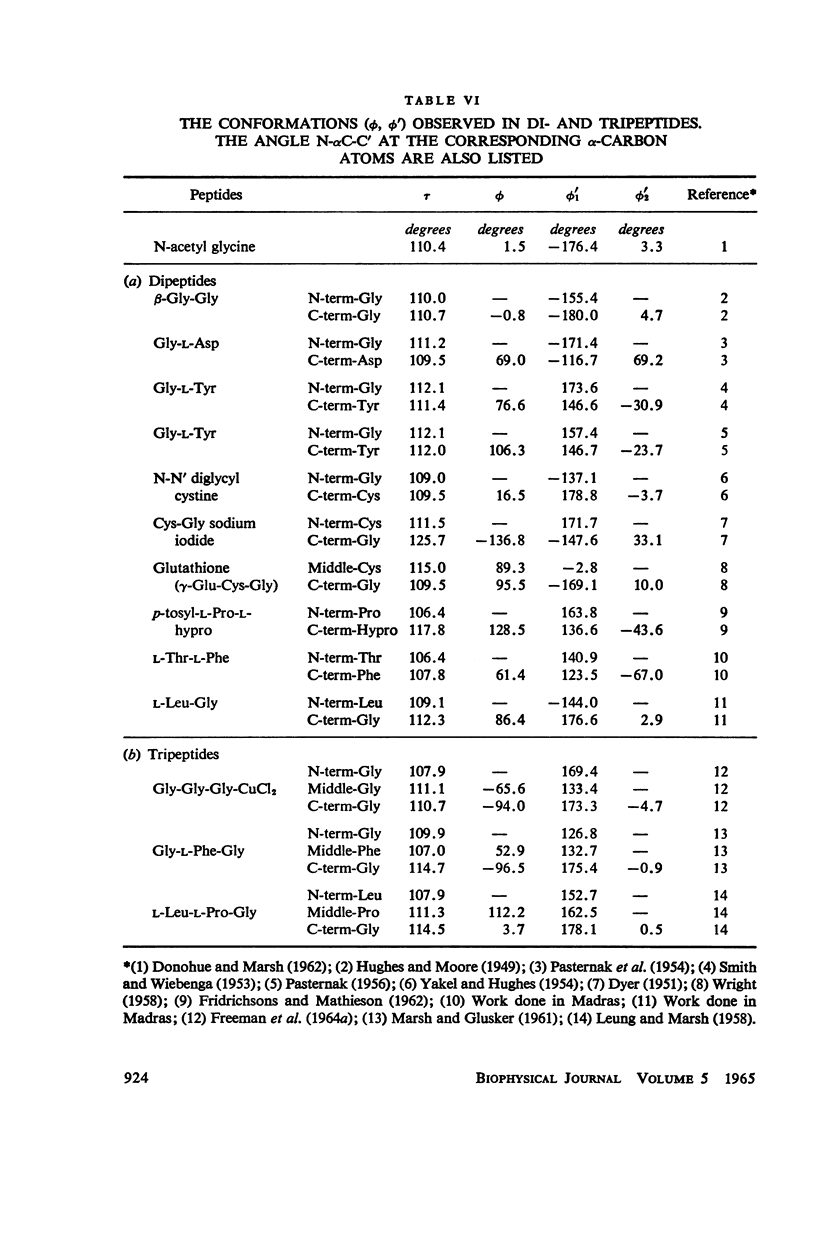
The conformation of a polypeptide or protein chain may be specified by stating the orientations of the two linked peptide residues at each alpha carbon atom in the chain, namely the two dihedral angles ϕ, ϕ′ about the single bonds N—αC and αC—C′ from a defined standard conformation. By using certain criteria of minimum contact distances between the various atoms, the allowed anges of (ϕ, ϕ′) have been worked out for three values of the angle N-αC-C′ (τ), namely 105, 110, and 115° for non-glycyl, and 110 and 115° for glycyl residues. The theory is compared with all the available crystallographic data (up to early 1965) on simple (di- and tri-) peptides, cyclic peptides, polypeptide and protein structures, and the observed data fully support the conclusions from theory. The effect of the gamma carbon atom, in its three possible positions, is also discussed, and is found to alter the outer limits of the allowed region of (ϕ, ϕ′) only slightly. The paper contains exhaustive references to the published data on these structures, using x-ray diffraction.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADBURY E. M., BROWN L., DOWNIE A. R., ELLIOTT A., FRASER R. D., HANBY W. E. The structure of the omegaform of poly-Beta-benzyl-L-aspartate. J Mol Biol. 1962 Aug;5:230–247. doi: 10.1016/s0022-2836(62)80086-2. [DOI] [PubMed] [Google Scholar]
- CRICK F. H., RICH A. Structure of polyglycine II. Nature. 1955 Oct 22;176(4486):780–781. doi: 10.1038/176780a0. [DOI] [PubMed] [Google Scholar]
- De Santis P., Giglio E., Liquori A. M., Ripamonti A. Van der Waals interaction and the stability of helical polypeptide chains. Nature. 1965 May 1;206(983):456–458. doi: 10.1038/206456a0. [DOI] [PubMed] [Google Scholar]
- KARTHA G., DE VRIES A. Structure of asparagine monohydrate. Nature. 1961 Dec 2;192:862–863. doi: 10.1038/192862a0. [DOI] [PubMed] [Google Scholar]
- KENDREW J. C., WATSON H. C., STRANDBERG B. E., DICKERSON R. E., PHILLIPS D. C., SHORE V. C. The amino-acid sequence x-ray methods, and its correlation with chemical data. Nature. 1961 May 20;190:666–670. doi: 10.1038/190666a0. [DOI] [PubMed] [Google Scholar]
- LINDLEY H., ROLLETT J. S. An investigation of insulin structure by model building techniques. Biochim Biophys Acta. 1955 Oct;18(2):183–193. doi: 10.1016/0006-3002(55)90054-8. [DOI] [PubMed] [Google Scholar]
- Low B. W., Grenville-Wells H. J. Generalized Mathematical Relationships for Polypeptide Chain Helices: The Coordinates of the II Helix. Proc Natl Acad Sci U S A. 1953 Aug;39(8):785–801. doi: 10.1073/pnas.39.8.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIZUSHIMA S. I., SHIMANOUCHI T. Possible polypeptide configurations of proteins from the viewpoint of internal rotation potential. Adv Enzymol Relat Subj Biochem. 1961;23:1–27. doi: 10.1002/9780470122686.ch1. [DOI] [PubMed] [Google Scholar]
- PAULING L., COREY R. B. Atomic coordinates and structure factors for two helical configurations of polypeptide chains. Proc Natl Acad Sci U S A. 1951 May;37(5):235–240. doi: 10.1073/pnas.37.5.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAULING L., COREY R. B., BRANSON H. R. The structure of proteins; two hydrogen-bonded helical configurations of the polypeptide chain. Proc Natl Acad Sci U S A. 1951 Apr;37(4):205–211. doi: 10.1073/pnas.37.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling L., Corey R. B. Two Rippled-Sheet Configurations of Polypeptide Chains, and a Note about the Pleated Sheets. Proc Natl Acad Sci U S A. 1953 Apr;39(4):253–256. doi: 10.1073/pnas.39.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMACHANDRAN G. N., RAMAKRISHNAN C., SASISEKHARAN V. Stereochemistry of polypeptide chain configurations. J Mol Biol. 1963 Jul;7:95–99. doi: 10.1016/s0022-2836(63)80023-6. [DOI] [PubMed] [Google Scholar]
- Zalkin A., Forrester J. D., Templeton D. H. Crystal and Molecular Structure of Ferrichrome A. Science. 1964 Oct 9;146(3641):261–263. doi: 10.1126/science.146.3641.261. [DOI] [PubMed] [Google Scholar]


