Abstract
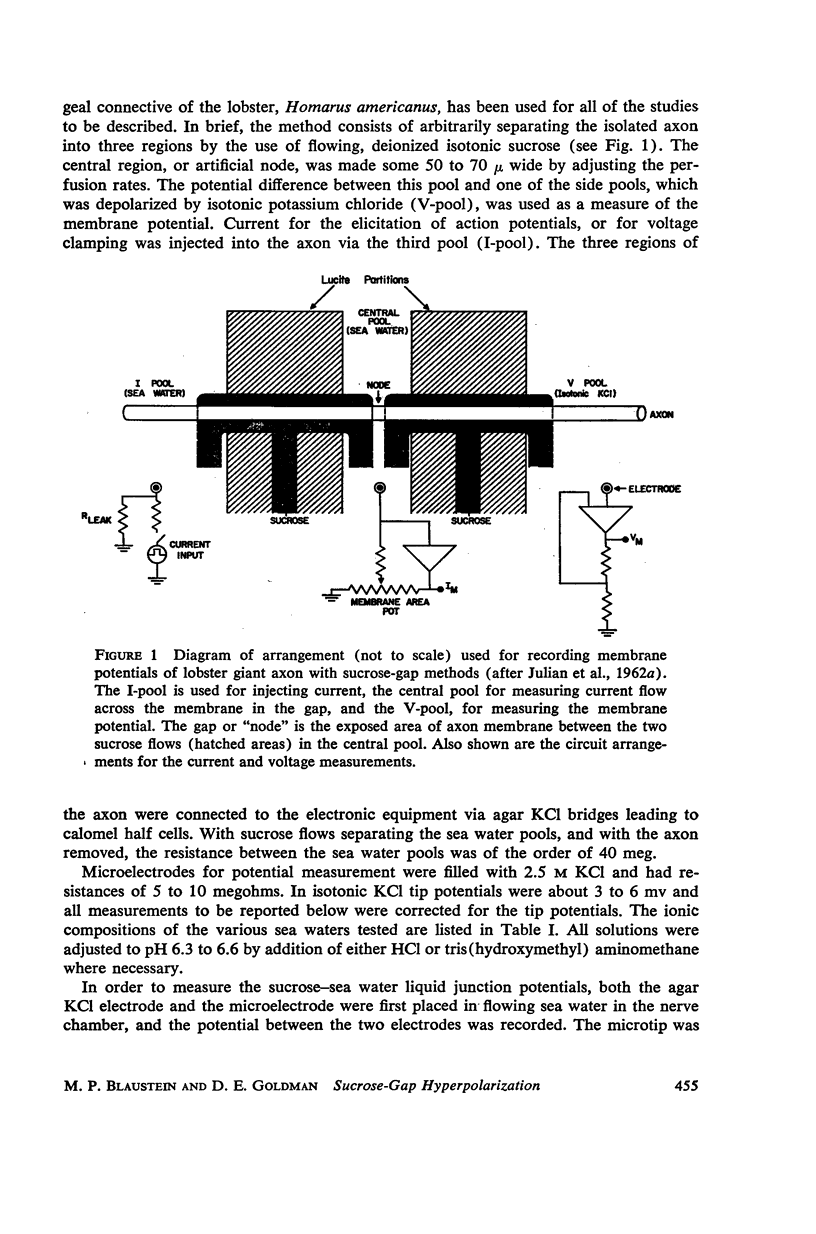
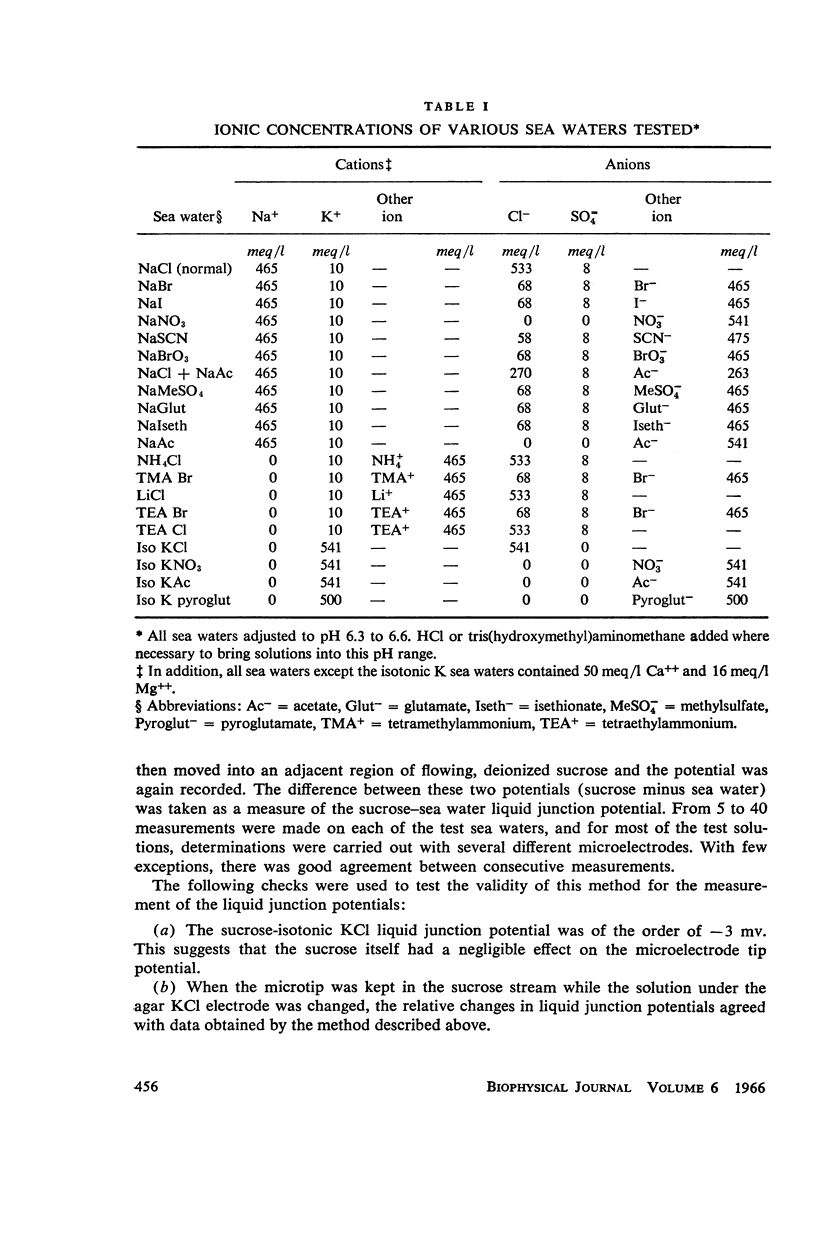
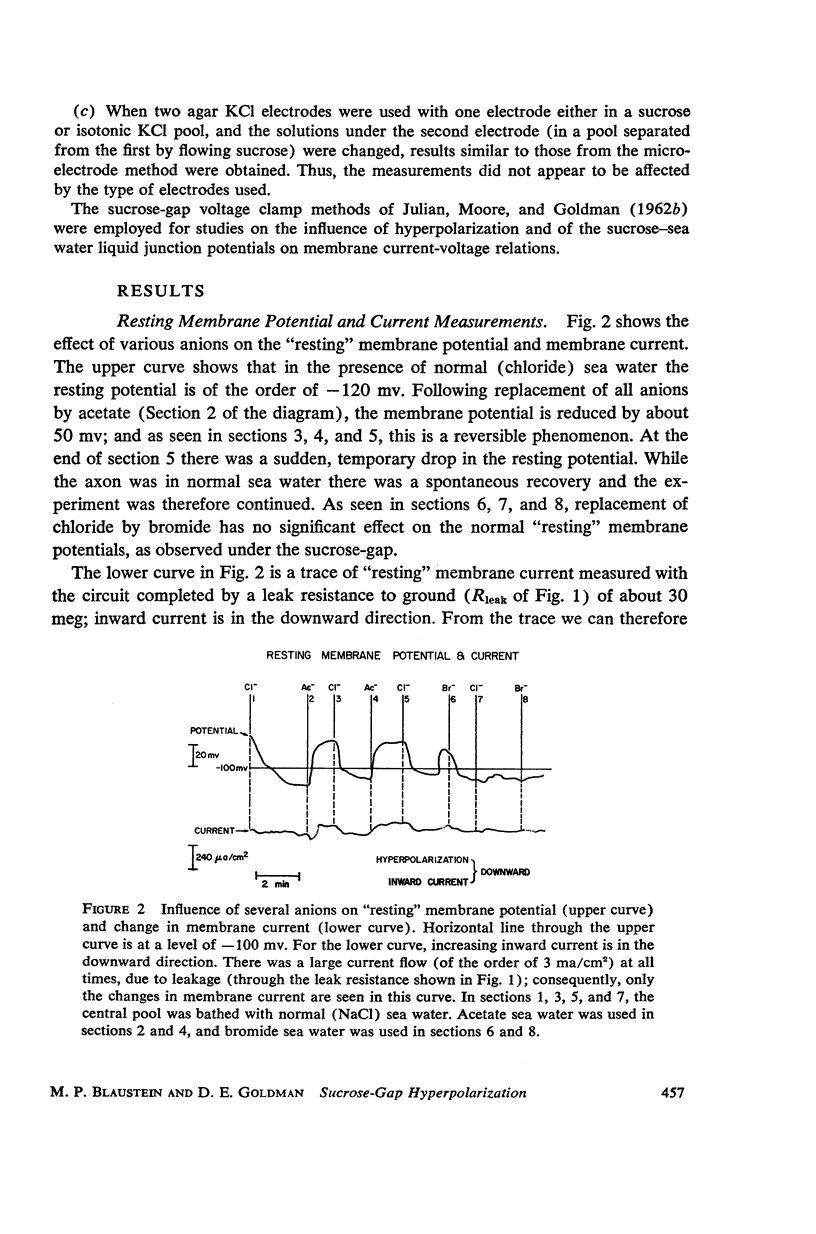
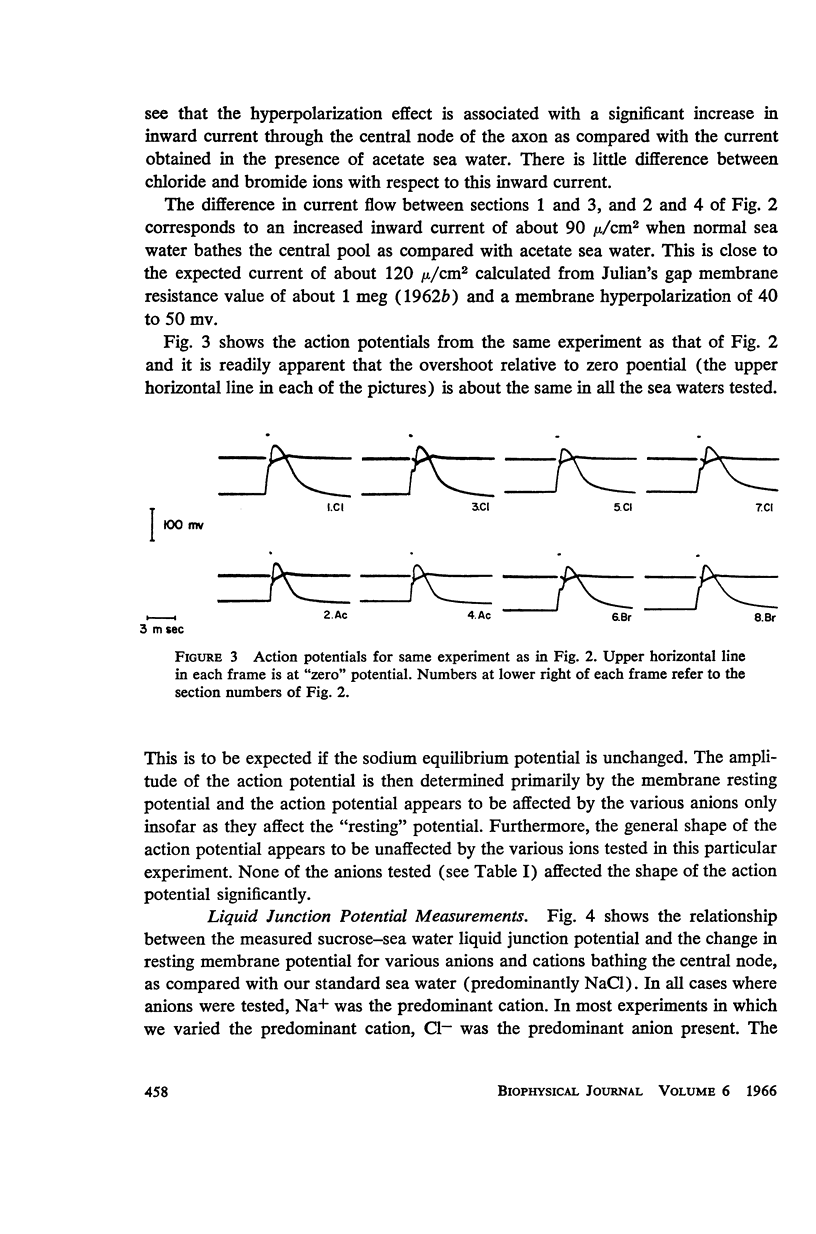
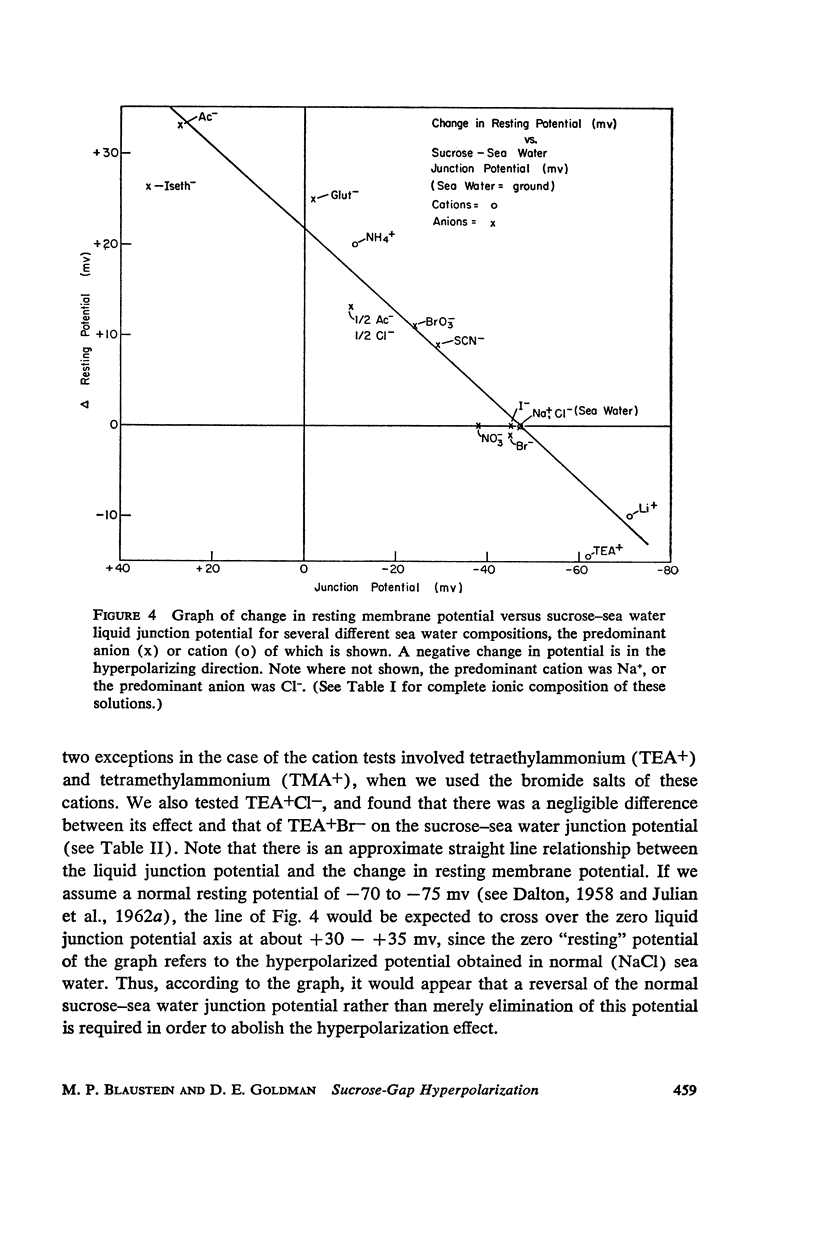
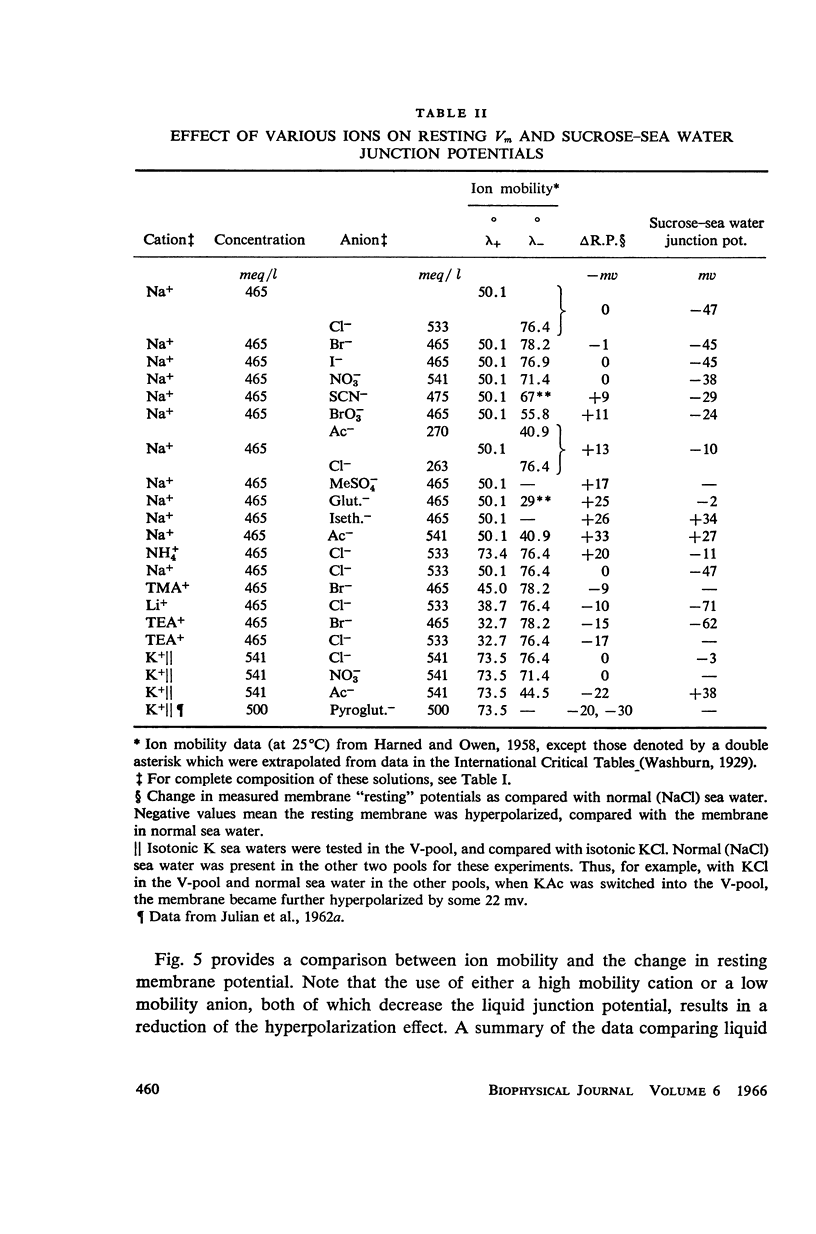
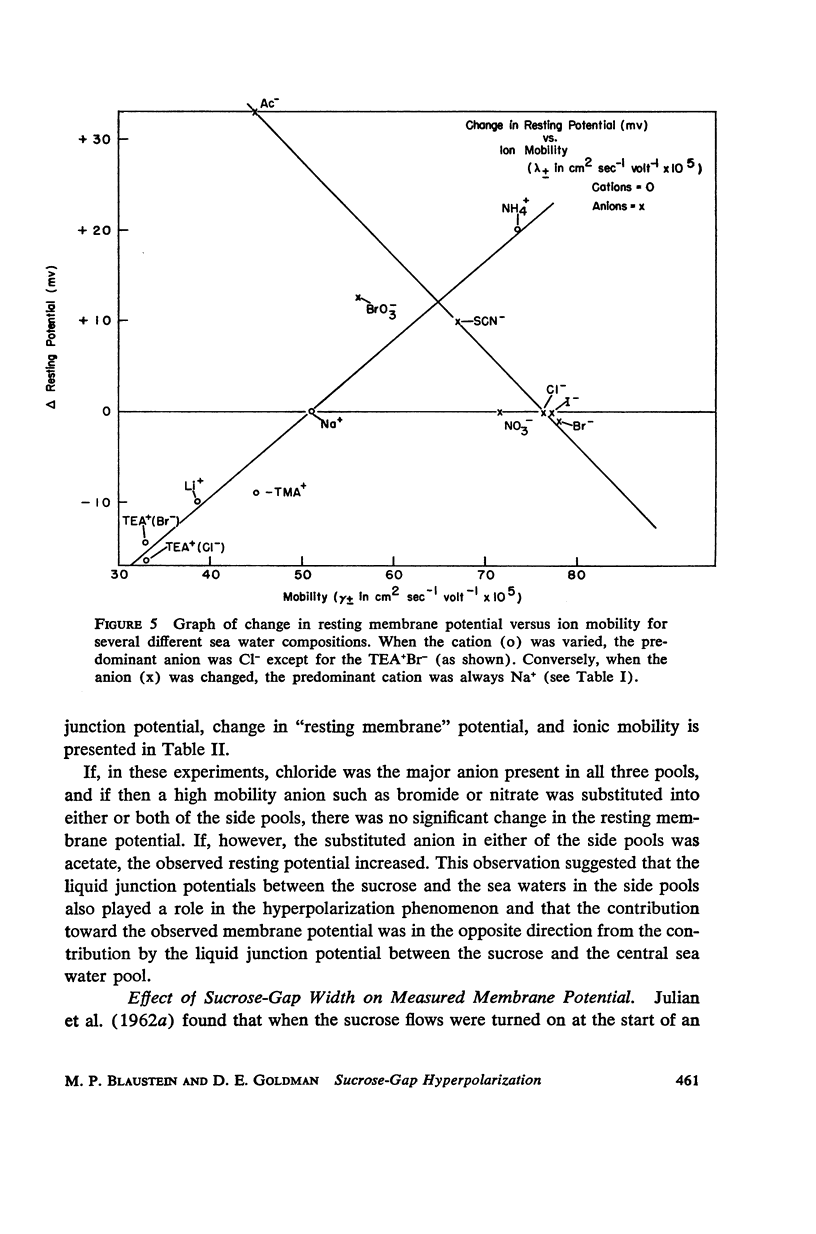
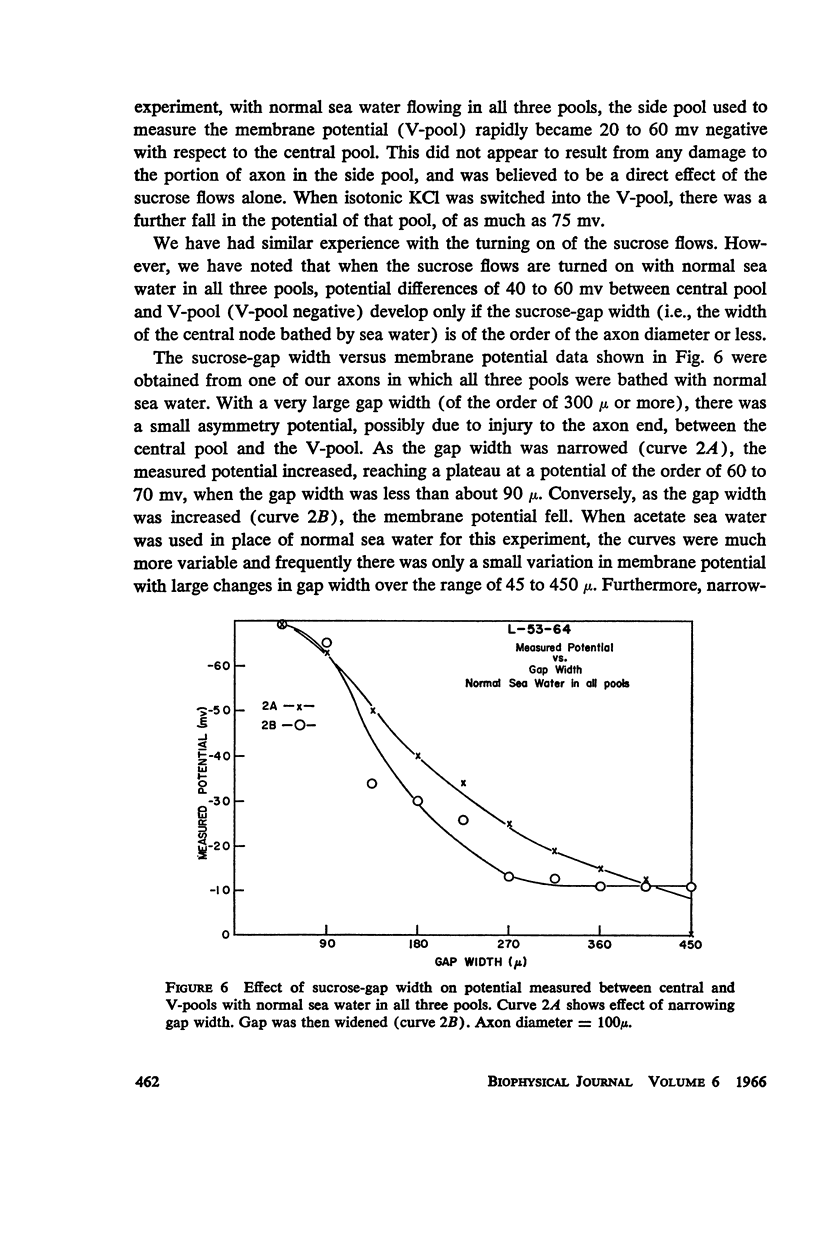
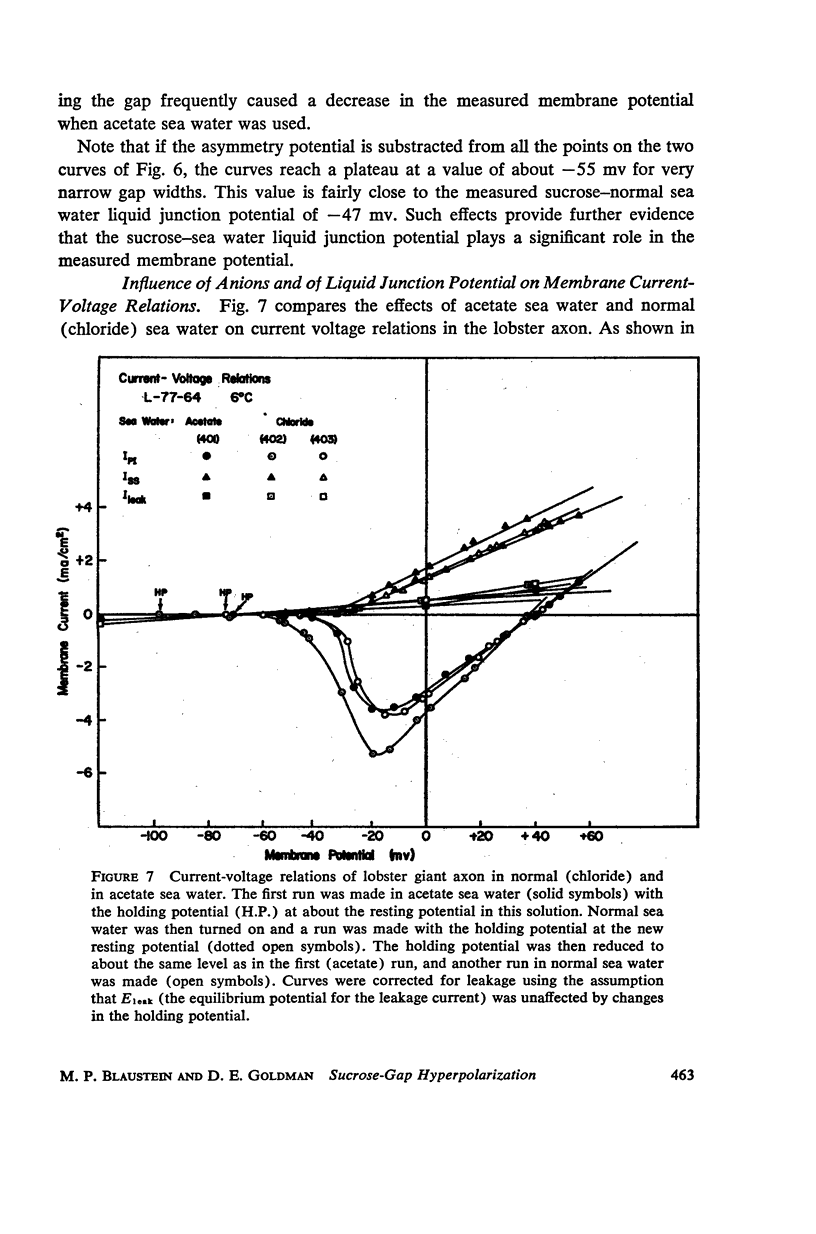
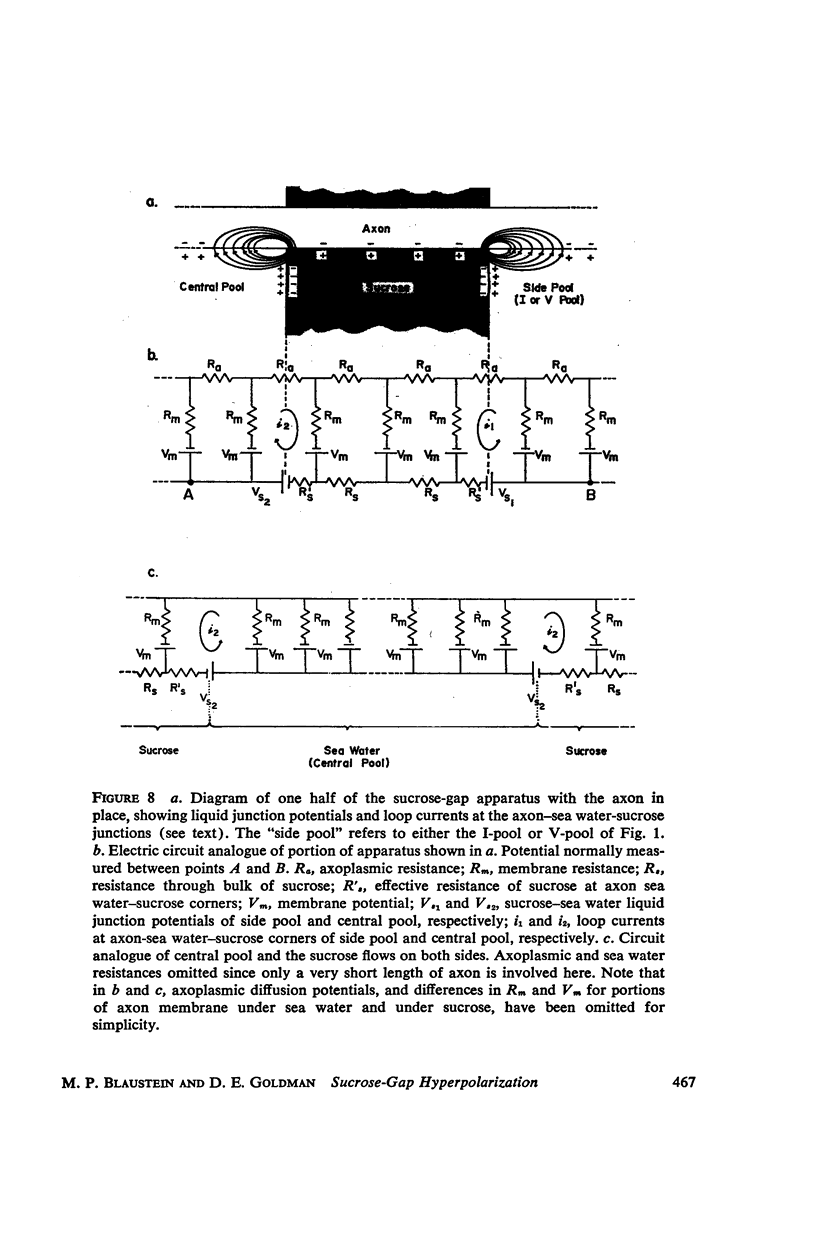
One of the disadvantages of the sucrose-gap method for measuring membrane potentials with extracellular electrodes is a membrane hyperpolarization of the order of 30 to 60 mv, as compared with the resting potential obtained with intracellular microelectrodes in the absence of a sucrose-gap. In the present study the contribution of the sucrose-sea water junction potential to this hyperpolarization effect has been evaluated by comparing the effects on the resting potential of several anion and cation substitutions in the sea water bathing the lobster giant axon under sucrose-gap. Measurements with microelectrodes demonstrate a significant liquid junction potential between sucrose and standard artificial sea water. The value of this liquid junction potential as well as the measured resting membrane potential varies as a function of the anions and cations substituted in the sea water. Both the liquid junction potential and the sucrose-gap-induced hyperpolarization can be eliminated by using a low mobility anion to replace most of the chloride in sea water while the normal cation content remains unchanged. These data provide evidence that loop currents at the sucrose-sea water-axon junctions are at least partly responsible for membrane hyperpolarization under a sucrose gap.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DALTON J. C. Effects of external ions on membrane potentials of a lobster giant axon. J Gen Physiol. 1958 Jan 20;41(3):529–542. doi: 10.1085/jgp.41.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Potassium permeability in myelinated nerve fibres of Xenopus laevis. J Physiol. 1962 Jan;160:54–61. doi: 10.1113/jphysiol.1962.sp006834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURUKAWA T., SUGIHARA T., TAKAGI T. Depolarization of end-plates by acetylcholine externally applied. Jpn J Physiol. 1956 Jun 15;6(2):98–107. doi: 10.2170/jjphysiol.6.98. [DOI] [PubMed] [Google Scholar]
- HASHIMURA S., OSA T. The effect of nitrate and thiocyanate ions on the resting and action potentials of cobalt-treated single node of Ranvier. Jpn J Physiol. 1963 Jun 15;13:219–230. doi: 10.2170/jjphysiol.13.219. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOROWICZ P. THE EFFECTS OF ANIONS ON EXCITABLE CELLS. Pharmacol Rev. 1964 Jun;16:193–221. [PubMed] [Google Scholar]
- JULIAN F. J., MOORE J. W., GOLDMAN D. E. Current-voltage relations in the lobster giant axon membrane under voltage clamp conditions. J Gen Physiol. 1962 Jul;45:1217–1238. doi: 10.1085/jgp.45.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JULIAN F. J., MOORE J. W., GOLDMAN D. E. Membrane potentials of the lobster giant axon obtained by use of the sucrose-gap technique. J Gen Physiol. 1962 Jul;45:1195–1216. doi: 10.1085/jgp.45.6.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE J. W., NARAHASHI T., ULBRICHT W. SODIUM CONDUCTANCE SHIFT IN AN AXON INTERNALLY PERFUSED WITH A SUCROSE AND LOW-POTASSIUM SOLUTION. J Physiol. 1964 Aug;172:163–173. doi: 10.1113/jphysiol.1964.sp007410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANES A. M. Electrochemical aspects of physiological and pharmacological action in excitable cells. I. The resting cell and its alteration by extrinsic factors. Pharmacol Rev. 1958 Mar;10(1):59–164. [PubMed] [Google Scholar]
- STAMPFLI R. A new method for measuring membrane potentials with external electrodes. Experientia. 1954 Dec 15;10(12):508–509. doi: 10.1007/BF02166189. [DOI] [PubMed] [Google Scholar]


