Abstract
Theiler's murine encephalomyelitis virus (TMEV) infection induces immune-mediated demyelinating disease in susceptible mouse strains and serves as a relevant infectious model for human multiple sclerosis. To investigate the pathogenic mechanisms, two strains of TMEV (DA and BeAn), capable of inducing chronic demyelination in the central nervous system (CNS), have primarily been used. Here, we have compared the T-cell responses induced after infection with DA and BeAn strains in highly susceptible SJL/J mice. CD4+ T-cell responses to known epitopes induced by these two strains were virtually identical. However, the CD8+ T-cell response induced following DA infection in susceptible SJL/J mice was unable to recognize two of three H-2Ks-restricted epitope regions of BeAn, due to single-amino-acid substitutions. Interestingly, T cells specific for the H-2Ks-restricted epitope (VP111-20) recognized by both strains showed a drastic increase in frequency as well as avidity after infection with DA virus. These results strongly suggest that the level and avidity of virus-specific CD8+ T cells infiltrating the CNS could be drastically different after infection with these two strains of TMEV and may differentially influence the pathogenic and/or protective outcome.
General properties of DA and BeAn viruses.
Theiler's murine encephalomyelitis virus (TMEV) is a common enteric pathogen in mice and belongs to the picornavirus family (14, 28). Two major subgroups of TMEV have been identified based on various biological characteristics such as neurovirulence and antigenicity. The first subgroup of TMEV includes GDVII and FA viruses, which cause rapid fatal encephalitis. The second subgroup, known as Theiler's original (TO) viruses, including the BeAn8386 and DA strains, causes a biphasic neurological disease upon access to the central nervous systems (CNS) of susceptible mice (5, 6, 13, 14). The early, acute (poliomyelitis) phase is characterized by flaccid limb paralysis and degeneration of neurons. The late phase is characterized by chronic, inflammatory demyelination. This myelin destruction in the late phase is accompanied by stripping of myelin lamellae by mononuclear processes (6, 13). Interestingly, however, the BeAn strain is known to induce a clinically undetectable level of the early-phase disease whereas DA induces significant polio-like symptoms (5, 6). Nevertheless, these two virus strains induce similar late-phase demyelinating disease and share greater than 93% identical amino acid residues in the capsid proteins (19, 21).
Although the major histocompatibility complex class II-restricted CD4+ T-cell response is critically important in susceptibility and/or resistance to TMEV-induced demyelinating disease, the major histocompatibility complex class I H-2D locus also influences susceptibility to the disease (12, 16, 24). As demyelination is closely linked to viral persistence (15, 26, 29), virus-specific CD8+ cytotoxic T cells perhaps play an important role in protection and/or pathogenesis (4). However, investigations assessing the role of these CD8+ T cells (2, 8, 22, 25) have drawn conflicting conclusions. Fully functional CD8+ T cells are necessary for manifestation of clinical symptoms after infection with TMEV strain DA (18, 23). However, such T cells from BeAn-infected mice appear to be involved in protection from demyelinating disease (1, 20, 22). Recently, the predominant and subdominant epitopes recognized by CNS-infiltrating CD8+ T cells from resistant C57BL/10 or C57BL/6 mice infected with either DA (3, 7, 10) or BeAn (17) have been identified. In addition, we have also recently identified one dominant (VP3159-166) and two subdominant (VP111-20 and VP3173-181) capsid protein epitopes recognized by CD8+ T cells from SJL/J mice infected with BeAn virus (11). However, it is not yet clear whether DA and BeAn strains induce differential CD4+ and CD8+ T-cell responses, and the potential differences in immune responses may account for the previously reported discrepancies in the protective and/or pathogenic outcome. Thus, the assessment of the level and specificity of immune T-cell responses induced by these viruses would be very helpful for understanding the potential mechanisms of pathogenesis and protection in TMEV-induced demyelinating diseases in susceptible mice.
CD4+ T-cell responses are similar in SJL/J mice infected with DA and those infected with BeAn.
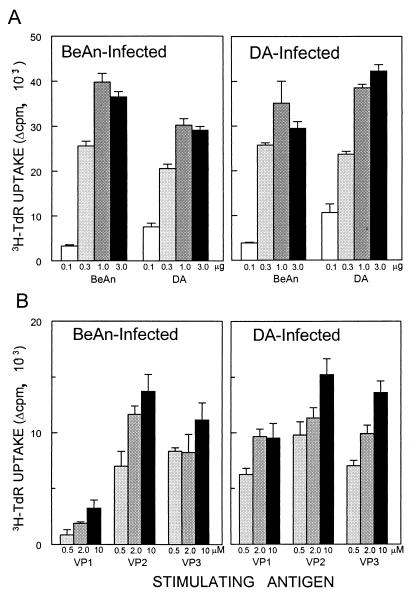
To compare the levels and the potential shared epitope recognition by Th cells induced by these TO viruses, SJL/J mice were infected with either DA or BeAn and then the levels of proliferative responses to DA, BeAn, and the predominant Th epitope peptides of BeAn were assessed (Fig. 1). Either virus (106 PFU) was administered at the right cerebral hemisphere of 6- to 8-week-old mice anesthetized with methoxyflurane. At 15 days postinfection, spleen cells (6 × 105 cells/well) were cultured in 96-well plates with RPMI 1640 containing 0.5% syngeneic mouse serum and 5 × 10−5 M 2-mercaptoethanol. Splenic T cells from DA- or BeAn-infected mice responded equally well to UV-inactivated DA and BeAn viruses interchangeably, as well as the individual Th epitope (VP1233-250, VP274-86, and VP324-37) peptides (9, 30, 31), despite the differences in one amino acid residue in the VP3 epitope region between the viruses. These results clearly indicate that the level of and epitope recognition by Th cells induced by DA infection are very similar to those of cells induced by BeAn infection.
FIG. 1.
T-cell proliferation responses by splenic T cells from SJL mice infected with BeAn or DA virus. Reactivity of T cells from mice infected with the BeAn or DA strain of TMEV was assessed at 15 days postinfection by proliferation assays in the presence of UV-inactivated BeAn, DA, or viral epitope peptides. Triplicate cultures were stimulated with different concentrations of UV-inactivated viruses (0.1 to 3 μg) (A) and TMEV T-cell epitopes (0.5 to 10 μM VP1233-250, VP274-86, and VP324-37) (B) for 72-h cultures, pulsed with 1 μCi of [3H]thymidine (TdR), and then harvested 18 h later. Measurements of [3H]thymidine uptake by the cells were determined in a scintillation counter and expressed as counts per minute. Background levels in the absence of specific antigens were less than 2,500 cpm.
H-2Db-restricted CTL responses to DA and BeAn are similar in resistant C57BL/6 mice.
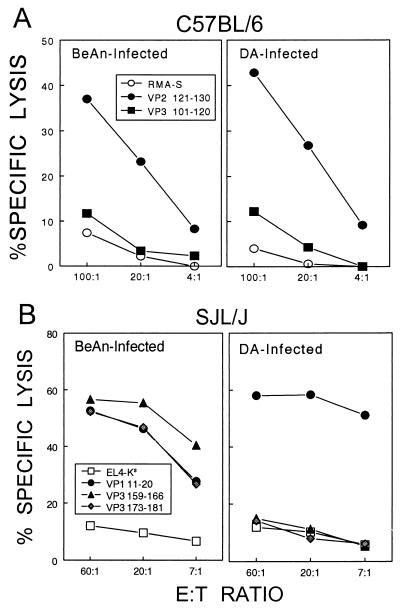
A predominant H-2Db-restricted cytotoxic T-lymphocyte (CTL) epitope region (VP2121-130) of DA is identical to that of BeAn (19, 21). In addition, subdominant H-2Db-restricted epitopes (VP2165-173 and VP3110-120) were recently identified with the BeAn strain (17), and these regions are also identical between DA and BeAn viruses. To compare the levels and recognition of these epitopes, cytolytic levels of epitope-loaded target cells for CNS-infiltrating CD8+ T cells from either DA- or BeAn-infected C57BL/6 mice were assessed with predominant and subdominant BeAn epitope peptides at 8 days postinfection (Fig. 2A). The results clearly indicate that the levels and recognition of the predominant (VP2121-130) as well as the subdominant (VP3110-120) CTL epitope are virtually identical after infection with DA and after infection with BeAn. This is not surprising, since these epitope region sequences are identical in the two strains of TMEV.
FIG. 2.
CTL specificity and cytotoxic function in resistant C57BL/6 and susceptible SJL mice infected with BeAn and DA viruses. Cytolytic function of CD8+ T cells in the CNS of virus-infected mice was assessed by the standard 51Cr release assay 8 days post-viral infection. Mononuclear cells from brains and spinal cords were prepared with a continuous Percoll gradient (Pharmacia, Piscataway, N.J.) as described previously (17) and used as effector cells. (A) Cytotoxic function of CTLs in resistant C57BL/6 mice. RMA-S cells loaded with predominant and subdominant CTL epitope peptides were used as target cells with various effector cell numbers. (B) Cytotoxic function of CTLs in susceptible SJL/J mice. EL-4Ks cells loaded with a predominant and two subdominant CTL epitope peptides were used as target cells. The EL-4Ks cell line was generated by electroporation of EL-4 cells as described previously (11). E:T ratio, effector/target cell ratio.
H-2Ks-restricted CTLs induced by DA infection in susceptible SJL/J mice fail to recognize two of three epitope regions identified with BeAn.
Since the susceptibility and pathogenic mechanisms have been studied extensively with prototypic, highly susceptible SJL/J mice, it would be of great value to compare the levels of and epitope recognition by CNS-infiltrating CD8+ T cells in this strain of mice. Recently, three major H-2Ks-restricted BeAn epitopes (VP3159-166 > VP3173-181 > VP111-20) recognized by CNS-infiltrating CD8+ T cells from SJL mice infected with BeAn have been identified (11). Hence, the levels and recognition of these epitopes by CD8+ T cells from DA and BeAn were compared by using the BeAn epitope peptides (Fig. 2B). Interestingly, only VP111-20-loaded target cells, not VP3159-166- or VP3173-181-loaded cells, were lysed over the background by CNS-infiltrating T cells from DA-infected mice. In fact, the level of lysis seen with VP111-20 by T cells from DA-infected mice was slightly greater than that for T cells from BeAn-infected mice. The sequence comparisons between BeAn and DA indicate an alteration (N versus V) at the P2 position of the predominant VP3159-166 epitope and a change (P versus A) at the P7 position of the intermediate VP3173-181 epitope, respectively. Thus, these results indicate that single-amino-acid changes in these two DA epitope regions prevented the recognition by and/or induction of CD8+ T cells reactive to the BeAn epitopes following infection with DA virus.
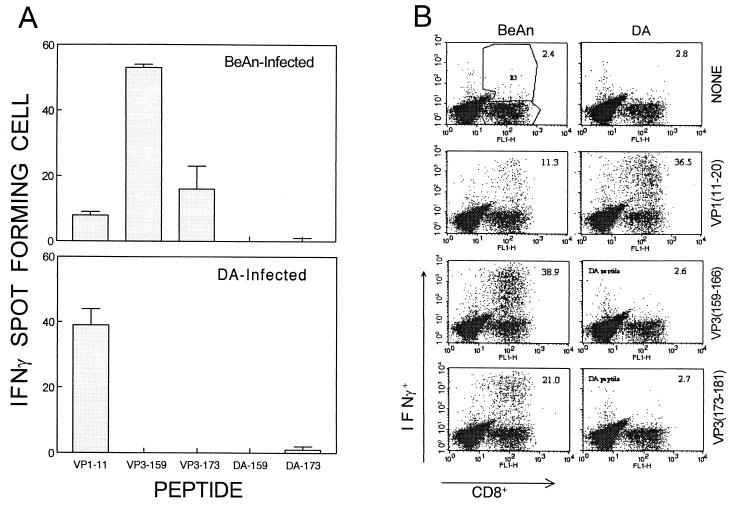
Failure of target cell lysis for the predominant and intermediate BeAn epitope peptides by CD8+ T cells from DA-infected mice could reflect poor epitope recognition due to amino acid sequence alterations or lack of CTL cell response. This possibility was examined with ELISPOT and intracellular cytokine staining (Fig. 3). For ELISPOT experiments, plates (Millipore, Bedford, Mass.) were precoated with 1 to 5 μg of anti-gamma interferon (IFN-γ) antibody per ml and then incubated for 18 h with 2 × 104 CNS-infiltrating lymphocytes plus 106 irradiated (3,000-rad) syngeneic spleen cells at 37°C under 5% CO2 in the presence of 2 μM peptides (17, 27). Intracellular IFN-γ staining was performed as described previously (11) and then analyzed on a Becton Dickinson FACSCalibur flow cytometer. Based on the lack of cytolytic function (data not shown) as well as IFN-γ production measured by ELISPOT and intracellular cytokine staining, CNS-infiltrating CD8+ T cells from DA-infected SJL/J mice failed to recognize the peptides representing the DA epitope sequences. These results clearly indicate that the sequence differences in these predominant and intermediate epitope regions of DA virus prevent the induction of CD8+ T-cell responses to these regions following DA infection in susceptible SJL/J mice. Consequently, the number of capsid epitopes recognized by CD8+ T cells from DA-infected SJL/J mice may be reduced to one, unless new epitopes are created by differences in the sequences at other regions. These differences in the epitope recognition between mice infected with DA and BeAn viruses may differentially affect the outcome for protection from and/or pathogenesis of TMEV-induced demyelinating disease.
FIG. 3.
IFN-γ production by CD8+ T cells infiltrating the CNS of SJL/J mice infected with BeAn and DA viruses. The levels and epitope specificity of CD8+ T cells infiltrating the CNS of virus-infected mice were assessed by measuring IFN-γ production upon stimulation with various peptides by ELISPOT assay (A) and intracellular cytokine staining (B). Mononuclear cell populations isolated from the brains and spinal cords of mice infected with viruses for 8 days were analyzed. Peptides (2 μM) representing both BeAn epitope regions (VP1-11, VP3-159, and VP3-173) and DA epitope regions (VP1-11, DA-159, and DA-173) were included.
Level of DA-induced CD8+ T cells against the common epitope (VP111-20) in SJL mice is significantly increased.
Because the epitopes recognized by CD8+ T cells induced by DA infection are drastically different from those recognized by cells induced by BeAn infection, the levels of CNS-infiltrating CD8+ T cells specific for the common epitope (VP111-20) were further compared by enumerating IFN-γ-producing cells generated in response to the epitope (Fig. 3). ELISPOT assays indicated that the number of VP111-20-reactive CD8+ T cells in DA-infected mice is increased compared to that in BeAn-infected SJL/J mice, although the total number of capsid-specific CD8+ T cells is much higher in BeAn-infected mice. This is more readily detectable by flow cytometric analysis following intracellular IFN-γ staining. A threefold increase, from 11 to 37%, is seen in the VP111-20-specific CD8+ T cells infiltrating the CNS of DA-infected mice compared to those for BeAn-infected mice. This is similar to the proportion (39%) of CD8+ T cells reactive to the predominant VP3159-166 epitope in BeAn-infected mice. None of the other epitope region peptides, regardless of the origin of the viruses, were able to stimulate cytokine production, confirming the lack of CD8+ T-cell responses to these two epitope regions. Since the numbers of infiltrating mononuclear cells were similar between BeAn- and DA-infected mice (1.6 × 106 versus 1.5 × 106 cells, respectively), this increase in the proportion of CD8+ T cells specific for VP111-20 in DA-infected mice represents an increase in the number of CD8+ T cells reactive to this epitope. More than 60% of the CNS-infiltrating CD8+ T cells can be accounted for as specific for these epitopes following BeAn infection, whereas only 37% can be done so following DA infection. Therefore, the overall relatively lower level of CTLs specific for TMEV in DA-infected SJL/J mice may be associated with susceptibility to the early acute-phase disease in DA-infected mice compared to BeAn-infected mice, despite the fact that the VP1-specific CTL level is increased in DA-infected mice. The possibility of CTL escape mutations is unlikely to be an important factor, since our preliminary sequence analysis of the epitope regions in the persisting viruses does not indicate any mutations. However, it is not yet clear whether the remainder of CD8+ T cells infiltrating the CNS recognize a new epitope region(s) of the capsid proteins, due to amino acid sequence differences, and hence the overall numbers of infiltrating CD8+ T cells are similar. Alternatively, it is conceivable that a larger proportion of CD8+ T cells from DA-infected SJL/J mice than from BeAn-infected mice recognize epitopes in the nonstructural proteins. Nevertheless, the CD8+ T-cell response to this epitope in DA-infected mice may have a selective advantage over such T cells reactive to other undefined epitopes, in the absence of reactivity to the other capsid epitopes. The lack of response to other capsid epitopes and/or an increased generation of VP111-20 peptide due to sequence differences in the flanking regions may facilitate a competitive advantage for the T-cell response to this epitope in DA-infected mice. Perhaps such a preferred expansion of CD8+ T cells reactive to VP111-20 may facilitate the recruitment of CD8+ T cells with higher avidity to the epitope.
Avidity of CD8+ T cells to VP111-20 is drastically altered in SJL/J mice infected with DA virus.
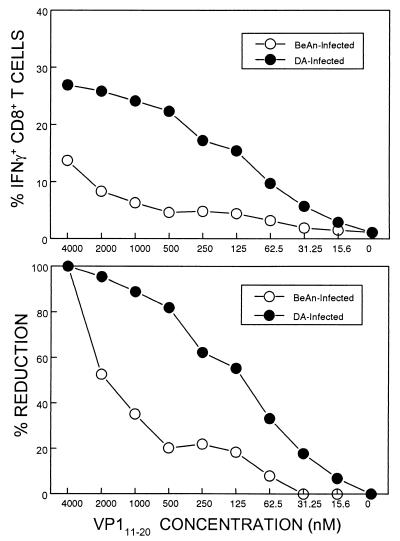
We have previously observed for SJL/J mice infected with BeAn that CNS-infiltrating CD8+ T cells reactive to the predominant epitope, VP3159-166, display relatively higher avidity than do CD8+ T cells reactive to the other epitopes, including VP111-20 (11). Because the level of CD8+ T cells reactive to VP111-20 was significantly increased in mice infected with DA, comparable to the level specific for the predominant epitope in BeAn-infected mice, the potential increase in the avidity of VP111-20-specific CD8+ T cells from the DA-infected mice was assessed (Fig. 4). The flow cytometric enumeration of IFN-γ-producing cells from BeAn- or DA-infected mice in response to various concentrations of VP111-20 peptides clearly demonstrates that VP111-20-specific CD8+ T cells from DA-infected mice have much greater avidity to the cognate peptide than do those from BeAn-infected SJL/J mice. For example, the level of IFN-γ-producing cells in BeAn-infected mice was reduced to ∼50% when the concentration of peptide was lowered from 4 to 2 μM, whereas that in DA-infected mice was reduced to less than 5%. These results strongly suggest that a subdominant CD8+ T-cell epitope of BeAn functions as a predominant epitope of DA virus by increasing the avidity as well as the proportion in the infiltrating CD8+ T-cell population.
FIG. 4.
Avidity toward VP111-20 peptide by CD8+ T cells from BeAn- and DA virus-infected SJL/J mice. Avidity to the peptide ligand was assessed by measuring IFN-γ production in response to various concentrations of VP111-20 by flow cytometric analysis following intracellular IFN-γ staining as described above.
The effect of these differences in the epitope recognition on the pathogenic mechanisms of and/or protection from TMEV-induced demyelinating disease is not yet clear. However, it would be interesting to speculate that this difference in the CD8+ T-cell responses may result in the induction of significant early-phase disease, a polio-like encephalomyelitis in SJL/J mice infected with DA compared to an undetectable level of disease in BeAn-infected mice. Although CD8+ T-cell infiltration is similar between mice infected with these viruses, overall a lower CD8+ T-cell response to capsid proteins in DA-infected mice may provide less efficient initial viral clearance, and this may lead to induction of encephalomyelitis due to elevated viral replication in the CNS. Nevertheless, our findings indicating the differences in the levels and recognition of capsid epitopes in these two representative pathogenic TMEV strains will provide an important tool for dissecting one of the important aspects of the immune response and its correlation with the pathogenic mechanisms.
Acknowledgments
This work was supported by grants NS23349, NS28752, and NS33008 from the USPHS and RG3126-A-4 from the National Multiple Sclerosis Society.
REFERENCES
- 1.Begolka, W. S., L. M. Haynes, J. K. Olson, J. Padilla, K. L. Neville, M. D. Canto, J. Palma, B. S. Kim, and S. D. Miller. 2001. CD8-deficient SJL mice display enhanced susceptibility to Theiler's virus infection and increased demyelinating pathology. J. Neurovirol. 7:409-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrow, P., P. Tonks, C. J. Welsh, and A. A. Nash. 1992. The role of CD8+ T cells in the acute and chronic phases of Theiler's murine encephalomyelitis virus-induced disease in mice. J. Gen. Virol. 73:1861-1865. [DOI] [PubMed] [Google Scholar]
- 3.Borson, N. D., C. Paul, X. Lin, W. K. Nevala, M. A. Strausbauch, M. Rodriguez, and P. J. Wettstein. 1997. Brain-infiltrating cytolytic T lymphocytes specific for Theiler's virus recognize H2Db molecules complexed with a viral VP2 peptide lacking a consensus anchor residue. J. Virol. 71:5244-5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bureau, J. F., X. Montagutelli, S. Lefebvre, J. L. Guenet, M. Pla, and M. Brahic. 1992. The interaction of two groups of murine genes determines the persistence of Theiler's virus in the central nervous system. J. Virol. 66:4698-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dal Canto, M. C., B. S. Kim, S. D. Miller, and R. W. Melvold. 1996. Theiler's murine encephalomyelitis virus (TMEV)-induced demyelination: a model for human multiple sclerosis. Methods 10:453-461. [DOI] [PubMed] [Google Scholar]
- 6.Dal Canto, M. C., and H. L. Lipton. 1975. Primary demyelination in Theiler's virus infection. An ultrastructural study. Lab. Investig. 33:626-637. [PubMed] [Google Scholar]
- 7.Dethlefs, S., N. Escriou, M. Brahic, S. van der Werf, and E. L. Larsson-Sciard. 1997. Theiler's virus and Mengo virus induce cross-reactive cytotoxic T lymphocytes restricted to the same immunodominant VP2 epitope in C57BL/6 mice. J. Virol. 71:5361-5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiette, L., C. Aubert, M. Brahic, and C. P. Rossi. 1993. Theiler's virus infection of β2-microglobulin-deficient mice. J. Virol. 67:589-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerety, S. J., W. J. Karpus, A. R. Cubbon, R. G. Goswami, M. K. Rundell, J. D. Peterson, and S. D. Miller. 1994. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus-induced demyelinating disease. V. Mapping of a dominant immunopathologic VP2 T cell epitope in susceptible SJL/J mice. J. Immunol. 152:908-918. [PubMed] [Google Scholar]
- 10.Johnson, A. J., M. K. Njenga, M. J. Hansen, S. T. Kuhns, L. Chen, M. Rodriguez, and L. R. Pease. 1999. Prevalent class I-restricted T-cell response to the Theiler's virus epitope Db:VP2121-130 in the absence of endogenous CD4 help, tumor necrosis factor alpha, gamma interferon, perforin, or costimulation through CD28. J. Virol. 73:3702-3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang, B. S., M. A. Lyman, and B. S. Kim. 2002. The majority of infiltrating CD8+ T cells in the central nervous system of susceptible SJL/J mice infected with Theiler's virus are virus specific and fully functional. J. Virol. 76:6577-6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, B. S., M. A. Lyman, B. S. Kang, H. K. Kang, H. G. Lee, M. Mohindru, and J. P. Palma. 2001. Pathogenesis of virus-induced immune-mediated demyelination. Immunol. Res. 24:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehrich, J. R., B. G. Arnason, and F. H. Hochberg. 1976. Demyelinative myelopathy in mice induced by the DA virus. J. Neurosci. 29:149-160. [DOI] [PubMed] [Google Scholar]
- 14.Lipton, H. L., and A. Friedmann. 1980. Purification of Theiler's murine encephalomyelitis virus and analysis of the structural virion polypeptides: correlation of the polypeptide profile with virulence. J. Virol. 33:1165-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipton, H. L., J. Kratochvil, P. Sethi, and M. C. Dal Canto. 1984. Theiler's virus antigen detected in mouse spinal cord 2 1/2 years after infection. Neurology 34:1117-1119. [DOI] [PubMed] [Google Scholar]
- 16.Lipton, H. L., and R. Melvold. 1984. Genetic analysis of susceptibility to Theiler's virus-induced demyelinating disease in mice. J. Immunol. 132:1821-1825. [PubMed] [Google Scholar]
- 17.Lyman, M. A., H. G. Lee, B. S. Kang, H. K. Kang, and B. S. Kim. 2002. Capsid-specific cytotoxic T lymphocytes recognize three distinct H-2Db-restricted regions of the BeAn strain of Theiler's virus and exhibit different cytokine profiles. J. Virol. 76:3125-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray, P. D., D. B. McGavern, X. Lin, M. K. Njenga, J. Leibowitz, L. R. Pease, and M. Rodriguez. 1998. Perforin-dependent neurologic injury in a viral model of multiple sclerosis. J. Neurosci. 18:7306-7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohara, Y., S. Stein, J. L. Fu, L. Stillman, L. Klaman, and R. P. Roos. 1988. Molecular cloning and sequence determination of DA strain of Theiler's murine encephalomyelitis viruses. Virology. 164:245-255. [DOI] [PubMed] [Google Scholar]
- 20.Palma, J. P., H.-G. Lee, B.-S. Kang, M. Dal Canto, S. D. Miller, and B. S. Kim. 2001. Enhanced susceptibility to Theiler's virus-induced demyelinating disease in perforin-deficient mice. J. Neuroimmunol. 116:125-135. [DOI] [PubMed] [Google Scholar]
- 21.Pevear, D. C., M. Calenoff, E. Rozhon, and H. L. Lipton. 1987. Analysis of the complete nucleotide sequence of the picornavirus Theiler's murine encephalomyelitis virus indicates that it is closely related to cardioviruses. J. Virol. 61:1507-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pullen, L. C., S. D. Miller, M. C. Dal Canto, and B. S. Kim. 1993. Class I-deficient resistant mice intracerebrally inoculated with Theiler's virus show an increased T cell response to viral antigens and susceptibility to demyelination. Eur. J. Immunol. 23:2287-2293. [DOI] [PubMed] [Google Scholar]
- 23.Rivera-Quinones, C., D. McGavern, J. D. Schmelzer, S. F. Hunter, P. A. Low, and M. Rodriguez. 1998. Absence of neurological deficits following extensive demyelination in a class I-deficient murine model of multiple sclerosis. Nat. Med. 4:187-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez, M., and C. S. David. 1985. Demyelination induced by Theiler's virus: influence of the H-2 haplotype. J. Immunol. 135:2145-2148. [PubMed] [Google Scholar]
- 25.Rodriguez, M., A. J. Dunkel, R. L. Thiemann, J. Leibowitz, M. Zijlstra, and R. Jaenisch. 1993. Abrogation of resistance to Theiler's virus-induced demyelination in H-2b mice deficient in beta 2-microglobulin. J. Immunol. 151:266-276. [PubMed] [Google Scholar]
- 26.Rodriguez, M., J. Leibowitz, and P. Lampert. 1983. Persistent infection of oligodendrocytes in Theiler's virus induced encephalomyelitis. Ann. Neurol. 13:426-433. [DOI] [PubMed] [Google Scholar]
- 27.Targoni, O. S., and P. V. Lehmann. 1998. Endogenous myelin basic protein inactivates the high avidity T cell repertoire. J. Exp. Med. 187:2055-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theiler, M., and S. Gard. 1940. Encephalomyelitis of mice. J. Exp. Med. 72:49-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamada, M., A. Zurbriggen, and R. S. Fujinami. 1990. The relationship between viral RNA, myelin-specific mRNAs, and demyelination in central nervous system disease during Theiler's virus infection. Am. J. Pathol. 137:1467-1479. [PMC free article] [PubMed] [Google Scholar]
- 30.Yauch, R. L., K. Kerekes, K. Saujani, and B. S. Kim. 1995. Identification of a major T-cell epitope within VP3 amino acid residues 24 to 37 of Theiler's virus in demyelination-susceptible SJL/J mice. J. Virol. 69:7315-7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yauch, R. L., and B. S. Kim. 1994. A predominant viral epitope recognized by T cells from the periphery and demyelinating lesions of SJL/J mice infected with Theiler's virus is located within VP1(233-244). J. Immunol. 153:4508-4519. [PubMed] [Google Scholar]