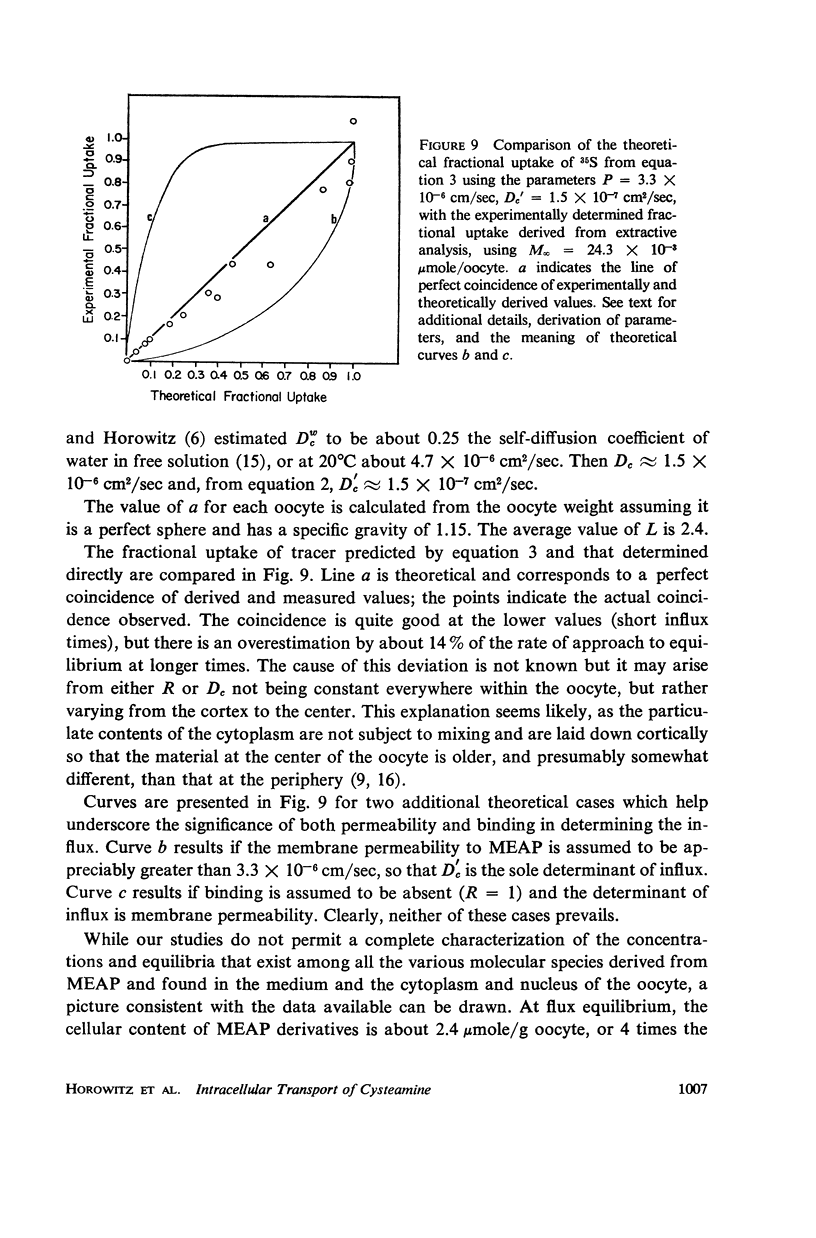
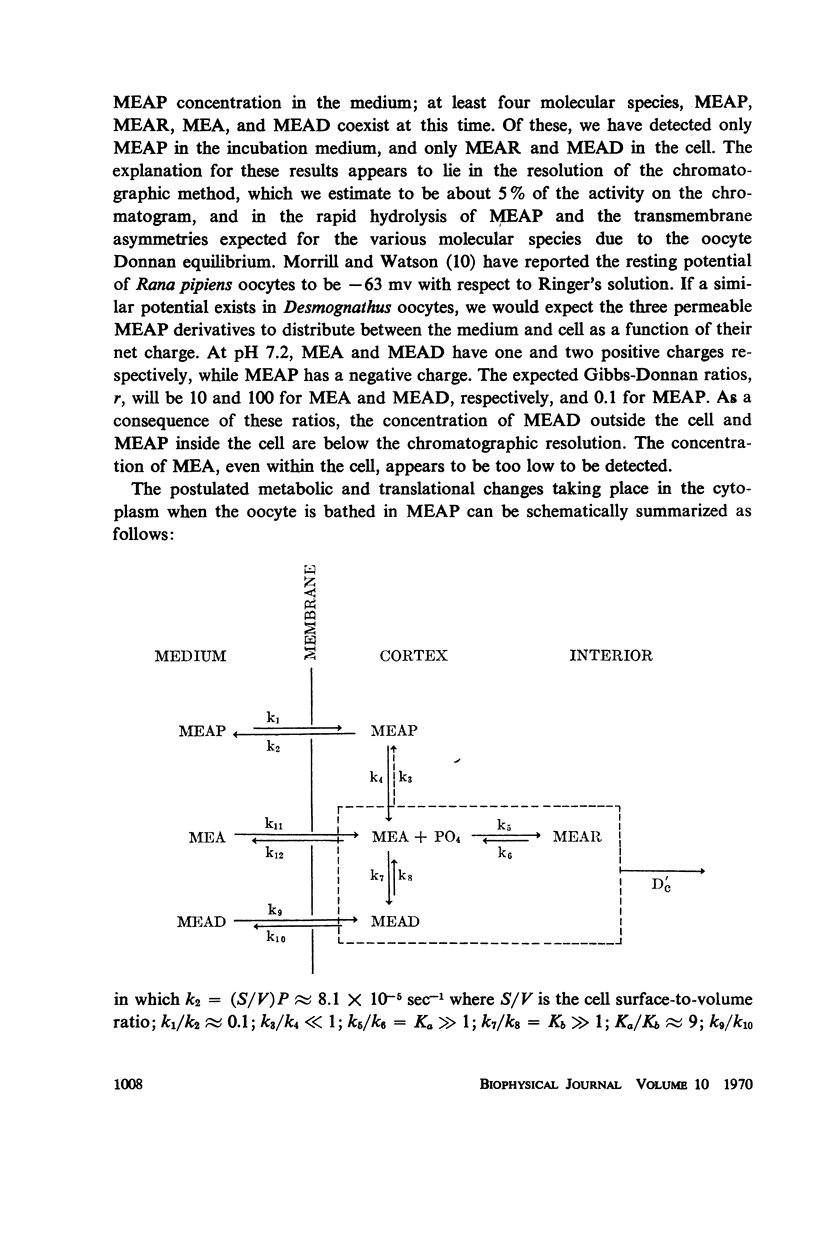
Abstract
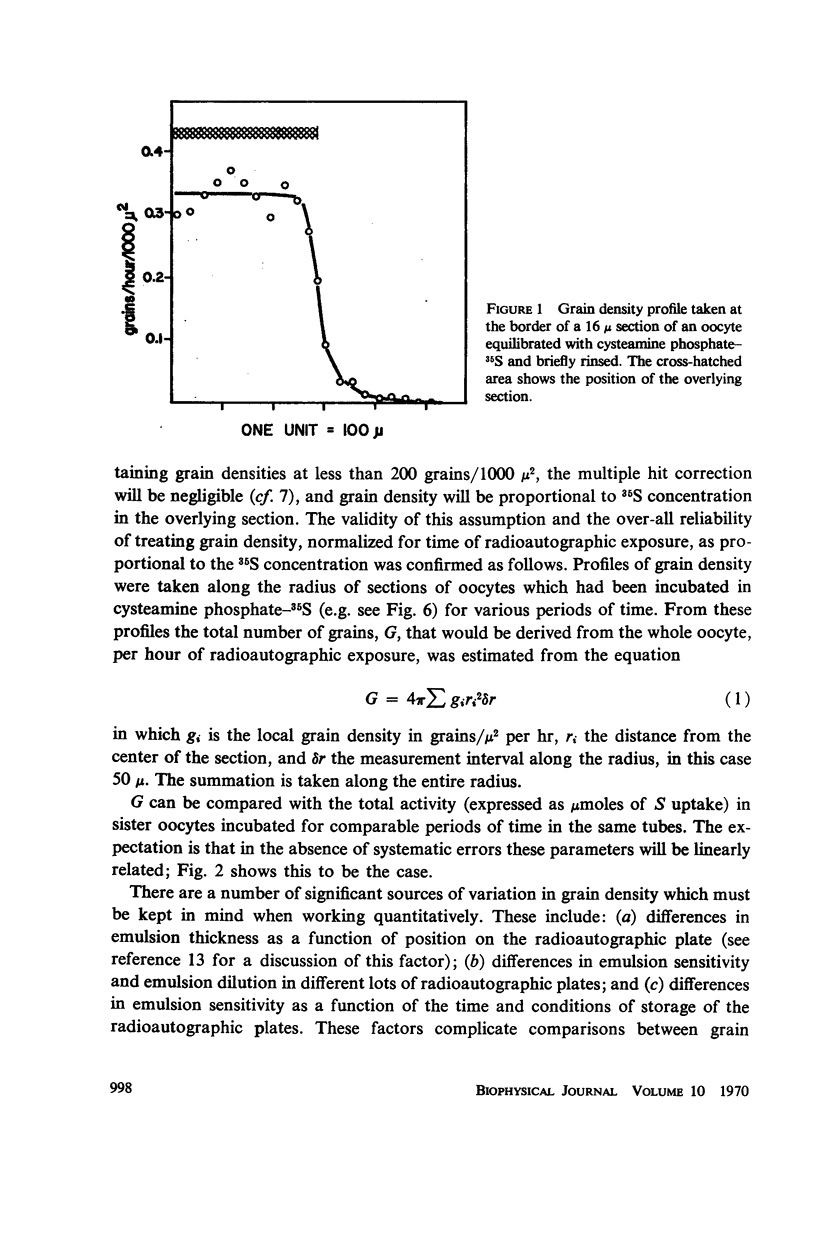
Radioautography and extractive techniques were used to analyze the transport of cysteamine phosphate and its derivatives in salamander oocytes. The quantitative relations among the processes involved — membrane permeation, enzymatic dephosphorylation, binding through mixed disulfide formation, and cytoplasmic diffusion — were elucidated. Within the detection limits, all of the intracellular material is present as dephosphorylated derivatives. Cytoplasmic diffusion is effectively slowed by binding (the “chromatographic” effect) and makes an appreciable contribution to cellular flux rates. As a consequence, one can observe by radioautography a cortical diffusion ring which spreads inward as a function of influx time, while also increasing in peak density because of the finite membrane permeability. Good agreement was found between the transport parameters determined by radioautography and those from influx data for the whole oocyte. The ratio of nuclear to cytoplasmic concentrations of the cysteamine phosphate derivatives at equilibrium is about 0.4. The nuclear membrane is, however, a negligible barrier to transport, and the asymmetry appears to arise primarily from the quantity and sulfhydryl content of the binding proteins in the two compartments.
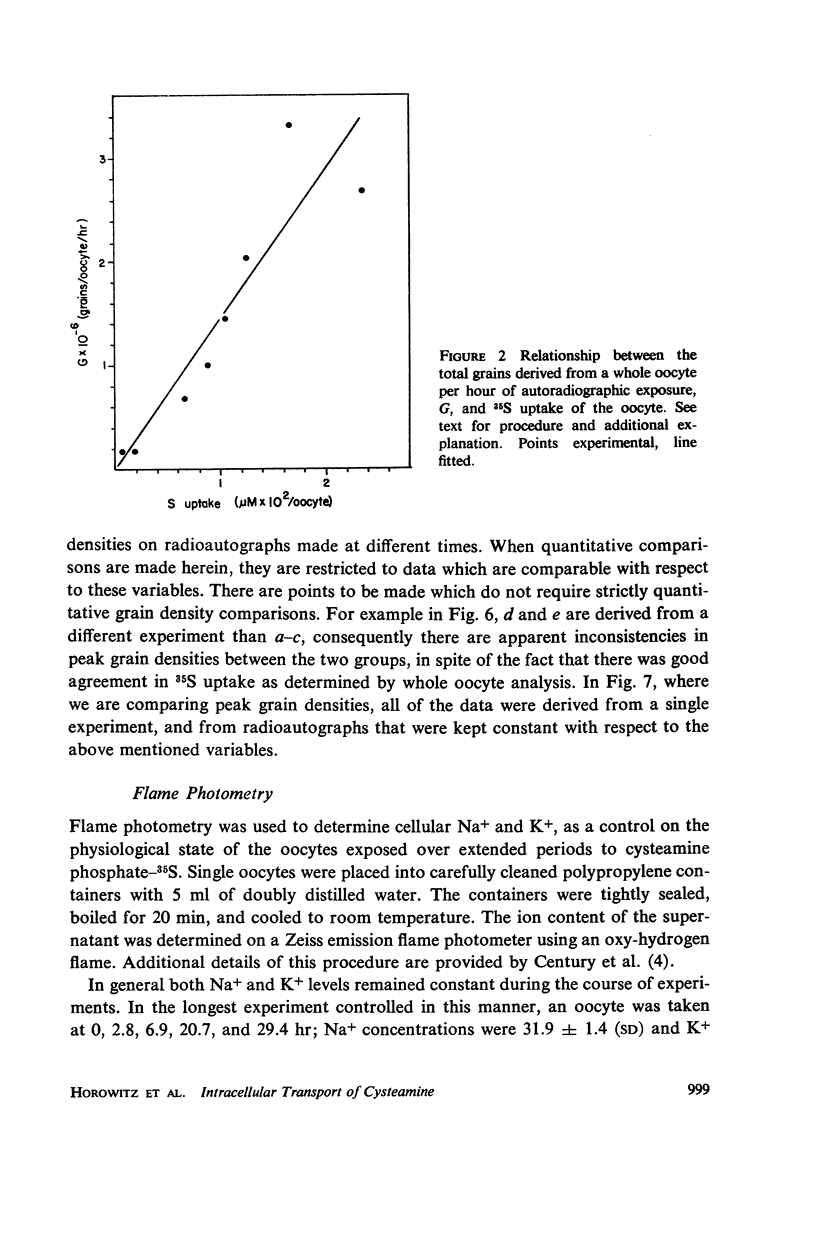
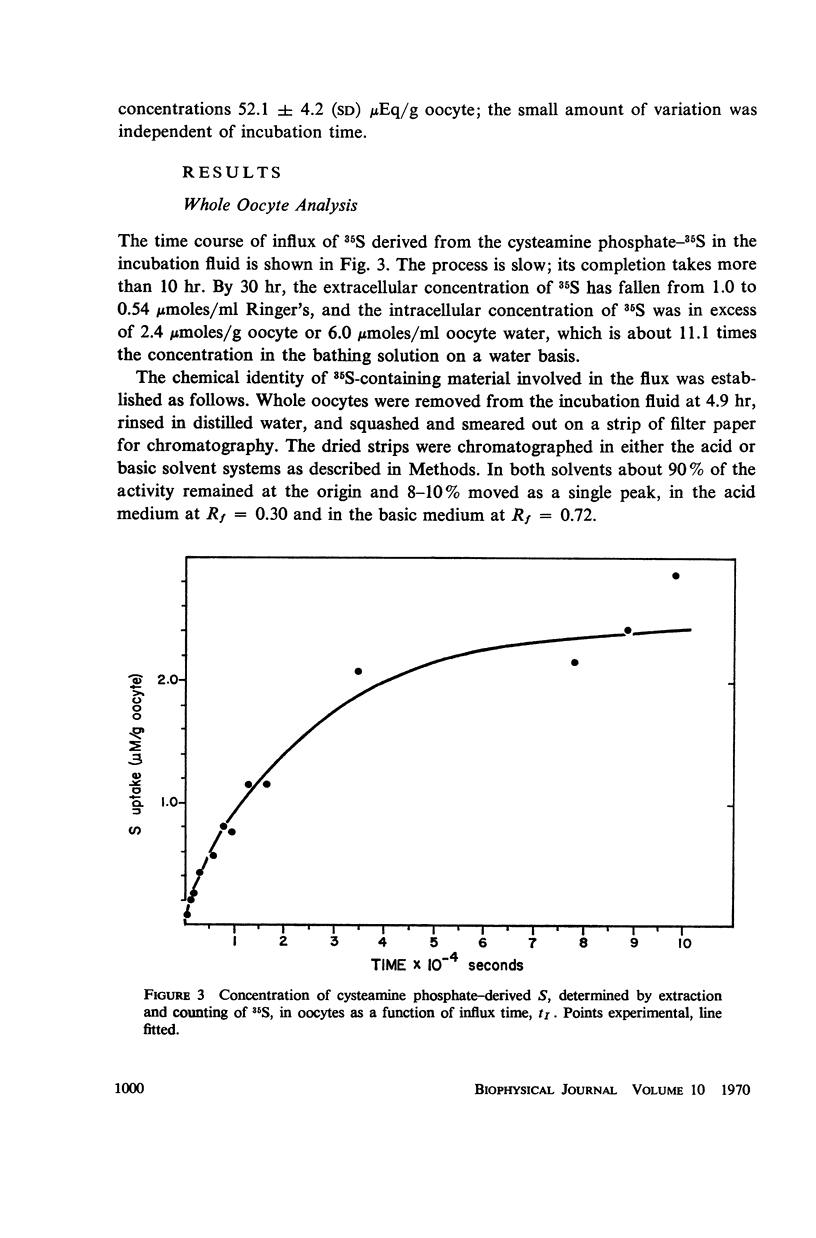
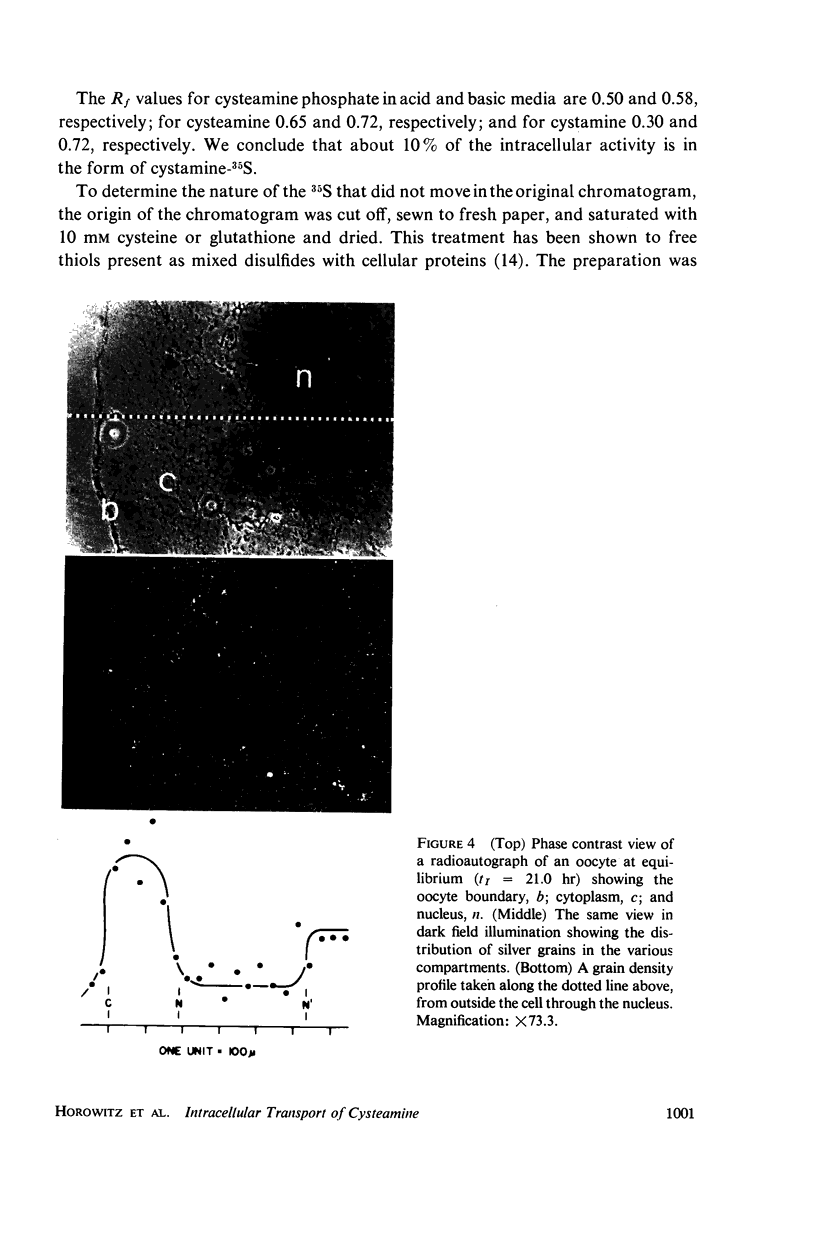
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleton T. C. Resolving power, sensitivity and latent image fading of soluble-compound autoradiographs. J Histochem Cytochem. 1966 May;14(5):414–420. doi: 10.1177/14.5.414. [DOI] [PubMed] [Google Scholar]
- Century T. J., Fenichel I. R., Horowitz S. B. The concentrations of water, sodium and potassium in the nucleus and cytoplasm of amphibian oocytes. J Cell Sci. 1970 Jul;7(1):5–13. doi: 10.1242/jcs.7.1.5. [DOI] [PubMed] [Google Scholar]
- Century T. J., Fenichel I. R., Horowitz S. B. The concentrations of water, sodium and potassium in the nucleus and cytoplasm of amphibian oocytes. J Cell Sci. 1970 Jul;7(1):5–13. doi: 10.1242/jcs.7.1.5. [DOI] [PubMed] [Google Scholar]
- Horowitz S. B., Fenichel I. R. Analysis of glycerol-3H transport in the frog oocyte by extractive and radioautographic techniques. J Gen Physiol. 1968 Jun;51(6):703–730. doi: 10.1085/jgp.51.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEMP N. E. Electron microscopy of growing oocytes of Rana pipiens. J Biophys Biochem Cytol. 1956 May 25;2(3):281–292. doi: 10.1083/jcb.2.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill G. A., Watson D. E. Transmembrane electropotential changes in amphibian eggs at ovulation, activation and first cleavage. J Cell Physiol. 1966 Feb;67(1):85–92. doi: 10.1002/jcp.1040670110. [DOI] [PubMed] [Google Scholar]
- NADLER N. J. Some theoretical aspects of radioautography. Can J Med Sci. 1951 Aug;29(4):182–194. doi: 10.1139/cjms51-024. [DOI] [PubMed] [Google Scholar]
- Neumann H. Substrate selectivity in the action of alkaline and acid phosphatases. J Biol Chem. 1968 Sep 25;243(18):4671–4676. [PubMed] [Google Scholar]



