Abstract
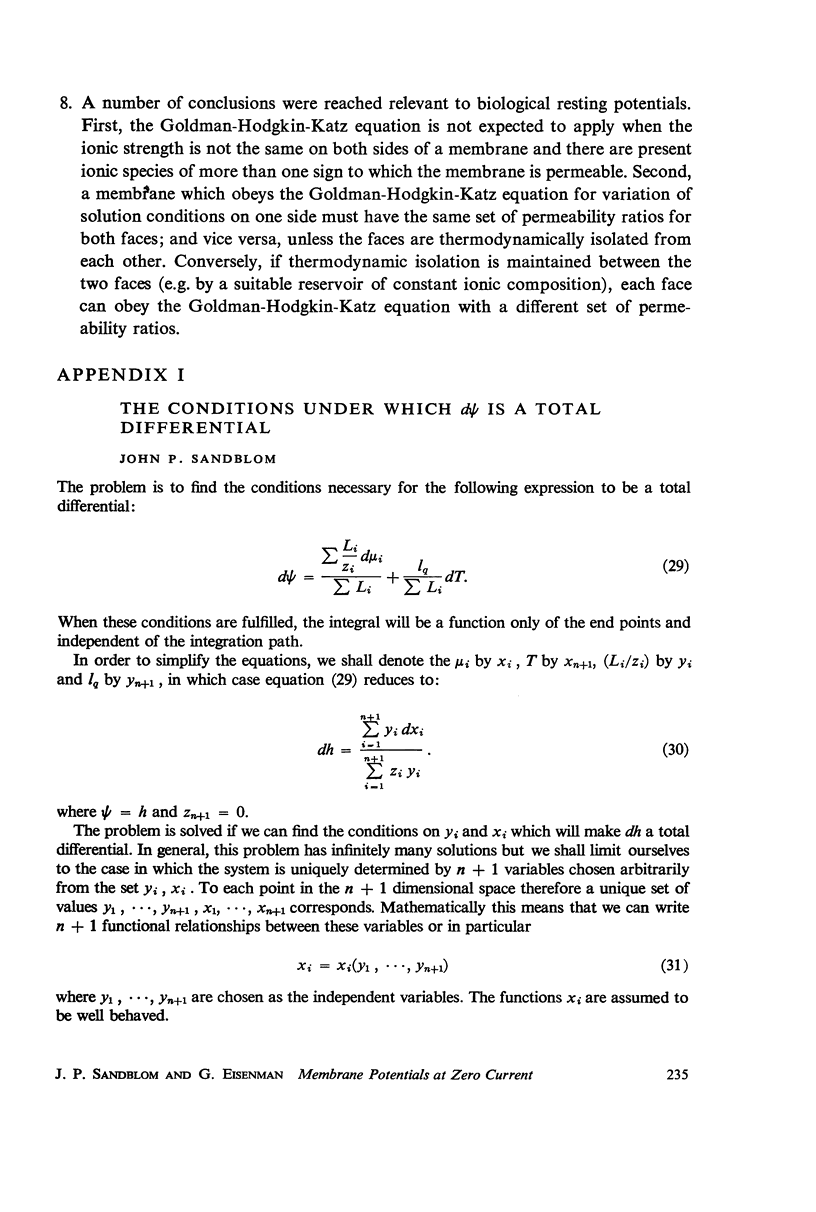
The possibility has been examined that the Goldman-Hodgkin-Katz equation for V0, the total membrane potential at zero current, can be derived with constant permeability ratios from a thermodynamic treatment. The flux equations have been integrated under zero current conditions subject only to the restriction that the total membrane potential should be independent of internal concentration profiles, which is the requirement for the premeability ratios to be phenomenological constants, independent of solution conditions. No assumptions have been made concerning the electric potential profile. It was found that a constant permeability ratio can only be characteristic of systems satisfying certain relationships between ionic conductances and chemical potentials. From these relationships it was possible to define the permeability ratio in terms of the thermodynamic properties of the membrane quite generally and to identify the permeability ratio as the product of mobility ratio and ratio of partition coefficients. Moreover, the ionic conductance ratio at any point in the membrane has been shown to be expressable explicitly in terms of the permeability ratio and the activities of an external solution which would be in equilibrium with the point under consideration. Lastly, a number of conclusions have been reached regarding the range of applicability of the Goldman-Hodgkin-Katz equation with constant permeability ratios.
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER P. F., HODGKIN A. L., MEVES H. THE EFFECT OF DILUTING THE INTERNAL SOLUTION ON THE ELECTRICAL PROPERTIES OF A PERFUSED GIANT AXON. J Physiol. 1964 Apr;170:541–560. doi: 10.1113/jphysiol.1964.sp007348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONTI F., EISENMAN G. THE NON-STEADY STATE MEMBRANE POTENTIAL OF ION EXCHANGERS WITH FIXED SITES. Biophys J. 1965 Mar;5:247–256. doi: 10.1016/s0006-3495(65)86714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Eisenman G. The steady-state properties of an ion exchange membrane with mobile sites. Biophys J. 1966 May;6(3):227–246. doi: 10.1016/S0006-3495(66)86653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein A., Mauro A. Equivalent Circuits as Related to Ionic Systems. Biophys J. 1963 May;3(3):215–237. doi: 10.1016/s0006-3495(63)86817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDLEY B. D., HOSHIKO T. THE EFFECTS OF ALKALI METAL CATIONS AND COMMON ANIONS ON THE FROG SKIN POTENTIAL. J Gen Physiol. 1964 Mar;47:749–771. doi: 10.1085/jgp.47.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandblom J. P. A method to relate steady-state ionic currents, conductances, and membrane potential in ion exchange membranes with unknown thermodynamic properties. Biophys J. 1967 May;7(3):243–265. doi: 10.1016/S0006-3495(67)86586-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEORELL T. Electrokinetic membrane processes in relation to properties excitable tissues. II. Some theoretical considerations. J Gen Physiol. 1959 Mar 20;42(4):847–863. doi: 10.1085/jgp.42.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]



