Abstract
Retrovirus Gag proteins are synthesized on free ribosomes, and are sufficient to govern the assembly and release of virus particles. Like type C retroviruses, human T-cell leukemia virus type 1 (HTLV-1) assembles and buds at the plasma membrane. After immunofluorescence staining, HTLV-1 Gag proteins appear as punctuated intracellular clusters, which suggests that they are associated either with intracellular membranes or with the plasma membrane. However, colocalization experiments using a panel of markers demonstrated that Gag proteins were not associated with the membranes involved in the secretory or endocytosis pathway. Small amounts of Gag proteins were detected at the plasma membrane and colocalized with the envelope glycoproteins. Moreover, Gag proteins were excluded from streptolysin-O permeabilized cells and in this respect behaved like cytoplasmic proteins. This suggests that the trafficking of HTLV-1 Gag proteins through the cytoplasm of the host cell is independent of any cell membrane system.
During the late stages of virus replication, retroviruses assemble and bud from a cell membrane, where they acquire their envelope (29). The membrane involved is usually the plasma membrane, except for spumaviruses, which bud from the endoplasmic reticulum (ER) (7). Retrovirus assembly and budding depend on the Gag proteins (8, 25), and these proteins alone are sufficient for the production of virus particles, again with the exception of spumaviruses (4, 7, 8, 25).
The intracellular loci where the self-association of Gag proteins occurs in type C and type D retroviruses are different (29). Type D retroviruses first assemble within the cytoplasm, where they form preassembled capsids visible by electron microscopy. These capsids are then transported to the plasma membrane for budding and release. In contrast, in type C retrovirus morphogenesis, the assembly of Gag proteins into electron-dense structures begins at the plasma membrane and occurs simultaneously with the virus budding process. A single amino acid substitution in Gag proteins, the R55W mutation, converts the type D Mason-Pfizer monkey virus into a C-type-like retrovirus, which assembles at the plasma membrane (27). This suggests that Gag proteins of type D retroviruses may carry an intracellular retention signal, which restricts their transportation to the cell surface (30, 43). This signal seems to be masked after the intracytoplasmic-assembly phase and to be disturbed by the R55W mutation (26, 30). However, the intracellular self-association of Gag proteins may not be restricted to type D retroviruses, because several findings suggest that type C human immunodeficiency virus type 1 (HIV-1) Gag proteins may also form intracellular assembly intermediate complexes but that they are not visible under electron microscopy (16–18, 31).
Gag proteins are translated on free ribosomes in the cytosol. They are bound to the inner face of the plasma membrane via a myristate added cotranslationally to their N terminus, via an N-terminal cluster of basic amino acids of Gag precursors, or via both (2, 9, 15, 24, 26, 27, 35, 44, 45). However, the intracellular pathway followed by Gag proteins of retroviruses before they reach the plasma membrane remains to be elucidated. Early data suggested that they could shuttle using secretion pathway vesicles, since budding is inhibited in the presence of monensin, a drug which blocks the secretory process (12, 14). However, further experiments did not confirm this (38). We were puzzled by the punctuated staining of Gag proteins of the type C human T-cell leukemia virus type 1 (HTLV-1) that we observed both in cells transfected with the XMT infectious provirus and in T cells chronically infected with HTLV-1 (15). Punctuated staining is usually observed with viral envelope proteins expressed at the plasma membrane or viral proteins which traffic via cargo vesicles of the secretory or the endocytic pathway. Moreover, when mutant HTLV-1 Gag proteins lacking their myristate membrane anchorage were tested under the same conditions, the staining was diffusely and regularly distributed throughout the cytoplasm (15). The combination of these observations suggests that the punctuated staining of HTLV-1 Gag proteins could reflect either the budding step of the virus at the plasma membrane or their traffic to the plasma membrane via intracellular compartments.
To address this question, we performed colocalization experiments using immunofluorescence staining of Gag proteins and of several well-established markers of cellular compartments or of the plasma membrane. HeLa cells cultured on glass slides were transfected with the XMT HTLV-1 provirus DNA (5), as previously described (15). For plasma membrane staining, we cotransfected the TX-O vector, which encodes the α chain of the interleukin-2 receptor (CD25), a protein which accumulates at the cell surface (11, 37), or the CMVenvLTR vector, which encodes the HTLV-1 envelope glycoproteins (Env) (3). Cells were fixed 48 h after transfection with 4% paraformaldehyde and quenched in 0.1 M glycine. For cell surface labeling, cells were then stained either with a mouse monoclonal antibody (MAb) directed to CD25 (7G7B6; ascitic fluid; 1/500 dilution) followed by cyanin 5-conjugated secondary antibodies (1/400 dilution; Jackson ImmunoResearch Laboratories, Inc.) or a pool of sera from patients infected by HTLV-1 (1/500 dilution) followed by Texas Red-conjugated secondary antibodies. Intracellular labeling was performed after permeabilizing the cells using phosphate-buffered saline containing 0.05% saponin and 0.2% bovine serum albumin. Gag proteins were then stained using an anti-Gag MAb directed against the HTLV-1 Gag matrix (MA) protein (6) (provided by C. Desgranges, INSERM U342, Paris, France), either biotinylated (costaining with CD25; 1/800 dilution) or in ascitic fluid (costaining with Env; 1/600 dilution) diluted in permeabilizing buffer, followed by fluorescein isothiocyanate (FITC)-conjugated streptavidin (1/1,000 dilution) or FITC-conjugated secondary antibodies, respectively. The anti-Gag MAb probably recognizes the MA domain of the Gag precursor, because similar staining was obtained with HTLV-1 wild-type and protease-defective proviruses and because no staining was obtained with a mutant provirus expressing only the MA protein (data not shown). For costaining of Gag proteins and intracellular markers, the cells were permeabilized immediately after fixing and quenching and the anti-Gag ascitic fluid was used simultaneously with rabbit sera directed to cellular compartments; labeled structures were then revealed by FITC-coupled goat anti-mouse immunoglobulin G and cyanin 3-coupled goat anti-rabbit antibodies, respectively. After being washed, the glass slides were mounted in Mowiol and observed under a confocal laser scanning microscope (MRC-1024; Bio-Rad Laboratories) with a ×60 objective and ×2 zoom (Nikon Inc.). The pinhole aperture was such that the optical section thickness was 0.6 μm. Bleedthrough from green to red channels was negligible. Images were processed using Laser Sharp software. Colocalization of two markers should appear either as yellow pixels (superposition of red and green channels) or as light blue (superposition of blue and green channels) after sections recorded at the same z level in each channel were merged.
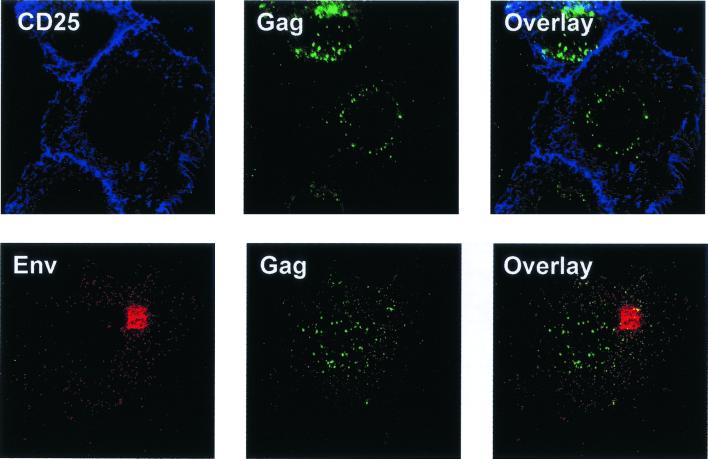
First, we tried to find out whether the punctuated staining was due to the localization of HTLV-1 Gag proteins at the plasma membrane, as observed for HIV-1 protease-defective mutants (13). The plasma membrane was labeled using two different markers: the α chain of the interleukin-2 receptor (CD25), which accumulates at the plasma membrane (37), and HTLV-1 envelope glycoproteins. This first immunostaining step was performed in the absence of permeabilization, so that only the CD25 proteins or HTLV-1 envelope glycoproteins exposed at the cell surface were labeled. Only a small amount of Gag protein was expressed at the plasma membrane, as shown by the low level of colocalization with the CD25 marker (Fig. 1), and most of the punctuated staining of HTLV-1 Gag proteins was intracellular. This was confirmed using another cell surface marker, the HTLV-1 envelope glycoprotein. Interestingly, the few Gag proteins which were found at the plasma membrane colocalized with the HTLV-1 envelope glycoproteins, suggesting that they were associated at the cell surface (Fig. 1).
FIG. 1.
Costaining of HTLV-1 Gag proteins and markers of the plasma membrane. Each row shows a single optical plane recorded in the red or blue and the green channels by confocal microscopy. (Left) Staining of CD25 was performed using the 7G7B6 MAb followed by cyanin 5-conjugated secondary antibodies; staining of HTLV-1 envelope glycoproteins used a pool of sera from patients infected by HTLV-1, followed by Texas red-conjugated secondary antibodies. Both staining procedures were performed before cell permeabilization and so only revealed proteins present on the plasma membrane. (Middle) After permeabilization, Gag proteins were revealed using either a purified and biotinylated monoclonal anti-Gag antibody followed by FITC-conjugated streptavidin (costaining with CD25), or an anti-Gag MAb in ascitic fluid, followed by FITC-conjugated secondary antibodies (costaining with Env). (Right) Overlay of fluorescences. Colocalizations appear either as yellow pixels (red and green) or as light blue pixels (blue and green).
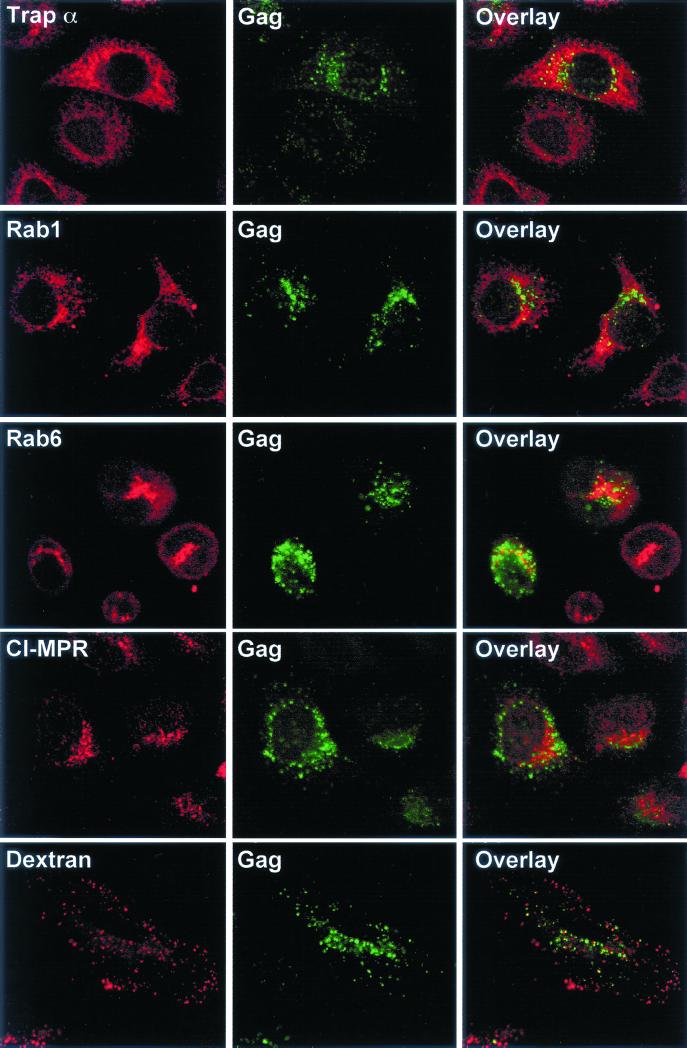
We then tried to find out whether Gag proteins could associate with membranes involved in the secretory pathway. We did this using the α-subunit of translocating chain-associated membrane protein (Trapα), a marker of the ER (41) (antibody provided by T. A. Rapoport, Harvard Medical School, Boston, Mass,); Rab1 and Rab6, two small GTPases associated with the intermediate compartment/cis-Golgi and the Golgi/trans-Golgi networks (TGN), respectively (10, 32); or the cation-independent mannose-6-phosphate receptor (CI-MPR), which cycles between the TGN, the plasma membrane, and the endosomes but which is mainly detected in the TGN at steady state (20) (antibody provided by B. Hoflack, Institut Pasteur, Lille, France). None of these markers seemed to be clearly colocalized with HTLV-1 Gag proteins (Fig. 2).
FIG. 2.
Costaining of HTLV-1 Gag proteins and markers of intracellular compartments. Each row shows a single optical plane recorded in both the red and green channels by confocal microscopy. (Left) Staining of intracellular compartments with rabbit sera, followed by cyanin 3-conjugated secondary antibodies. Anti-Trapα was used to reveal ER, anti-Rab1 was used to reveal the intermediate compartment/cis-Golgi network, and anti-Rab6 was used to reveal the Golgi/TGN. Anti-CI-MPR was used to reveal a pathway between the TGN, the cell surface, and the endosomes. It stains mainly the TGN at steady state. Texas Red-conjugated dextran revealed the whole endocytic pathway. (Middle) Gag proteins were labeled using an anti-Gag MAb, followed by FITC-conjugated secondary antibodies. (Right) Overlay of red and green fluorescences. Colocalizations appear as yellow pixels.
We then tested for the presence of Gag proteins along the endocytosis pathway. To do this, we performed colocalization with Texas red-conjugated dextran (Lys-fixable Texas red-conjugated dextran; 70 kDa; Molecular Probes, Inc.), a molecule that follows the endocytic pathway up to the lysosomes, so that the whole pathway can be stained. A 2-mg/ml solution of dextran in culture medium was added to XMT HTLV-1 provirus-transfected cells for 30 min at 37°C before they were fixed and stained using the anti-Gag MAb. We did not observe any significant colocalization between dextran and Gag proteins (Fig. 2). These negative findings were confirmed in colocalization experiments involving Gag proteins with markers of early and recycling endosomes (cyanin 3-conjugated transferrin) and of late endosomes/lysosomes (CD63) (data not shown).
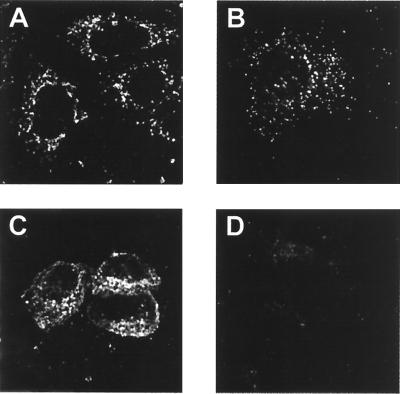
To test the propensity of HTLV-1 Gag proteins to associate with intracellular membranes, we permeabilized living XMT HTLV-1 provirus-transfected cells with 1 IU of streptolysin-O bacterial toxin (SLO; Murex Diagnostics Ltd.) in 115 mM potassium acetate buffer for 10 min at 37°C. This toxin allows cytoplasmic proteins to leave the cells but prevents proteins associated with intracellular membranes from doing so. The cells were then fixed and stained for Gag proteins as described above. After exposure, Gag proteins were no longer detected in the permeabilized cells (compare Fig. 3B and D). This was confirmed by means of the biochemical detection of Gag proteins after separating cytosolic and membrane proteins by SLO treatment as described in reference 15; 95% of the Gag proteins were detected in the cytosol fraction, and 5% were detected in the membrane fraction (data not shown). Arf1 (rabbit sera provided by B. Geny, INSERM U332, ICGM, Paris, France), a myristoylated protein anchored to the outer membranes of the ER and the Golgi compartments (36), was still stained (Fig. 3A and C).
FIG. 3.
Effect of SLO permeabilization on the intracellular distribution of HTLV-1 Gag proteins. (A and C) Arf1 staining of untreated (A) and SLO-permeabilized (C) XMT-transfected HeLa cells. (B and D) Gag staining of untreated (B) and SLO-permeabilized (D) XMT-transfected HeLa cells.
We conclude that, although HTLV-1 Gag proteins appear as intracellular clusters, giving rise to punctuated immunofluorescence staining, (i) they do not associate with any known intracellular membrane involved in the secretory or the endocytic pathway and (ii) they leave SLO-permeabilized cells in a manner similar to that for cytoplasmic proteins. Taken together, these findings suggest that the trafficking of the HTLV-1 Gag proteins is independent of membranes of the host cell at any stage from their synthesis in the cytoplasm to their budding at the plasma membrane locus, except during the budding step.
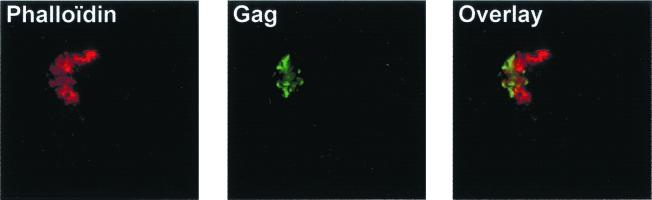
Finally, we carried out tests to find out whether HTLV-1 Gag proteins can travel with the cytoskeleton. First, we looked for an association of Gag with microtubules using an FITC-conjugated MAb to α-tubulin (Sigma) but did not detect any colocalization of these two proteins (data not shown). We then performed costaining of F-actin (rhodamine-phalloidin; Molecular Probes) and Gag proteins. In chronically infected C91/PL cells, Gag proteins were not uniformly distributed in the cells but clustered at a pole (Fig. 4, left). Actin was concentrated at the same pole of the cells (Fig. 4, middle) but did not colocalize with Gag proteins (Fig. 4, right). The polar distribution of Gag and actin proteins was also found for two other cell lines chronically infected with HTLV-1, MT2 and HUT102 (data not shown).
FIG. 4.
Costaining of Gag and actin proteins in C91/PL chronically HTLV-1-infected T cells. A single optical plane recorded in both the red and green channels by confocal microscopy is shown. (Left) F-actin staining using rhodamine-phalloidin. (Middle) Gag protein staining. Gag proteins were labeled using an anti-Gag MAb, followed by FITC-conjugated secondary antibodies. (Right) Overlay of red and green fluorescences. Colocalizations appear as yellow pixels.
In this study, we did tests to find out whether the association of Gag proteins with intracellular or plasma membranes could account for the punctuated staining of HTLV-1 Gag proteins observed in both chronically infected and XMT HTLV-1 provirus-transfected cells. All retrovirus Gag proteins contain membrane binding signals, consisting of an N-terminal myristate, a stretch of basic amino acids, or both. HTLV-1 Gag proteins anchor to the cell surface mainly via the N-terminal myristate (15). Myristoylation, however, does not predict which kind of membrane will be the final destination of the protein, because it is an anchoring signal rather than a targeting signal. Myristoylated proteins are anchored on different cell membranes. For instance, the Src protein anchors to the plasma membrane, whereas Arf1 is bound to ER and Golgi membranes (36) and NADH-cytochrome b5 reductase is associated with mitochondrial outer membranes (1). Our colocalization experiments do not support the hypothesis that Gag proteins bind to compartments involved in the secretory or the endocytic pathway. Moreover, in vivo fractionation using SLO permeabilization showed that HTLV-1 Gag proteins behaved like cytoplasmic proteins, whereas the myristoylated Arf1 protein remained bound to membranes. Thus, the association of HTLV-1 Gag proteins with membranes which we observed using subcellular biochemical fractionations (15) probably occurs only at the plasma membrane, and HTLV-1 Gag proteins are most probably not carried to the plasma membrane via a vesicular transport pathway. Some myristoylated HIV-1 MA protein mutants are targeted to the Golgi apparatus (21). They are processed normally and bud in the Golgi apparatus, but they are then assembled with no mature envelope glycoproteins. To achieve correct assembly, it may be necessary for Gag proteins to interact with no membrane other than the plasma membrane. Finally, our data show that, at the plasma membrane, HTLV-1 Gag proteins colocalize with the envelope glycoproteins, a process which probably optimizes viral transmission.
Our findings concerning HTLV-1 Gag proteins are consistent with some data obtained for Moloney murine leukemia virus Gag proteins (38), although conflicting data for this virus have been reported elsewhere (12, 14). As was suggested in the HIV-1 model (22, 34, 46), the late association between HTLV-1 Gag proteins and membranes could be controlled via a myristyl switch, which first masks the myristic acid while the Gag proteins transit through the cytoplasm and then exposes it during late assembly steps at the plasma membrane, thereby permitting the anchorage to the membrane required for budding.
If the punctuated staining of HTLV-1 Gag proteins is not due to interactions with intracellular membranes, it may correspond to an early assembly step of Gag precursors in the cytoplasm. Early intracellular assembly of Gag proteins has been reported for HIV-1 (17) on the basis of biochemical evidence of the formation of Gag complexes (16). These data suggest that, like type D retroviruses, type C retroviruses may preassemble in the cytoplasm, the only difference being that the cytoplasmic complexes they form are not dense enough to be visible by electron microscopy. If early assembly also occurs for HTLV-1 Gag proteins, the diffuse staining observed with HTLV-1 Gag mutant proteins that lack the myristoylation signal could reflect impaired early assembly rather than a defect in early membrane association.
It is unlikely that the punctuated staining of Gag proteins reflects a dead-end accumulation of misfolded or misassembled Gag proteins, which never reach the plasma membrane or which are dissociated from it after inefficient budding, although this possibility cannot be completely ruled out (40). Our observations mean that the population of HTLV-1 Gag proteins in cells chronically infected and those transfected with HTLV-1 provirus that attains a conformation compatible with intracellular transport and budding either have a short half-life or constitute a small proportion of Gag proteins. This hypothesis implies that HTLV-1 is a very inefficient virus. It is noteworthy that an accumulation of misfolded proteins is also observed for HTLV-1 envelope glycoproteins in the ER (28).
Finally, this does not explain which motor Gag proteins use when they traffic toward the plasma membrane. Cytoskeleton-associated proteins, or the cytoskeleton itself, may be implicated in several steps of the retrovirus replication cycle, including transport of Gag proteins to the cell surface, assembly, and budding. The Gag proteins of several other retroviruses are associated with KIF-4, a cellular motor protein (39), or actin (19, 42). HIV-1 particle production is partially inhibited by cytochalasin D, which alters actin structures (33), and budding occurs in T lymphocytes, where actin-rich pseudopods form (23). We observed no colocalization of HTLV-1 Gag proteins and microtubules or actin. In chronically infected cells, however, actin is polarized at a pole of the cells, where HTLV-1 Gag proteins are also clustered, which suggests active intracellular trafficking of HTLV-1 Gag proteins. The motor involved in trafficking of HTLV-1 Gag protein toward the cell surface, however, remains to be identified, and it is possible that Gag proteins themselves act as motor-like proteins and are self-propelling.
Acknowledgments
We thank T. A. Rapoport (Harvard Medical School), B. Hoflack (Institut Pasteur, Lille, France), B. Geny (INSERM U332, ICGM), and C. Desgranges (INSERM U342, Paris, France) for providing us with monoclonal antibodies or sera.
This work was supported by a grant from the Agence Nationale de Recherche sur le SIDA, and Isabelle Le Blanc received a fellowship from the Fondation pour la Recherche Médicale.
REFERENCES
- 1.Borgese, N., D. Aggujaro, P. Carrera, G. Pietrini, and M. Bassetti. 1996. A role for N-myristoylation in protein targeting: NADH-cytochrome b5 reductase requires myristic acid for association with outer mitochondrial but not ER membranes. J. Cell Biol. 135:1501–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryant, M., and L. Ratner. 1990. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc. Natl. Acad. Sci. USA 87:523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delamarre, L., A. R. Rosenberg, C. Pique, D. Pham, and M.-C. Dokhélar. 1997. A novel human T-leukemia virus type 1 cell-to-cell transmission assay permits definition of SU glycoprotein amino acids important for infectivity. J. Virol. 71:259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delchambre, M., D. Gheysen, D. Thines, C. Thiriart, E. Jacobs, E. Verdin, M. Horth, A. Burny, and F. Bex. 1989. The Gag precursor of simian immunodeficiency virus assembles into virus-like particles. EMBO J. 8:2653–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derse, D., J. Mikovits, M. Polianova, B. K. Felber, and F. Ruscetti. 1995. Virions released from cells transfected with a molecular clone of human T-cell leukemia virus type I give rise to primary and secondary infections of T cells. J. Virol. 69:1907–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebersold, A., N. Noraz, J. Grange, M. Gasmi, M. P. Grange, S. Souche, R. Mamoun, and C. Desgranges. 1993. Production and characterization of a monoclonal antibody directed against HTLV-1 p19: use in a specific capture enzyme immunoassay. Hybridoma 12:185–195. [DOI] [PubMed] [Google Scholar]
- 7.Fischer, N., M. Heinkelein, D. Lindemann, J. Enssle, C. Baum, E. Werder, H. Zentgraf, J. G. Muller, and A. Rethwilm. 1998. Foamy virus particle formation. J. Virol. 72:1610–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gheysen, D., E. Jacobs, F. de Foresta, C. Thiriart, M. Francotte, D. Thines, and M. De Wilde. 1989. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell 59:103–112. [DOI] [PubMed] [Google Scholar]
- 9.Göttlinger, H. G., J. G. Sodroski, and W. A. Haseltine. 1989. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 86:5781–5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goud, B., A. Zahraoui, A. Tavitian, and J. Saraste. 1990. Small GTP-binding protein associated with Golgi cisternae. Nature 345:553–556. [DOI] [PubMed] [Google Scholar]
- 11.Grange, M. P., V. Blot, L. Delamarre, I. Bouchaert, A. Rocca, A. Dautry-Varsat, and M.-C. Dokhélar. 2000. Identification of two intracellular mechanisms leading to reduced expression of oncoretroviral envelope glycoproteins at the cell surface. J. Virol. 74:11734–11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen, M., L. Jelinek, S. Whiting, and E. Barklis. 1990. Transport and assembly of Gag proteins into Moloney murine leukemia virus. J. Virol. 64:5306–5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermida-Matsumoto, L., and M. D. Resh. 2000. Localization of human immunodeficiency virus type 1 Gag and Env at the plasma membrane by confocal imaging. J. Virol. 74:8670–8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones, T. A., G. Blaug, M. Hansen, and E. Barklis. 1990. Assembly of gag-β-galactosidase proteins into retrovirus particles. J. Virol. 64:2265–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Blanc, I., A. R. Rosenberg, and M.-C. Dokhélar. 1999. Multiple functions for the basic amino acids of the human T-cell leukemia virus type 1 matrix protein in viral transmission. J. Virol. 73:1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, Y. M., B. Liu, and X. F. Yu. 1999. Formation of virus assembly intermediate complexes in the cytoplasm by wild-type and assembly defective mutant human immunodeficiency virus type 1 and their association with membranes. J. Virol. 73:5654–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, Y. M., and X. F. Yu. 1998. Identification and characterization of virus assembly intermediate complexes in HIV-1-infected CD4+ T cells. Virology 243:78–93. [DOI] [PubMed] [Google Scholar]
- 18.Lingappa, J. R., R. L. Hill, M. L. Wong, and R. S. Hegde. 1997. A multistep, ATP-dependent pathway for assembly of human immunodeficiency virus capsids in a cell-free system. J. Cell Biol. 136:567–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, B., R. Dai, C. J. Tian, L. Dawson, R. Gorelick, and X. F. Yu. 1999. Interaction of the human immunodeficiency virus type 1 nucleocapsid with actin. J. Virol. 73:2901–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meresse, S., and B. Hoflack. 1993. Phosphorylation of the cation-independent mannose 6-phosphate receptor is closely associated with its exit from the trans-Golgi network. J. Cell Biol. 120:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ono, A., D. Demirov, and E. O. Freed. 2000. Relationship between human immunodeficiency virus type 1 Gag multimerization and membrane binding. J. Virol. 74:5142–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paillart, J. C., and H. G. Göttlinger. 1999. Opposing effects of human immunodeficiency virus type 1 matrix mutations support a myristyl switch model of Gag membrane targeting. J. Virol. 73:2604–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearce-Pratt, R., D. Malamud, and D. M. Phillips. 1994. Role of the cytoskeleton in cell-to-cell transmission of human immunodeficiency virus. J. Virol. 68:2898–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rein, A., R. McClure, N. R. Rice, R. B. Luftig, and A. M. Schultz. 1986. Myristylation site in Pr65 gag is essential for virus particle formation by Moloney murine leukemia virus. Proc. Natl. Acad. Sci. USA 83:7246–7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhee, S. S., H. X. Hui, and E. Hunter. 1990. Preassembled capsids of type D retroviruses contain a signal sufficient for targeting specifically to the plasma membrane. J. Virol. 64:3844–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhee, S. S., and E. Hunter. 1987. Myristylation is required for intracellular transport but not for assembly of D-type retrovirus capsids. J. Virol. 61:1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhee, S. S., and E. Hunter. 1990. A single amino acid substitution within the matrix protein of a type D retrovirus converts its morphogenesis to that of a C retrovirus. Cell 63:77–86. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg, A. R., L. Delamarre, C. Pique, I. Le Blanc, G. Griffith, and M.-C. Dokhélar. 1999. Early assembly step of a retroviral envelope glycoprotein: analysis using a dominant negative assay. J. Cell Biol. 145:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakalian, M., and E. Hunter. 1998. Molecular events in the assembly of retrovirus particles. Adv. Exp. Med. Biol. 440:329–339. [DOI] [PubMed] [Google Scholar]
- 30.Sakalian, M., and E. Hunter. 1999. Separate assembly and transport domains within the Gag precursor of Mason-Pfizer monkey virus. J. Virol. 73:8073–8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakalian, M., S. D. Parker, R. A. Weldon, and E. Hunter. 1996. Synthesis and assembly of retrovirus Gag precursors into immature capsids in vitro. J. Virol. 70:3706–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saraste, J., U. Lahtinen, and B. Goud. 1995. Localization of the small GTP-binding protein rab1p to early compartments of the secretory pathway. J. Cell Sci. 108:1541–1552. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki, H., M. Nakamura, T. Ohno, Y. Matsuda, Y. Yuda, and Y. Nonomura. 1995. Myosin-actin interaction plays an important role in human immunodeficiency virus type 1 release from host cells. Proc. Natl. Acad. Sci. USA 92:2026–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spearman, P., R. Horton, L. Ratner, and I. Kuli-Zade. 1997. Membrane binding of human immunodeficiency virus type 1 matrix protein in vivo supports a conformational myristyl switch mechanism. J. Virol. 71:6582–6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spearman, P., J. J. Wang, N. Vander Heyden, and L. Ratner. 1994. Identification of human immunodeficiency virus type 1 Gag protein domains essential to membrane binding and particle assembly. J. Virol. 68:3232–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stearns, T., M. C. Willingham, D. Botstein, and R. A. Kahn. 1990. ADP-ribosylation factor is functionally and physically associated with the Golgi complex. Proc. Natl. Acad. Sci. USA 87:1238–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subtil, A., M. Delepierre, and A. Dautry-Varsat. 1997. An α-helical signal in the cytosolic domain of the interleukin 2 receptor β chain mediates sorting towards degradation after endocytosis. J. Cell Biol. 136:583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suomalainen, M., K. Hultenby, and H. Garoff. 1996. Targeting of Moloney murine leukemia virus gag precursor to the site of virus budding. J. Cell Biol. 135:1841–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang, Y., U. Winkler, E. O. Freed, T. A. Torrey, W. Kim, H. Li, S. P. Goff, and H. C. Morse. 1999. Cellular motor protein KIF-4 associates with retroviral Gag. J. Virol. 73:10508–10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tritel, M., and M. D. Resh. 2000. Kinetic analysis of human immunodeficiency virus type 1 assembly reveals the presence of sequential intermediates. J. Virol. 74:5845–5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiedmann, M., T. V. Kurzchalia, E. Hartmann, and T. A. Rapoport. 1987. A signal sequence receptor in the endoplasmic reticulum membrane. Nature 328:830–833. [DOI] [PubMed] [Google Scholar]
- 42.Wilk, T., B. Gowen, and S. D. Fuller. 1999. Actin associates with the nucleocapsid domain of the human immunodeficiency virus Gag polyprotein. J. Virol. 73:1931–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yasuda, J., and E. Hunter. 2000. Role of matrix protein in the type D retrovirus replication cycle: importance of the arginine residue at position 55. Virology 268:533–538. [DOI] [PubMed] [Google Scholar]
- 44.Yuan, X., X. Yu, T.-H. Lee, and M. Essex. 1993. Mutations in the N-terminal region of human immunodeficiency virus type 1 matrix protein block intracellular transport of the Gag precursor. J. Virol. 67:6387–6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou, W., L. J. Parent, J. W. Wills, and M. D. Resh. 1994. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J. Virol. 68:2556–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou, W., and M. D. Resh. 1996. Differential membrane binding of the human immunodeficiency virus type 1 matrix protein. J. Virol. 70:8540–8548. [DOI] [PMC free article] [PubMed] [Google Scholar]