Abstract
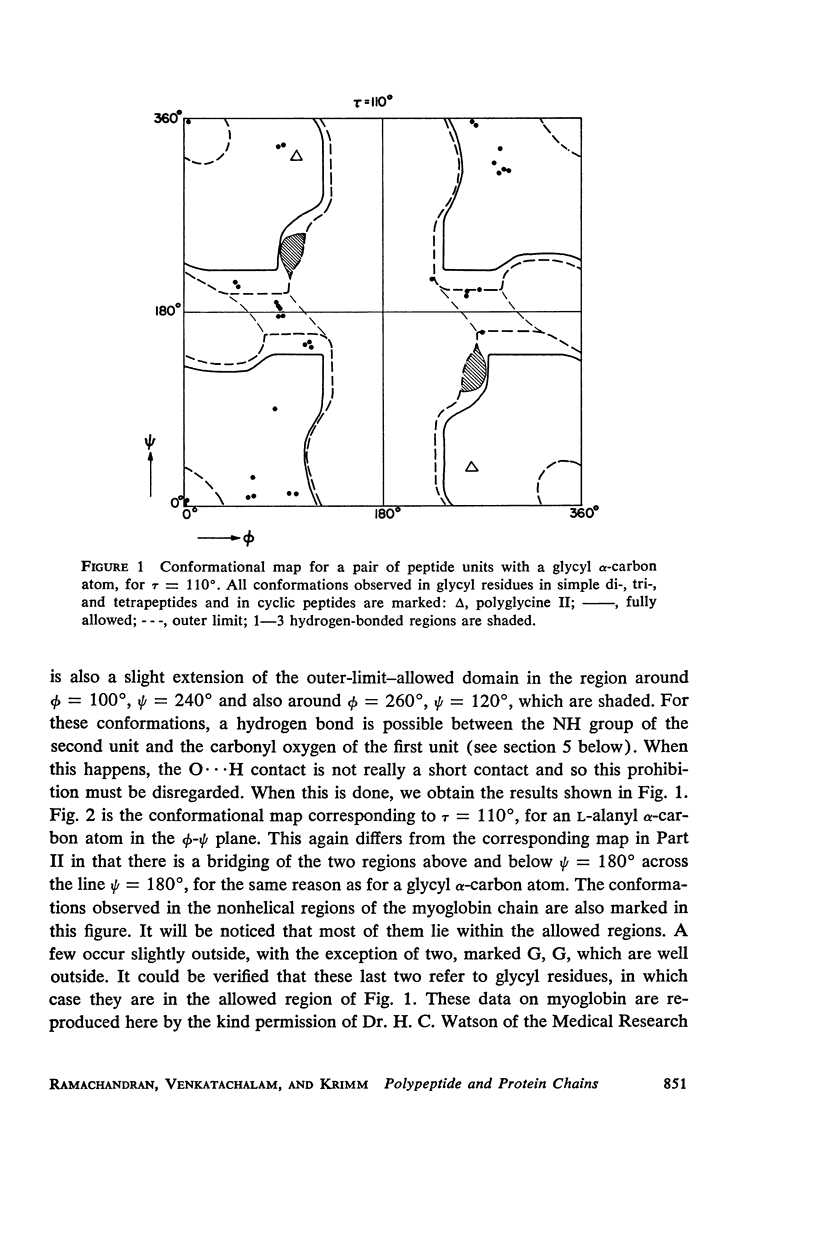
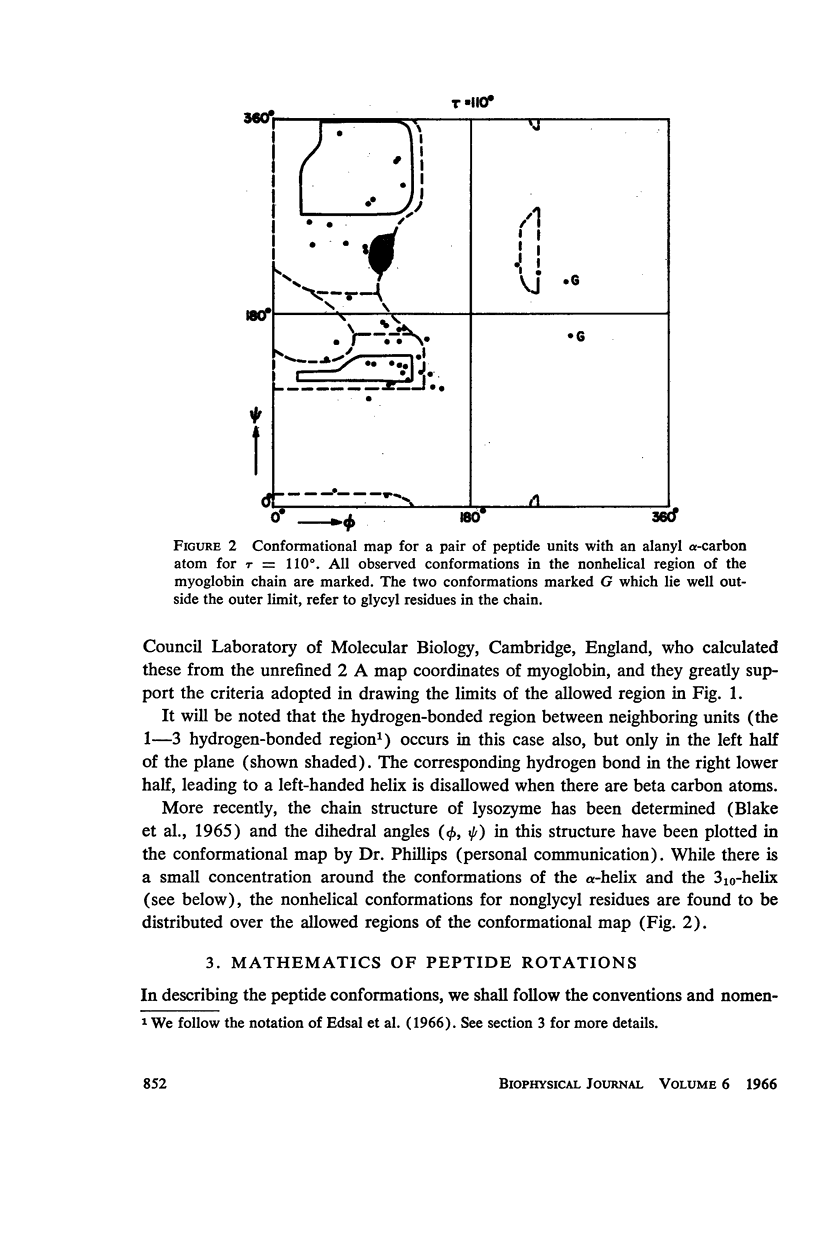
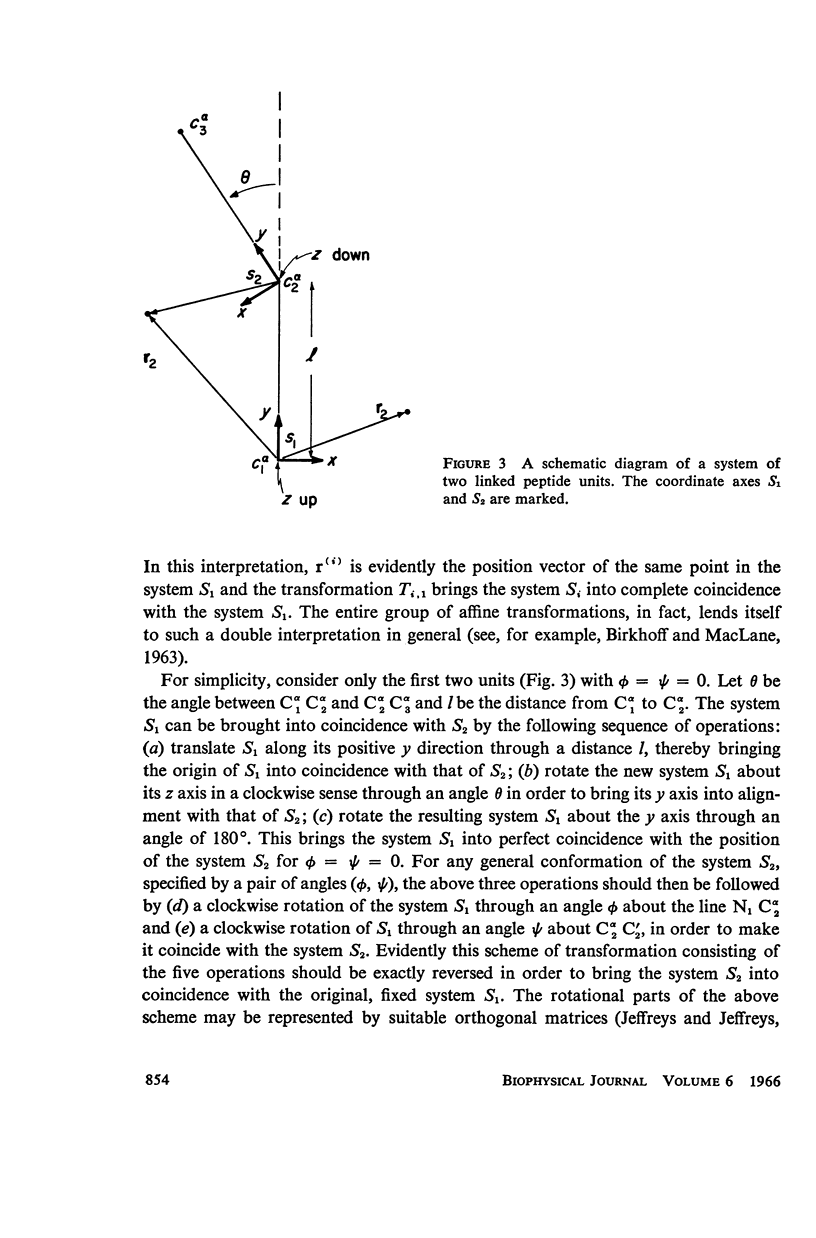
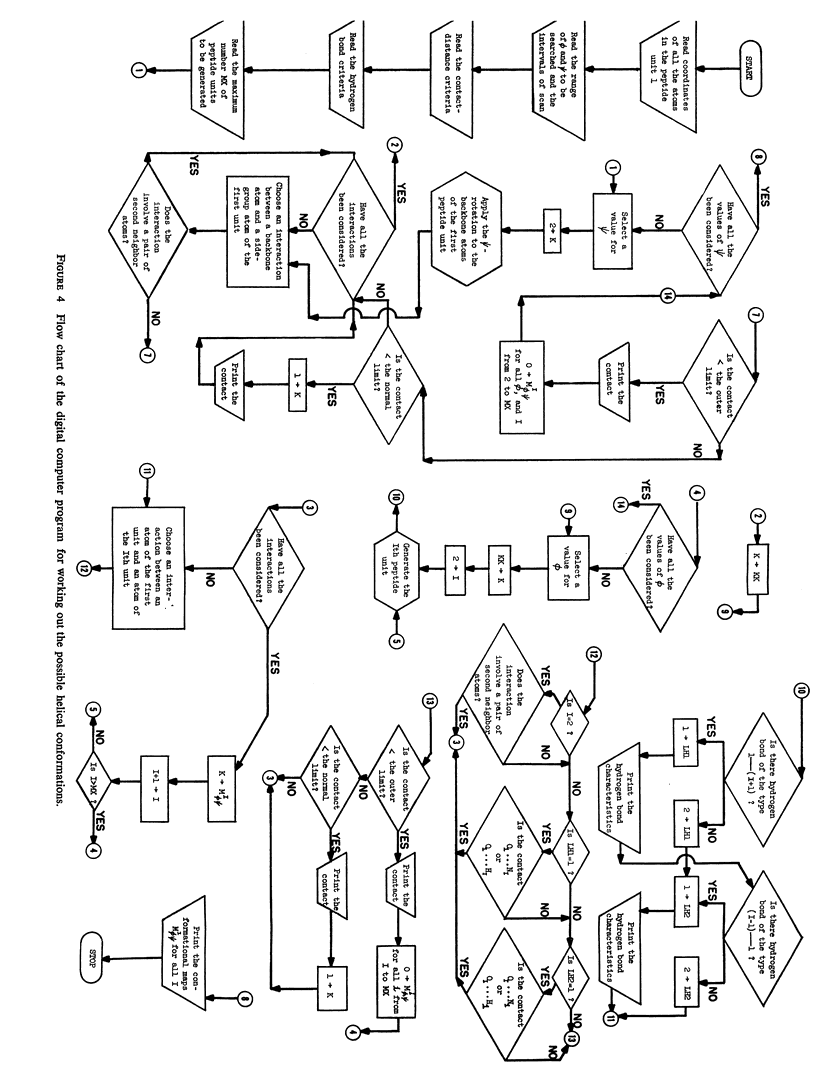
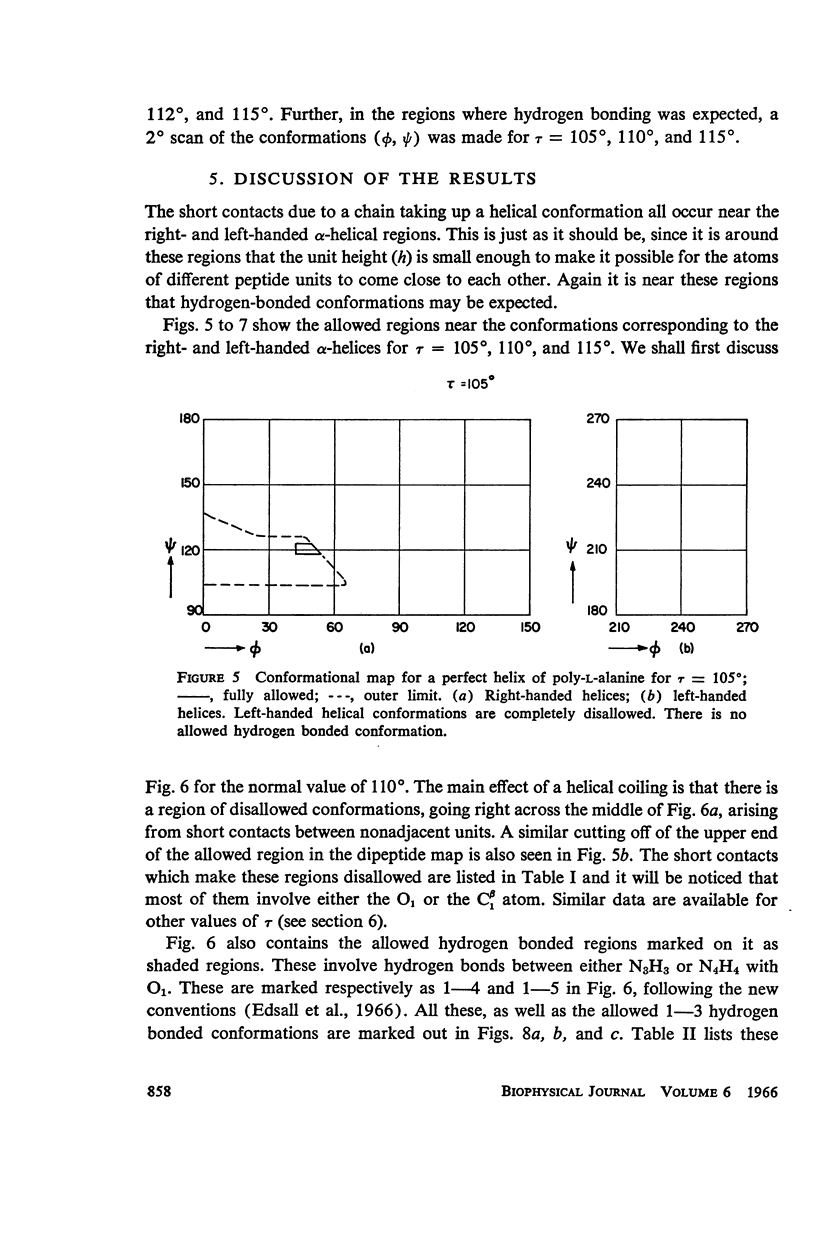
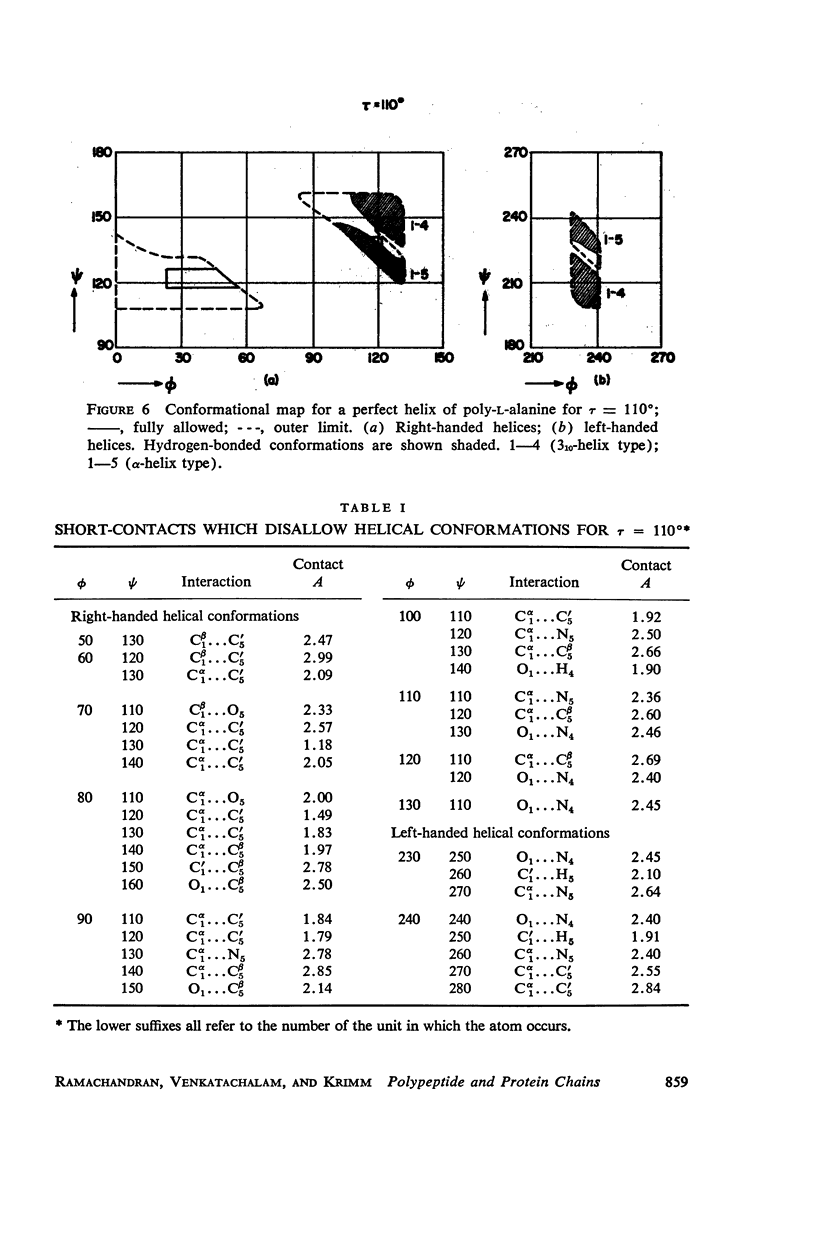
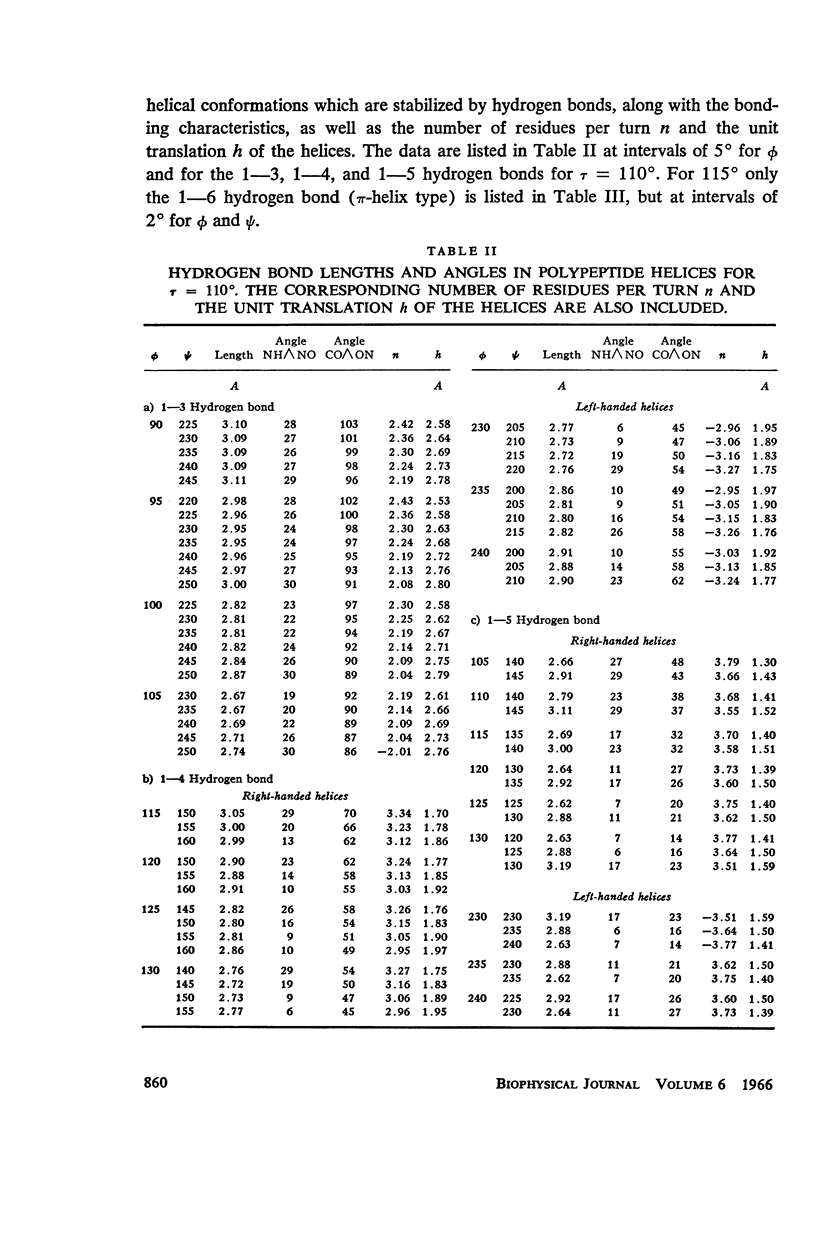
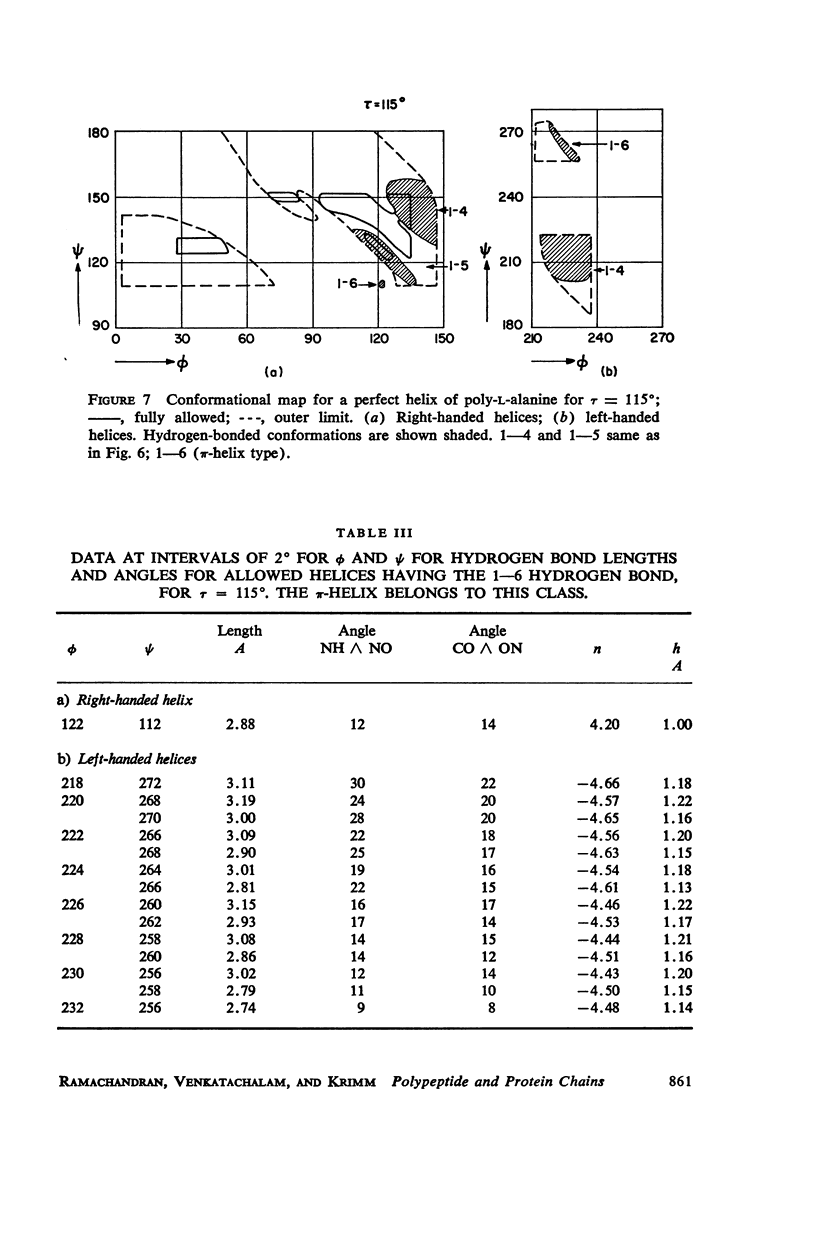
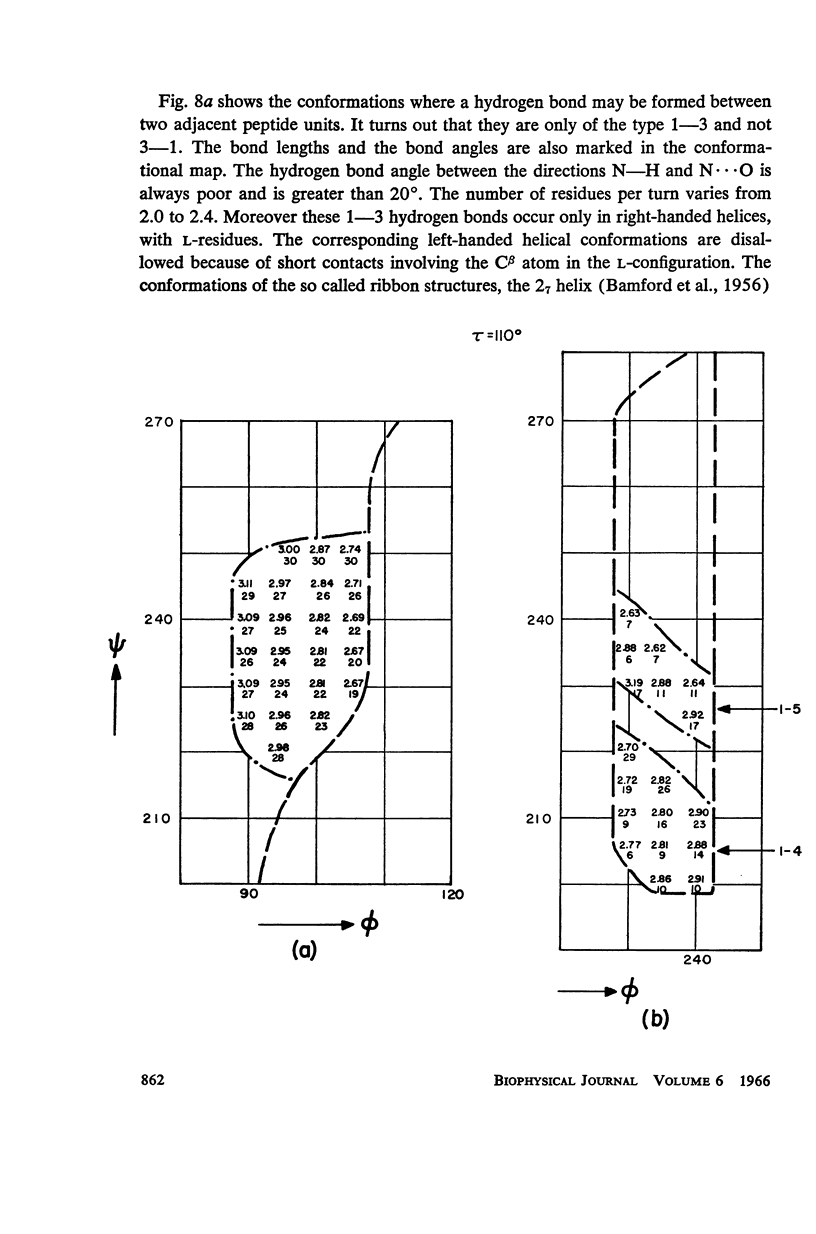
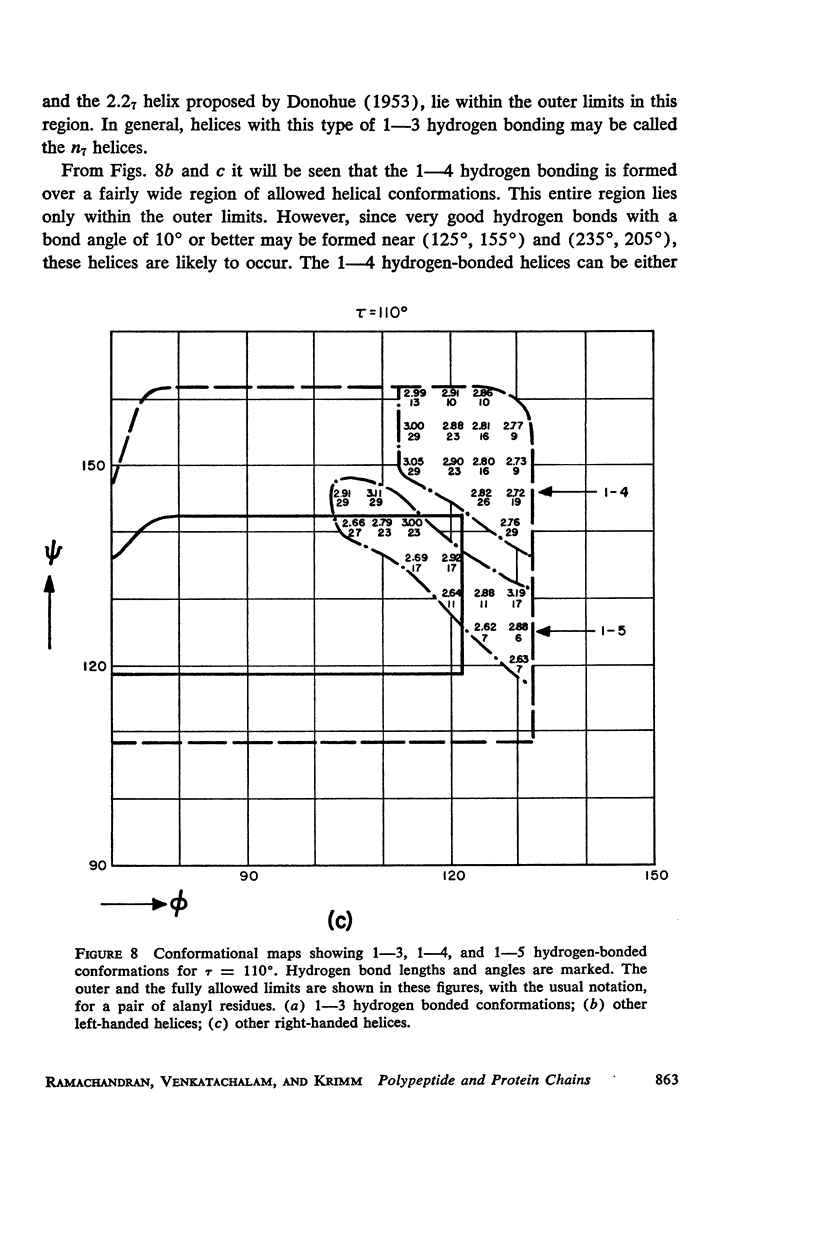
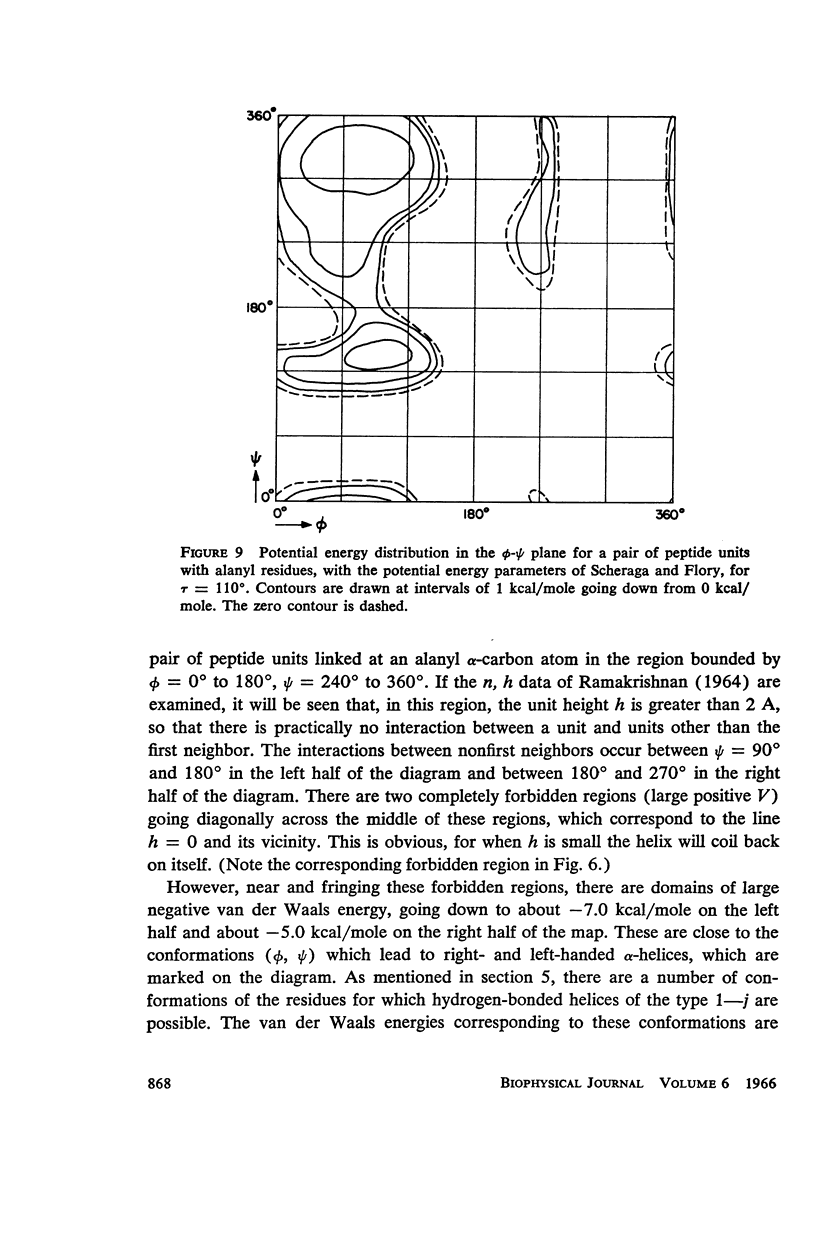
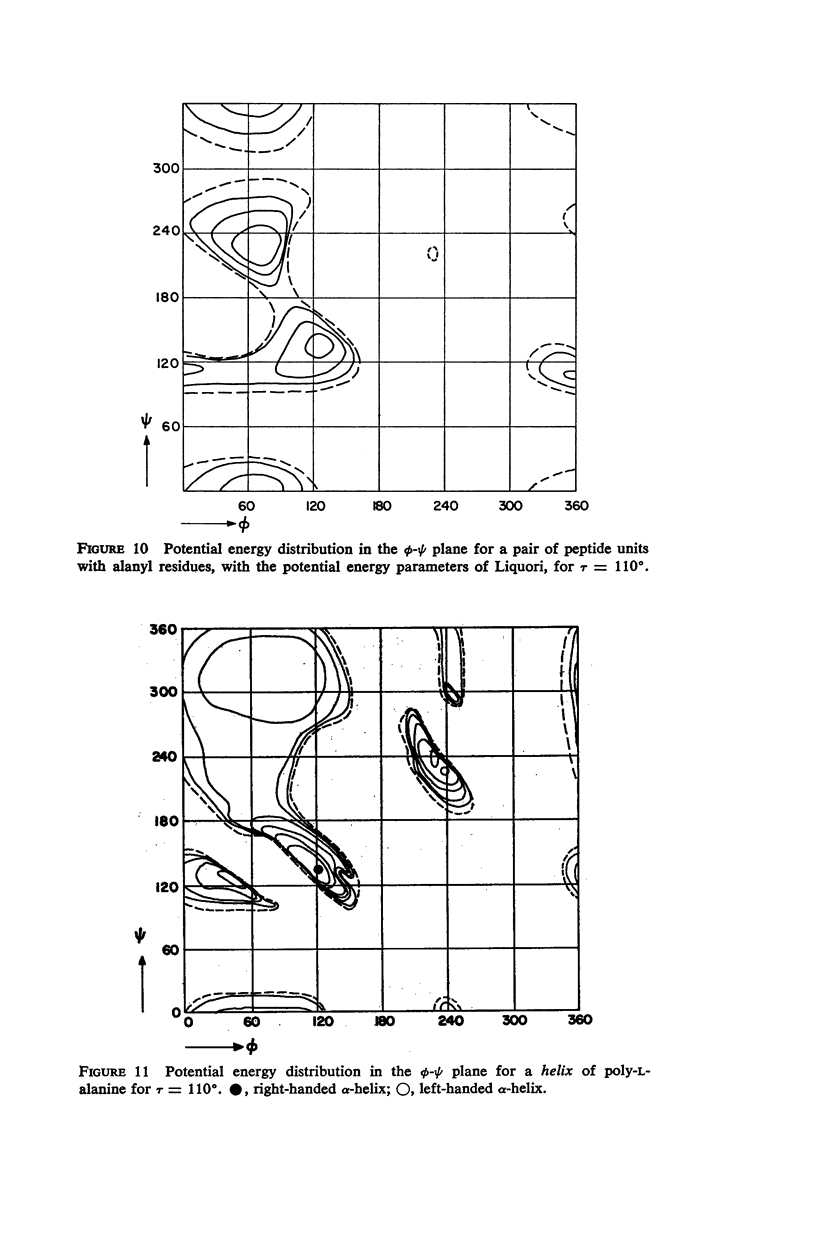
The previous study, for a pair of peptide units, of the conformations which are allowed on the basis of stereochemical criteria of van der Waals contacts has been extended to the analysis of possible conformations of helical polypeptide chains. Computer methods have been developed which select conformations on the basis of both satisfactory interatomic contacts as well as the formation of good intramolecular hydrogen bonds. Such programs have been used to map the allowed dihedral angle pairs (ϕ, ψ) for helical polypeptide chains. This survey has been made for values of the N—Ca—C′ angle (τ) of 105°, 110°, and 115°, from which the significant influence of this angle in determining allowed helical conformations can be demonstrated. Calculations have also been carried out using potential energy functions for the interaction between nonbonded atoms. The potential energy contour maps obtained in this manner are basically similar to the conformational maps calculated by the first method.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADBURY E. M., BROWN L., DOWNIE A. R., ELLIOTT A., FRASER R. D., HANBY W. E. The structure of the omegaform of poly-Beta-benzyl-L-aspartate. J Mol Biol. 1962 Aug;5:230–247. doi: 10.1016/s0022-2836(62)80086-2. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Koenig D. F., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Structure of hen egg-white lysozyme. A three-dimensional Fourier synthesis at 2 Angstrom resolution. Nature. 1965 May 22;206(4986):757–761. doi: 10.1038/206757a0. [DOI] [PubMed] [Google Scholar]
- De Santis P., Giglio E., Liquori A. M., Ripamonti A. Van der Waals interaction and the stability of helical polypeptide chains. Nature. 1965 May 1;206(983):456–458. doi: 10.1038/206456a0. [DOI] [PubMed] [Google Scholar]
- Edsall J. T., Flory P. J., Kendrew J. C., Liquori A. M., Nemethy G., Ramachandran G. N., Scheraga H. A. A proposal of standard conventions and nomenclature for the description of polypeptide conformation. J Biol Chem. 1966 Feb 25;241(4):1004–1008. [PubMed] [Google Scholar]
- RAMACHANDRAN G. N., RAMAKRISHNAN C., SASISEKHARAN V. Stereochemistry of polypeptide chain configurations. J Mol Biol. 1963 Jul;7:95–99. doi: 10.1016/s0022-2836(63)80023-6. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan C., Ramachandran G. N. Stereochemical criteria for polypeptide and protein chain conformations. II. Allowed conformations for a pair of peptide units. Biophys J. 1965 Nov;5(6):909–933. doi: 10.1016/S0006-3495(65)86759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT R. A., SCHERAGA H. A. METHOD FOR CALCULATION INTERNAL ROTATION BARRIERS. J Chem Phys. 1965 Mar 15;42:2209–2215. doi: 10.1063/1.1696269. [DOI] [PubMed] [Google Scholar]



