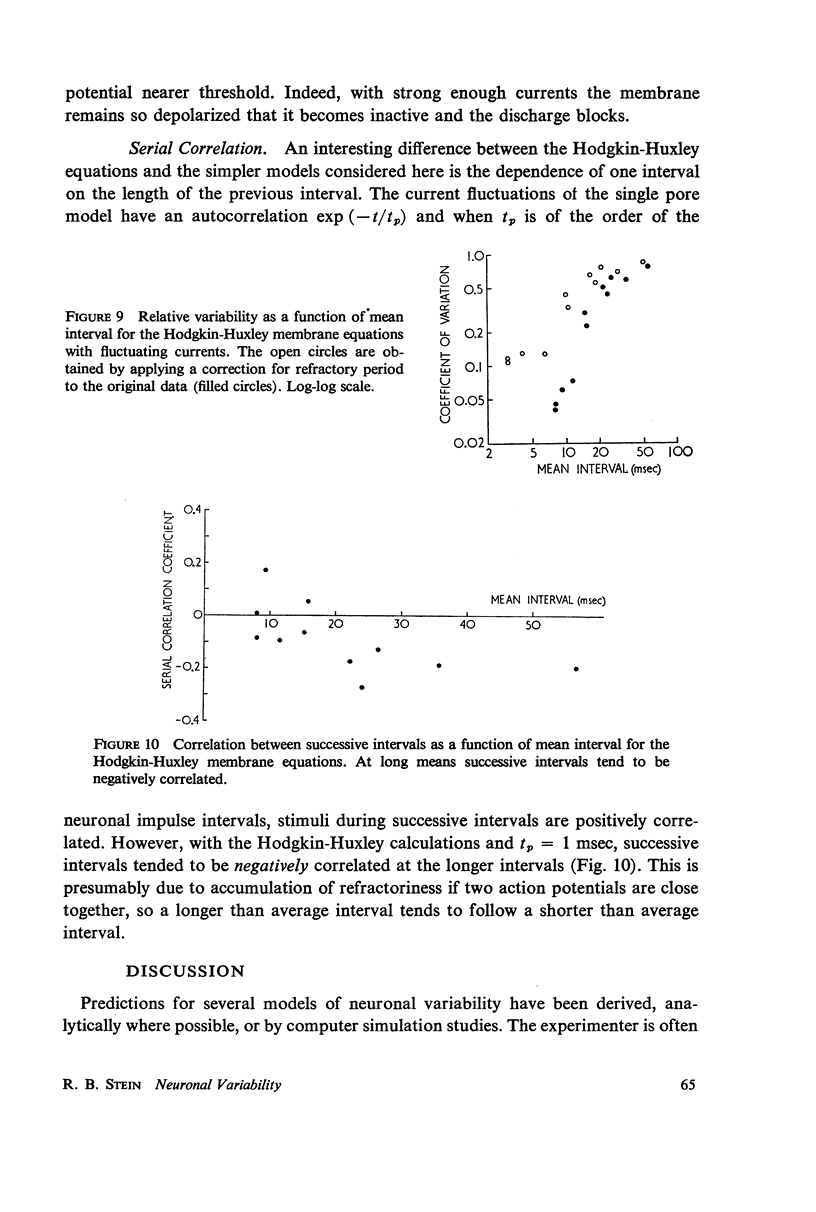
Abstract
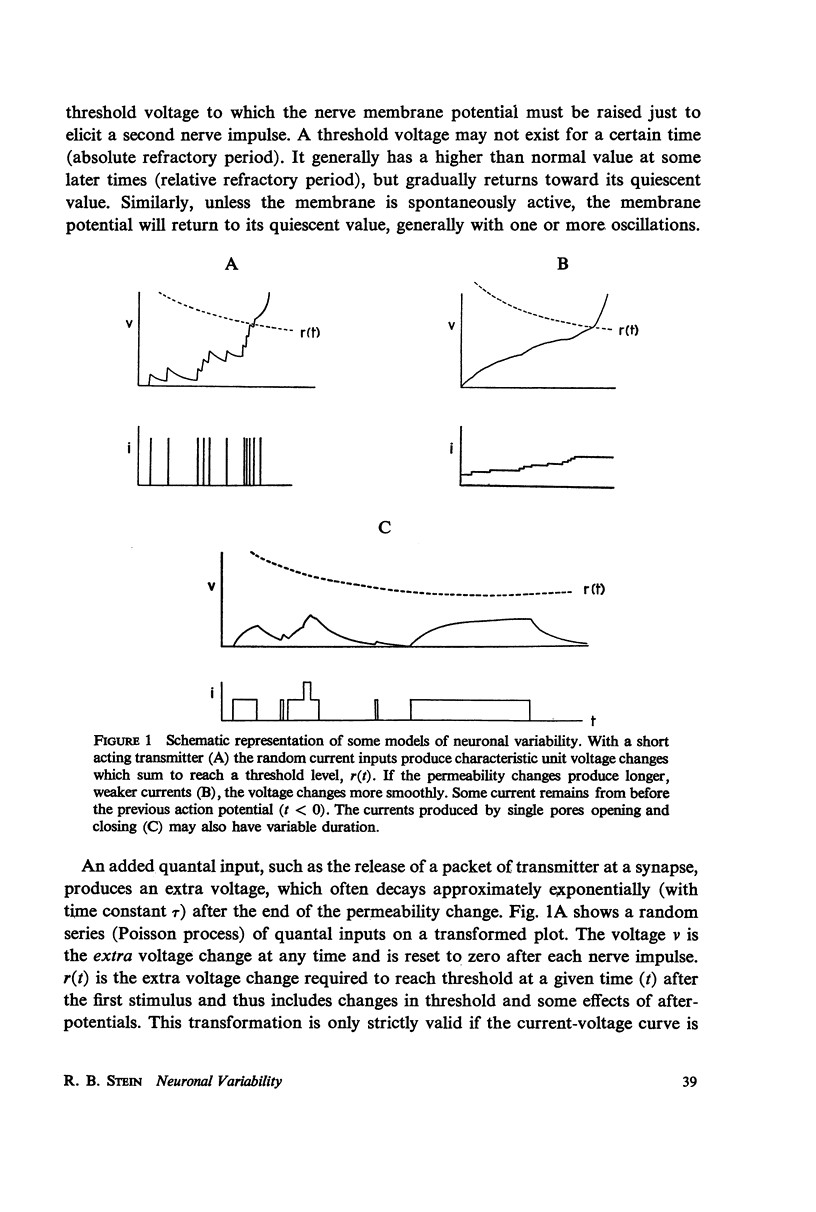
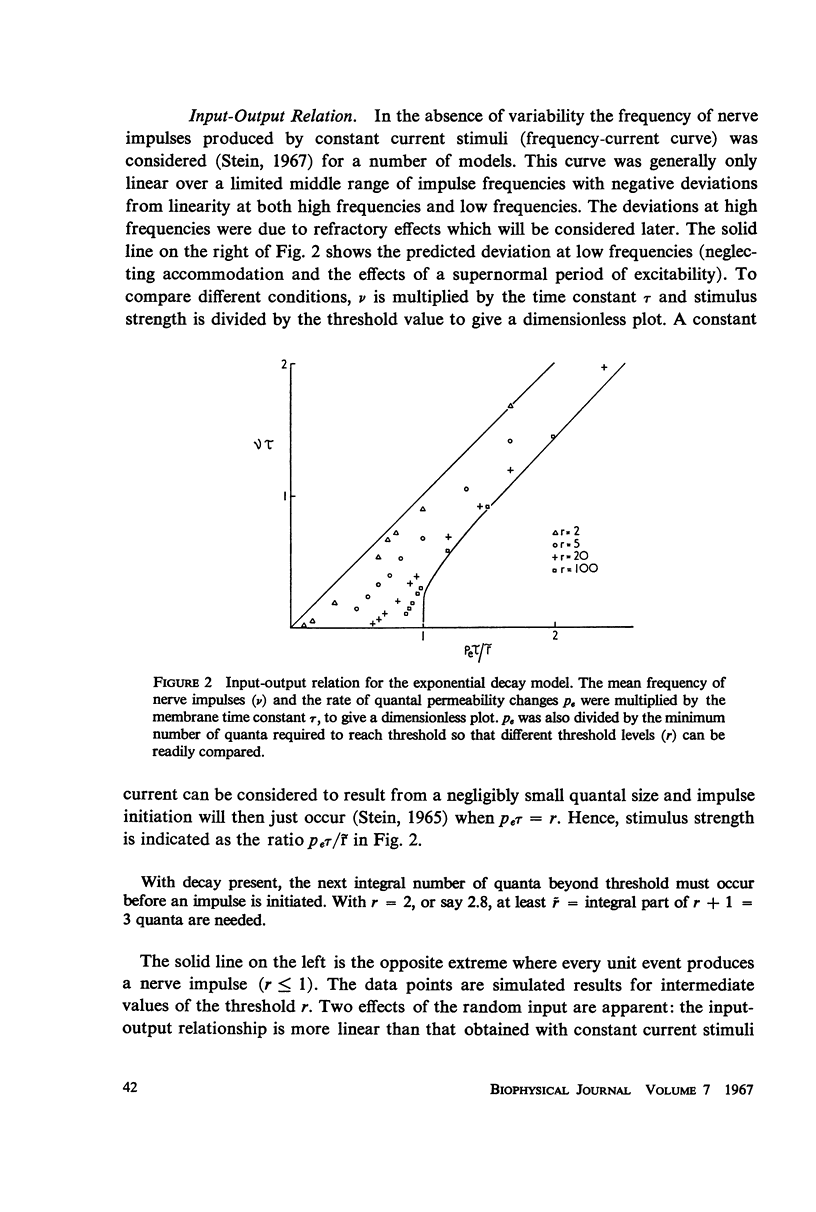
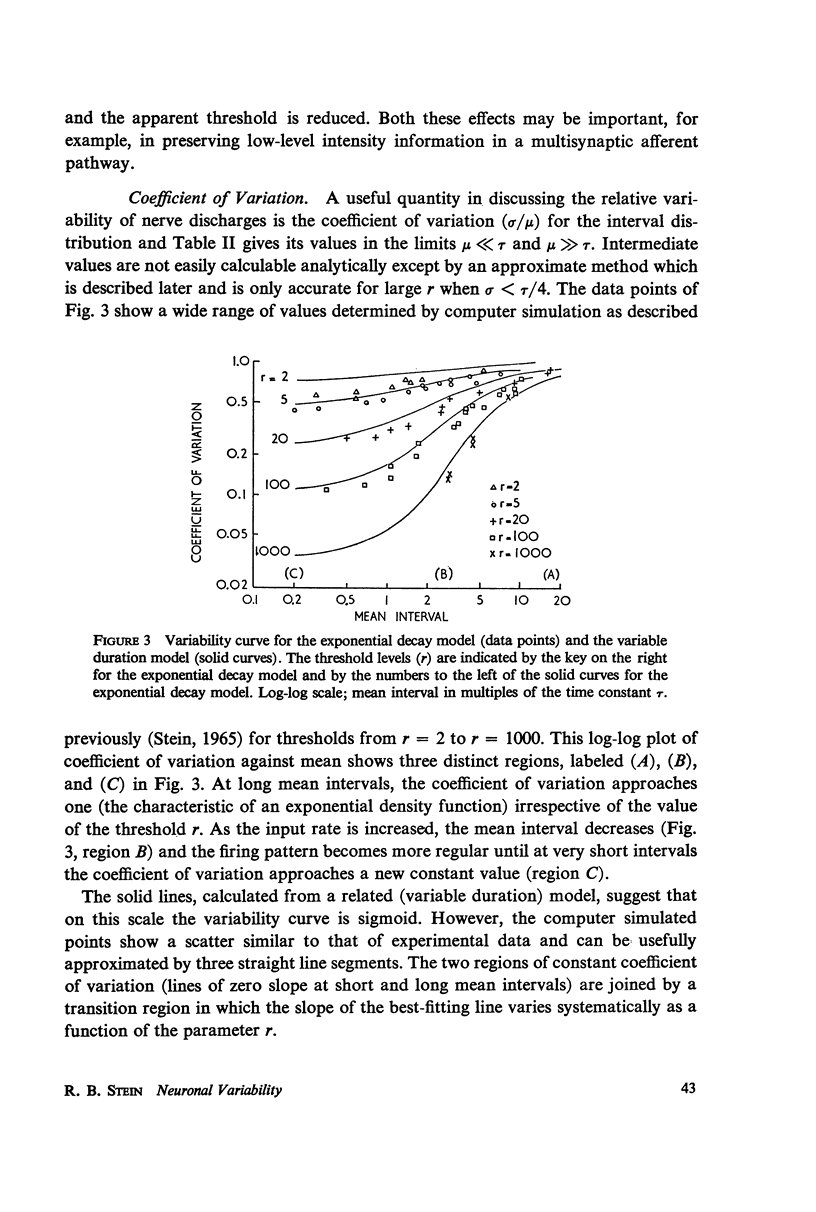
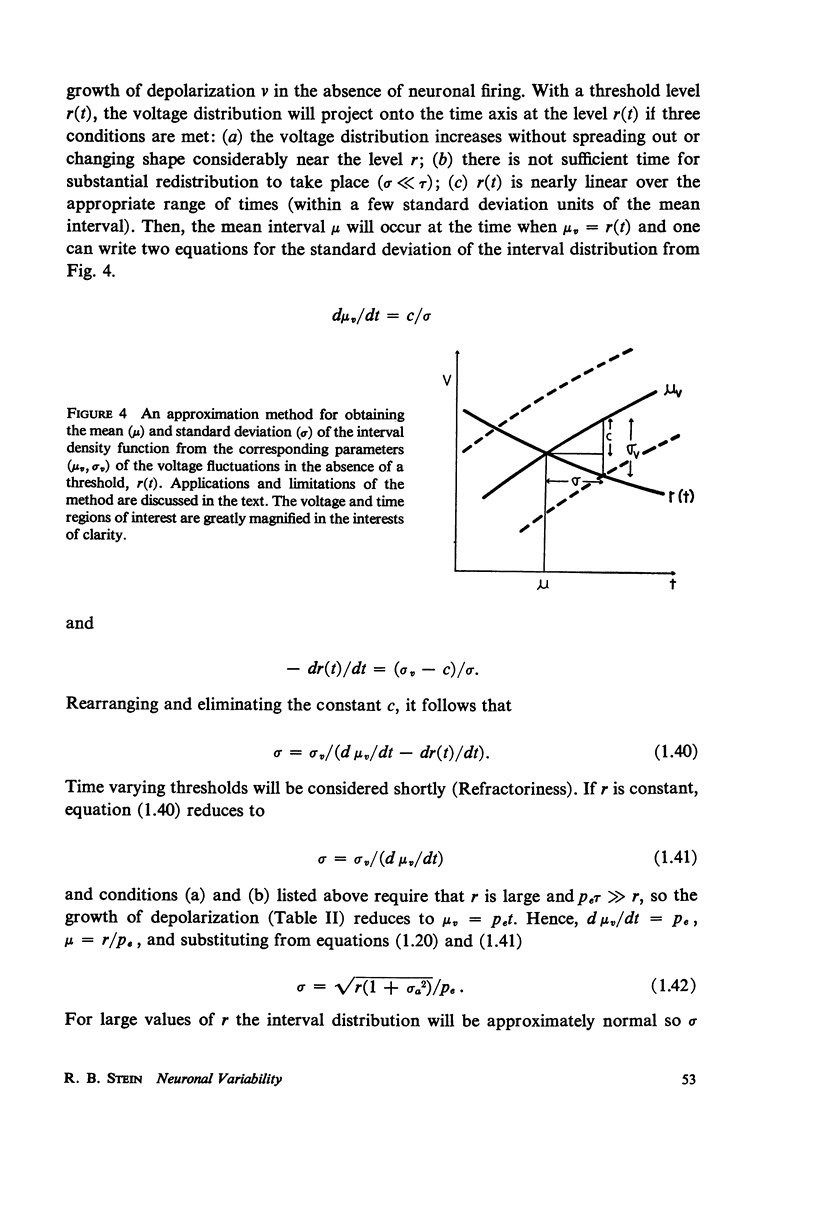
The pattern of nerve action potentials produced by unit permeability changes (quantal inputs) occurring at random is considered analytically and by computer simulation methods. The important parameters of a quantal input are size and duration. Varying both the mean and the probability density function of these parameters has calculable effects on the distribution of interspike intervals. Particular attention is paid to the relation between the mean rate of excitatory inputs and the mean frequency of nerve action potentials (input-output curve) and the relation between the coefficient of variation for the interval distribution and the mean interval (variability curve). In the absence of action potentials one can determine the parameters of the voltage distribution including the autocorrelation function and the power spectrum. These parameters can sometimes be used to approximate the variability of interspike intervals as a function of the threshold voltage. Different neuronal models are considered including one containing the Hodgkin-Huxley membrane equations. The negative feedback inherent in the Hodgkin-Huxley equations tends to produce a small negative serial correlation between successive intervals. The results are discussed in relation to the interpretation of experimental results.
Full text
PDF






















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADOLPH A. R. SPONTANEOUS SLOW POTENTIAL FLUCTUATIONS IN THE LIMULUS PHOTORECEPTOR. J Gen Physiol. 1964 Nov;48:297–322. doi: 10.1085/jgp.48.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian E. D., Zotterman Y. The impulses produced by sensory nerve-endings: Part II. The response of a Single End-Organ. J Physiol. 1926 Apr 23;61(2):151–171. doi: 10.1113/jphysiol.1926.sp002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., LEVICK W. R., WILLIAMS W. O. STATISTICAL ANALYSIS OF THE DARK DISCHARGE OF LATERAL GENICULATE NEURONES. J Physiol. 1964 Apr;170:598–612. doi: 10.1113/jphysiol.1964.sp007352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman-Thorson M. Source mechanisms for unit activity in isolated crayfish central nervous system. J Gen Physiol. 1966 Mar;49(4):597–612. doi: 10.1085/jgp.49.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braitenberg V. What can be learned from spike interval histograms about synaptic mechanisms. J Theor Biol. 1965 May;8(3):419–425. doi: 10.1016/0022-5193(65)90020-2. [DOI] [PubMed] [Google Scholar]
- Derksen H. E. Axon membrane voltage fluctuations. Acta Physiol Pharmacol Neerl. 1965;13(4):373–466. [PubMed] [Google Scholar]
- Derksen H. E., Verveen A. A. Fluctuations of resting neural membrane potential. Science. 1966 Mar 18;151(3716):1388–1389. doi: 10.1126/science.151.3716.1388. [DOI] [PubMed] [Google Scholar]
- GERSTEIN G. L., MANDELBROT B. RANDOM WALK MODELS FOR THE SPIKE ACTIVITY OF A SINGLE NEURON. Biophys J. 1964 Jan;4:41–68. doi: 10.1016/s0006-3495(64)86768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDBERG J. M., ADRIAN H. O., SMITH F. D. RESPONSE OF NEURONS OF THE SUPERIOR OLIVARY COMPLEX OF THE CAT TO ACOUSTIC STIMULI OF LONG DURATION. J Neurophysiol. 1964 Jul;27:706–749. doi: 10.1152/jn.1964.27.4.706. [DOI] [PubMed] [Google Scholar]
- Geisler C. D., Goldberg J. M. A stochastic model of the repetitive activity of neurons. Biophys J. 1966 Jan;6(1):53–69. doi: 10.1016/S0006-3495(66)86639-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopen M. T. Probabilistic firing of neurons considered as a first passage problem. Biophys J. 2008 Dec 31;6(4):435–451. doi: 10.1016/S0006-3495(66)86668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. A STUDY OF SPONTANEOUS MINIATURE POTENTIALS IN SPINAL MOTONEURONES. J Physiol. 1963 Sep;168:389–422. doi: 10.1113/jphysiol.1963.sp007199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G. P., Perkel D. H., Segundo J. P. Statistical analysis and functional interpretation of neuronal spike data. Annu Rev Physiol. 1966;28:493–522. doi: 10.1146/annurev.ph.28.030166.002425. [DOI] [PubMed] [Google Scholar]
- POGGIO G. F., VIERNSTEIN L. J. TIME SERIES ANALYSIS OF IMPULSE SEQUENCES OF THALAMIC SOMATIC SENSORY NEURONS. J Neurophysiol. 1964 Jul;27:517–545. doi: 10.1152/jn.1964.27.4.517. [DOI] [PubMed] [Google Scholar]
- RALL W. Membrane potential transients and membrane time constant of motoneurons. Exp Neurol. 1960 Oct;2:503–532. doi: 10.1016/0014-4886(60)90029-7. [DOI] [PubMed] [Google Scholar]
- Stein R. B., Mattews P. B. Differences in variability of discharge frequency between primary and secondary muscle spindle afferent endings of the cat. Nature. 1965 Dec 18;208(5016):1217–1218. doi: 10.1038/2081217a0. [DOI] [PubMed] [Google Scholar]
- VERVEEN A. A. Axon diameter and fluctuation in excitability. Acta Morphol Neerl Scand. 1962;5:79–85. [PubMed] [Google Scholar]



