Abstract
Norwalk-like viruses (NLVs) are a diverse group of single-stranded, nonenveloped, positive-polarity RNA viruses and are the leading cause of epidemic acute gastroenteritis in the United States. In this study, the major capsid gene of Norwalk virus, the prototype NLV, has been cloned and expressed in mammalian cells using a Venezuelan equine encephalitis (VEE) replicon expression system. Upon infection of baby hamster kidney (BHK) cells with VEE replicon particles (VRPs), the Norwalk virus capsid proteins self-assemble to generate high titers of Norwalk virus-like particles (VLPs) that are morphologically and antigenically analogous to wild-type Norwalk virus. Mice inoculated subcutaneously with VRPs expressing the Norwalk virus capsid protein (VRP-NV1) developed systemic and mucosal immune responses to Norwalk VLPs, as well as heterotypic antibody responses to the major capsid protein from another genogroup I NLV strain (NCFL) isolated from a recent outbreak. A second Norwalk virus capsid clone (NV2) containing three amino acid codon mutations from the NV1 clone was also expressed using VEE replicons (VRP-NV2), but upon infection of BHK cells failed to confer VLP self-assembly. Mice inoculated with VRP-NV2 elicited reduced systemic and mucosal immune responses to Norwalk VLPs, demonstrating the importance and potential utility of endogenous VLP presentation for maximum immune induction. Inoculation with either VRP-NV1 or VRP-NV2 resulted in serum antibody responses far superior to the induction in mice dosed orally with VLPs that were prepared using the VEE-NV1 replicon construct, a regimen similar to current models for NLV vaccination. Expression of NLV VLPs in mammalian cells offers a powerful approach for the design of novel NLV vaccines, either alone or in combination with current vaccination models.
Norwalk-like viruses (NLVs) are the most frequent cause of epidemic gastroenteritis in the United States, infecting an estimated 23 million people each year, and have accounted for as much as 96% of selected outbreaks of acute, nonbacterial gastroenteritis (20, 40, 47). Persons of all ages are affected, and it is unclear whether infection typically confers long-term immunity (38). NLV outbreaks commonly occur within families, nursing homes, day care centers, schools, hospitals, and in the military (1, 20, 40, 42). Infection with NLVs usually results in an episode of acute, self-limited gastroenteritis, with symptoms rarely more serious than nausea, vomiting, diarrhea, and low-grade fever (40), and most outbreaks are likely not reported (47). However, using refined molecular detection methodologies, recent studies have extended the public health significance of NLV infections and suggest that NLVs are a common cause of severe diarrhea in children (22, 53). Although further studies are necessary, NLVs may cause as much as 20% of endemic diarrheal disease in family settings in developed countries (42).
The mortality attributable to NLV infection remains unclear, but fatalities among infected elderly may not be uncommon. It has been suggested that NLV infections cause approximately 50,000 hospitalizations and 310 deaths in the United States each year (22, 47), although additional studies are needed to elucidate the potential of NLVs for causing severe disease. In developing countries, the overall extent of NLV-associated disease burden remains to be resolved, but infection can lead to malnutrition in young children and infants (53, 55). An effective vaccine would be useful for health care providers, food handlers, military personnel, and other at-risk populations to prevent the significant morbidity and potential mortality associated with NLV infection (18, 45, 63).
Because no reliable tissue culture or animal model for NLV infection is available to date, studies into the biology of NLVs have relied on recombinant molecular approaches. NLVs are classified as members of the family Caliciviridae. The genome of Norwalk virus, the prototype NLV, consists of a positive-sense, single-stranded RNA of approximately 7.5 kb in length that is organized into three open reading frames (ORFs) (for a recent review, see reference 11). ORF1 encodes the Norwalk virus nonstructural proteins, including putative helicase, poliovirus-like 3C-protease, and polymerase proteins (37). ORF2 and ORF3 encode structural proteins, with ORF2 encoding the major capsid protein, and ORF3 encoding a minor structural protein assumed to associate with the capsid protein, possibly to initiate encapsidation of the genome (21, 36).
Based on sequence homologies in regions of the polymerase and capsid genes, the genetically diverse NLVs can be divided into GI and GII genogroups, with seven to eight clusters assigned to each genogroup (66). Isolates differ by ≈30% amino acid identity in the major capsid protein within each genogroup, and by more than 50% between isolates in different genogroups (24). The extensive antigenic variability in the major capsid protein of NLVs correlates with the typical lack of heterotypic immune protection across NLV genogroups after infection (reviewed in reference 46).
The most informative studies of Norwalk virus structure and immunogenicity have stemmed from the production of recombinant baculoviruses expressing the Norwalk virus ORF2-encoded capsid protein, which upon infection of insect Sf9 cells results in the expression and self-assembly of intact Norwalk virus-like particles (Bac-Nor) (36). This accomplishment led to the first NLV human vaccine trials (2). Oral inoculation of Bac-Nor particles induces antigen-specific serum immunoglobulin G (IgG) and mucosal IgA responses in mice and boosts antibody levels in humans previously exposed to NLVs (2, 3). A second novel method for NLV vaccine design followed these experiments and used transgenic tobacco and potato plants to express and deliver Norwalk virus capsid proteins after ingestion (44).
While these oral vaccine strategies mimic the natural route of NLV infection, serum and mucosal immune responses to Norwalk virus capsid proteins are modest and below the levels exhibited after wild-type Norwalk virus infection (18, 45, 63). Most recently it has been demonstrated that Bac-Nor could induce similar levels of systemic and mucosal immune induction when administered to mice intranasally (27). It is not clear whether these current vaccine approaches will elicit protective immunity to Norwalk virus or protection against heterotypic NLV strains. Additionally, although the baculovirus- and plant-expressed virus-like particles (VLPs) appear to be morphologically and antigenically identical to wild-type Norwalk virus capsid proteins (25), it is important to consider possible subtle differences attributable to posttranslational modification patterns or protein folding in the nonmammalian versus mammalian cell systems. Based on these criteria, it is appropriate to consider complementary approaches for NLV vaccine design.
Venezuelan equine encephalitis virus (VEE) and other alphaviruses have been studied extensively for their use as vectors to express heterologous proteins, both in vitro and in vivo (reviewed in references 61 and 65). The 5′ two-thirds of the VEE genome encodes nonstructural proteins required for transcription and replication, whereas the 3′ one-third encodes structural proteins under the control of an internal 26S promoter (39), a property that has been exploited for the production of VEE replicons. The internal 26S promoter drives transcription of downstream genes, generating subgenomic mRNAs at concentrations nearly 10 times that of full-length genomic RNAs, resulting in an abundance of protein production (61, 65).
Using cDNA clones, VEE replicons are constructed by replacing VEE structural genes with sequences encoding a protein(s) of interest. Cotransfection of baby hamster kidney (BHK) cells with the replicon RNA and helper RNAs encoding VEE structural genes, which package the replicon RNA, results in the production and budding of VEE viral replicon particles (VRPs) from the transfected cells. The ensuing VRPs serve as one-hit, nonpackaging virus vectors that can deliver an expression system to produce ample concentrations of the heterologous protein in a wide range of mammalian cell lines. VEE replicons are currently being used for immunology, molecular, and cell biology research applications and are also a promising new tool for the design of novel vaccines for human use (16, 32, 61, 65).
We have recently demonstrated expression of large quantities of Norwalk VLPs in mammalian cells using a VEE replicon expression system (VRP-NV1) (Baric et al., submitted for publication). To evaluate the potential utility of the VEE replicon system for NLV vaccine design, three main objectives were pursued with the experiments described in this paper: to determine whether inoculation of mice with VRP-NV1 results in systemic, mucosal, and/or heterotypic immune responses to Norwalk VLPs; to compare the systemic and mucosal immune responses in mice inoculated with VRP-NV1 with those in mice inoculated with a second VRP construct (VRP-NV2), which expresses Norwalk virus capsid proteins that do not self-assemble into VLPs in vitro; and to compare the immune responses in mice inoculated with either VRP construct with those in mice orally inoculated with Norwalk VLPs, a regimen similar to current NLV vaccine approaches.
Our results demonstrate that VEE replicons expressing intact Norwalk VLPs induce the highest levels of systemic and mucosal immune responses in mice compared with the other tested vaccination regimens. Also, we observed that serum antibody elicited in mice inoculated with VRP-NV1 cross-reacts with the capsid protein from a heterotypic GI NLV strain (NCFL) isolated from a recent outbreak that occurred during a football game in North Carolina (4). The VEE-based vaccination method described in this paper offers a powerful complementary approach to current NLV vaccine strategies and, as a model system, provides a useful technique for generating VLPs in mammalian cells for future applications beyond NLV research and vaccine design.
MATERIALS AND METHODS
Virus and cells.
Norwalk virus antiserum and fecal specimens containing Norwalk virus were obtained from a human challenge study with the Norwalk virus NV8FIIa inoculum (Moe et al., unpublished data). The prechallenge immune status and response in terms of serum antibody titers, virus shedding, and clinical symptoms in all volunteers have been determined and will be described in more detail elsewhere. The NCFL viral strain was obtained from a specimen from a recent NLV outbreak that occurred during a football game in North Carolina (4).
Baby hamster kidney (BHK) cells were maintained in αMEM containing 7% fetal clone II (HyClone Laboratories; Logan, Utah), 10% tryptose phosphate broth, kanamycin (0.25 μg/ml) and gentamicin (0.05 μg/ml). Cultures were maintained at 37°C in a CO2 incubator.
Cloning and sequencing.
NV1 and NV2 capsid clones were obtained by reverse transcription (RT)-PCR of high-titer Norwalk virus fecal samples from NV8FIIa-infected volunteers as described (Baric et al., submitted). The NCFL capsid clone was obtained by RT-PCR of RNA extracted from the stool of an individual infected from a recent NLV outbreak (4). The NCFL capsid gene was cloned using procedures for mRNA isolation from stool and PCR conditions based on methods previously described for amplification of the NV2 capsid clone (Baric et al., submitted). Briefly, mRNA was isolated from a 20% stool mixture using an Oligotex Direct mRNA Minikit (Qiagen; Valencia, Calif.). RT reactions were performed with a 5-μl aliquot of the purified mRNA using Superscript II reverse transcriptase (Invitrogen Life Technologies; Carlsbad, Calif.) according to the manufacturers’ directions and either primer NW-550 (5′-GTACAGCATACACACAAG(A/G)) or NW-3′AscI (GACATTCGGCGCGCCTTATCGGCGCAGACCAAGCCTAC). PCRs designed to amplify the NCFL capsid gene by overlapping extension PCR were performed using an Expand Long Template PCR system (Roche Molecular Biochemicals; Indianapolis, Ind.). Reaction A amplified ≈550 bases of the 5′ end of the NCFL capsid gene using the primers NW-550 and VEE-NW5′ (5′-AGTCTAGTCCGCCAAGATGATGATGGCGTCTAAGGACGC-3′). Reaction B amplified the region from bp ≈550 to the 3′ end of the ORF2 sequence using the primers NW-3′AscI and NW + 550 (GCG(C/T)CTTGTGTGTATGCTGTAC). An additional PCR using 1 μl from each of reactions A and B and the VEE-NW5′ and NW-3′AscI primers amplified the full-length capsid. The ≈1.6-kb fragment was ligated overnight at 15°C into the pGEM-T vector system (Promega; Madison, Wis.). One tenth of the ligation reaction was transformed into competent Escherichia coli DH5α cells and plated on YT/ampicillin/X-Gal (5-bromo-4-chloro-3-indolyl-β-dS-galactopyranoside)/isopropylthiogalactopyranoside (IPTG) plates, followed by selection and screening of white colonies. All NLV clones were confirmed by restriction digest and sequence nalyses.
Production of VEE replicon particles.
The procedure for insertion of the NV1, NV2, and NCFL capsid genes into the multicloning site of the VEE replicon expression vector, pVR21, and subsequent production of VEE viral replicon particles (VRPs) was adapted from a method previously described by Pushko et al. for the production of influenza virus hemagglutinin (HA)-expressing VEE replicons (57). Briefly, NV1, NV2, and NCFL capsid sequences were inserted downstream of the 26S promoter of pVR21 by overlapping extension PCR, using primers and conditions as described (Baric et al., submitted). Full-length T7 transcripts of linearized VEE replicon cDNAs encoding NV1, NV2, and NCFL capsids were generated in vitro using an mMessage mMachine kit (Ambion; Austin, Tex.) and mixed with two helper packaging mRNAs encoding the VEE capsid and attenuated glycoprotein-3014 proteins (17). Transcripts were cotransfected into BHK cells by electroporation of 800-μl suspensions of 107 cells/ml in phosphate-buffered saline (PBS) using three electrical pulses of 850 V at 25 μF with a Bio-Rad Gene Pulser II electroporator (Bio-Rad; Hercules, Calif.).
Electroporation efficiencies ranging from 60 to 80% were measured using Sindbis virus replicon RNAs encoding green fluorescent protein (data not shown) (Sindbis virus replicons kindly provided by Charles Rice; Rockefeller University, New York, N.Y.). The transfected BHK cells were seeded into 75-cm2 flasks and incubated at 37°C for 36 h. VRPs were harvested 30 h posttransfection from BHK cell supernatants and pelleted at 27,000 rpm for 3 h (using a Beckman SW28 rotor) through a 20% sucrose cushion. The resulting VRPs encoding NV1, NV2, or NCFL are, respectively, designated VRP-NV1, VRP-NV2, and VRP-NCFL.
Production and harvesting of NLV virus-like particles.
Flasks (75 or 175 cm2) of BHK cells were infected with VRPs at a multiplicity of infection (MOI) of approximately 2. At 33 to 36 h postinfection, the supernatants were collected and centrifuged at 2,000 × g to collect any nonadherent cells and cell debris, and the pellets and cell monolayers were lysed by three freeze-thaw cycles. The supernatants and lysed cell suspensions were clarified by centrifugation at 15,000 × g to remove cell debris, followed by ultracentrifugation at 27,000 rpm for 3 h through a 20% sucrose cushion.
Electron microscopy (EM) analysis of putative Norwalk VLP (VEE-Nor) suspensions was performed using a negative staining technique as described (Baric et al., submitted). Protein concentrations were determined by protein assay using bovine serum albumin (BSA) as a protein standard (Bio-Rad).
Immunofluorescence analysis.
To monitor transfections and titer VRPs by immunofluorescence analysis (IFA), BHK cells were grown on four- or eight-well LabTek chamber slides (Nalge Nunc International; Naperville, Ill.), infected with serially diluted VRP-NV1, VRP-NV2, or VRP-NCFL, and incubated in a 37°C CO2 incubator. At 24 h postinfection, slides were fixed in acetone-methanol (1:1) and stored at 4°C for at least 1 h. When examining transfected cells, approximately 105 electroporated cells were seeded onto four- or eight-well chamber slides. The slides were incubated and fixed as described above. Fixed cells were rehydrated in PBS (pH 7.2) and incubated with a 1:100 dilution (in PBS) of appropriate antisera for 1 h at ambient temperature. After three washes in PBS, a 1:100 dilution of either goat anti-human or goat anti-mouse IgG-fluorescein isothiocyanate (FITC) conjugate (Sigma; St. Louis, Mo.) was added, and the slide was incubated for 30 min at ambient temperature, followed by three additional PBS washes. Slides were examined and photographed under a Zeiss LSM110 confocal fluorescent microscope. Images were digitized and assembled using Photoshop 5.5 (Adobe Systems Inc.).
Mouse inoculations and sample collection.
Four- to six-week-old male isogenic BALB/c mice were obtained from Charles River Laboratories (Wilmington, Mass.), and all mice were maintained in the same environment and fed the same diet. Mice for VRP-NV1, VRP-NV2, and oral dosing were analyzed in groups of four, and groups of two mice were inoculated with either VRP-HA (expressing influenza virus HA) or PBS as negative controls.
VRPs were inoculated by footpad injection, and 75-μg suspensions of VEE-Nor were administered by oral gavage according to the inoculation schedule indicated in Table 1. Serum was obtained by tail bleed and collected in serum separator tubes (Becton Dickinson Vacutainer Systems; Franklin Lakes, N.J.). Fecal pellets obtained from all mouse groups were processed in the same manner. Fresh pellets were obtained and immediately diluted 1:2 (wt/vol) in PBS containing 0.1% Tween, 0.1 mg/ml thimerisol, and 0.1 mg/ml soybean trypsin inhibitor (Sigma). Samples were then vortexed vigorously for 30 s, held on ice for 15 min, vortexed again, kept on ice for an additional 15 min, then finally vortexed for 1 min. Samples were clarified twice by centrifugation at 10,000 × g and immediately stored at −70°C until tested. Prior to testing, thawed fecal extracts were centrifuged at 10,000 × g for 5 min to remove any additional particulate matter.
TABLE 1.
Mouse inoculation schedule
| Immunization group (n) | Primary inoculum | Route of inoculationa | Boost inoculum | Route of inoculation | Serum IgG (μg/ml) (SD)b | Intestinal IgA (ng/ml) (SD)c |
|---|---|---|---|---|---|---|
| NV1:NV1 (4) | VRP-NV1 | FP | VRP-NV1 | FP | 3506.7(1277) | 581.6(331) |
| NV1:VEE-Nor (4) | VRP-NV1 | FP | VEE-Nor | Oral | 377.1(110) | NR |
| VEE-Nor: VEE-Nor (4) | VEE-Nor | Oral | VEE-Nor | Oral | 2.6(3),44.8d | NR |
| NV2:NV2 (4) | VRP-NV2 | FP | VRP-NV2 | FP | 303.1(193) | NR |
| PBS:PBS (2) | PBS | FP | PBS | FP | NR | N/A |
| HA:HA (2) | VRP-HA | FP | VRP-HA | FP | 6.2e | NR |
Footpad (FP) inoculations were with 10 μl containing 107 infectious units of VRPs in sterile PBS or 10 μl of PBS as a negative control; 75 μg of VEE-Nor suspensions in PBS was administered by oral gavage. VRP-HA expresses an influenza virus HA protein (15).
Mean (standard deviation) VEE-Nor specific IgG for all mice on experimental day 44 as in Fig. 3. NR, no response detected.
Mean (standard deviation) VEE-Nor specific IgA for all mice on experimental day 28 as in Fig. 4. N/A, not applicable, used to correct for background.
Titer on day 28 from one mouse that responded to 200-μg doses of VEE-Nor out of a total of four inoculated.
Titer from one mouse obtained at this time point; the second did not respond.
Western blot analysis of Norwalk virus capsid proteins.
Flasks of BHK cells at ≈90% confluence were infected with either VRP-NV1 or VRP-NV2 (in PBS containing 1% fetal bovine serum) at an MOI of approximately 10 or mock infected with dilution buffer. At 30 h postinfection, cells were collected using a cell scraper and pelleted by centrifugation. After two washes with PBS, cell pellets were resuspended in hot (≈80°C) sodium dodecyl sulfate (SDS) gel loading buffer, boiled for 5 min, and clarified, and total cellular protein concentrations were determined by standard protein assay.
Samples were normalized to equivalent protein amounts and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot using standard protocols (58). Gels were either stained with Coomassie brilliant blue or transferred to nitrocellulose blotting paper using a Bio-Rad Transblot apparatus. Blots were blocked with 2% BSA in TBS-Tween (0.05%) and probed with antiserum (1:1,000 in block solution) from a Norwalk virus-infected volunteer. Antibody-reactive protein was visualized using a goat anti-human IgG-alkaline phosphatase-conjugated secondary antibody (Sigma) and 5-bromo-4-chloro-3-indolylphosphate (BCIP)/nitro blue tetrazolium (NBT) substrate (Roche Molecular Biochemicals; Indianapolis, Ind.).
ELISA analysis.
Serum and fecal extracts were analyzed by enzyme-linked immunosorbent assay (ELISA) to detect antibodies to VEE-Nor. Briefly, 96-well polyvinyl chloride (PVC) microtiter plates (Falcon 353911, Becton Dickinson) were coated with 100 μl of a 1-μg/ml VEE-Nor solution in 0.05 M sodium bicarbonate buffer, pH 9.6, for 4 h at ambient temperature. Plates were blocked overnight at 4°C with 5% milk in PBS (5% Blotto) for serum analysis or blocked in 10% Blotto for fecal extract analysis. Serum samples were diluted in 5% Blotto based on a previously determined optical density (OD) linear range (1:50 minimum starting dilution for IgG, 1:20 for IgM), and fecal extract samples were diluted 1:1 in 1% Blotto containing 0.5% fetal calf serum (FCS). Serum or fecal samples were added to antigen-coated wells and twofold serially diluted. To reduce the possibility of assay variation between mouse groups due to testing conditions, fecal and serum samples from different groups were tested on the same plates.
Samples were incubated at 37°C for 2 h, washed five times with PBS-Tween (0.05%), followed by incubation with 100 μl of either goat anti-mouse IgG (1:5,000), IgM (1:5,000), or IgA (1:3,000) alkaline phosphatase-conjugated secondary antibodies (Sigma) in sample dilution buffer for 1 h at 37°C. Plates were washed and developed with 150 μl of p-nitrophenyl-phosphate (Sigma-FAST tablets, Sigma), and the optical density (OD) at 405 nm was determined using an ELISA plate reader (Molecular Devices; Sunnyvale, Calif.).
To quantitate serum IgG or IgM or fecal IgA titers, a standard curve was created for each microtiter plate tested by coating a series of wells on the plate with serially diluted, reagent-grade mouse IgG, IgM, or IgA standard (Sigma). Data were analyzed using Softmax Pro software (Molecular Devices), and results, statistical analysis, and graphs were processed using Microsoft Excel. The linear portion of each standard curve (R2≥ 0.95) was used to calculate antibody levels, and sample dilutions with ODs that fit in the standard curve were corrected and averaged to generate standard units of VEE-Nor-specific serum IgG, IgM, or fecal IgA units.
To correct for high background levels inherent in the intestinal IgA ELISA, average IgA values obtained for each time point from mock-infected mice were subtracted from corresponding time points for each mouse for all experimental groups (average background for each time point = 220 ng/μl). Only individual plate blanks (no primary antibody added) were used to correct for serum IgM and IgG titers. Student’s paired and unpaired t tests were performed for statistical analysis, with P < 0.05 considered significant.
RESULTS
Cloning and expression of NLV capsid proteins from VEE replicons.
We previously cloned and sequenced the ≈1.6-kb Norwalk virus ORF2 capsid genes, NV1 and NV2, from the stools of NV8FIIa-infected human volunteers (Baric et al., submitted). The NV1 clone is identical to the wild-type Norwalk virus ORF2 amino acid sequence (M. Estes, personal communication), whereas NV2 contains three amino acid variations from the wild-type sequence (Fig. 1a.
FIG. 1.
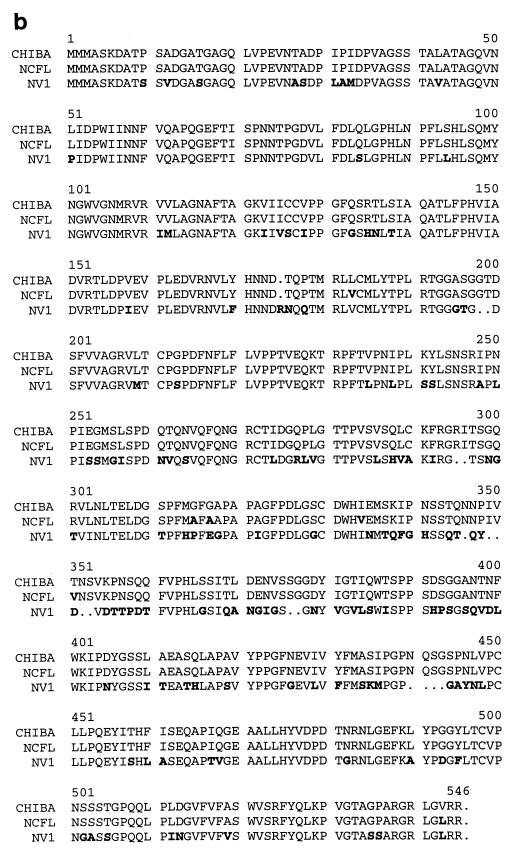
NLV capsid clones. NV1, NV2, and NCFL NLV capsid genes were cloned from fecal specimens and sequenced. NV1 and NV2 were isolated from human volunteers infected with the NV8FIIa challenge inoculum (Moe et al., unpublished data), and NCFL was isolated from an infected patient from a recent NLV outbreak that occurred during a football game in North Carolina (4). Amino acid variations and identities to Chiba virus and Norwalk virus capsid (37, 62) are indicated in panel a. Panel b shows an amino acid alignment of NV1, NCFL, and Chiba capsids. Differences from the Chiba reference are indicated in boldface. Note that the designated amino acid numbers in panel b might be shifted due to the alignment.
To study the induction of heterotypic immunity, a capsid clone from a second GI NLV strain from a recent outbreak (4) was cloned and sequenced (NCFL). The NCFL capsid protein is nearly 99% identical in amino acid sequence to a GI.4 Chiba reference strain (62), and approximately 72% identical to the Norwalk virus capsid (Fig. 1a). All three NLV capsid genes were inserted into the VEE replicon vector plasmid pVR21 (VEE-NV1, VEE-NV2, and VEE-NCFL, respectively). VEE viral replicon particles of the NV1 and NV2 clones were generated by coelectroporating T7 transcripts of either VEE-NV1 or VEE-NV2 with transcripts of two helper plasmids encoding the VEE capsid and attenuated 3014-glycoprotein genes. The 3014-glycoprotein variant confers increased heparan sulfate attachment of VEE, resulting in an attenuated phenotype in vivo (5, 17). The resulting VEE viral replicon particles were harvested by ultracentrifugation through a 20% sucrose cushion and titered by immunofluorescence analysis using antiserum from a Norwalk virus-infected volunteer (VRP-NV1 and VRP-NV2, respectively).
To characterize NCFL capsid protein expression from VEE, BHK cells were electroporated with T7 transcripts encoding VEE-NCFL and analyzed by IFA using antiserum from an NCFL-infected patient (4). As controls for capsid protein expression, BHK cells were infected with either VRP-NV1 or VRP-NV2 or mock infected with dilution buffer, followed by IFA using Norwalk virus antiserum. IFA results clearly demonstrated that all three NLV capsid proteins are expressed from VEE and recognized by human antiserum, suggesting that antigenic sites were retained in the NV1, NV2, and NCFL capsid proteins (Fig. 2A).
FIG. 2.
Expression and self-assembly of NLV capsid proteins. BHK cells were either infected with VEE VRPs expressing NV1 or NV2, mock infected, or transfected with VEE replicon RNA expressing the NCFL capsid gene. (A) IFA for NV1, NV2, and NCFL capsid protein expression. Cells were examined in a fluorescent microscope for NLV capsid expression using human antiserum to either Norwalk virus (NV1, NV2, and Mock) or the NCFL isolate (NCFL) and goat anti-human IgG-FITC-conjugated secondary antibody. (B) BHK cell lysates were examined for evidence of NLV VLP self-assembly. Putative VLPs were collected by ultracentrifugation through sucrose gradients and analyzed by negative-stain electron microscopy. Scale bar, 100 nm. (C) NV1 and NV2 capsid proteins from VRP-infected BHK cell lysates were analyzed by Western blot using human antiserum to Norwalk virus. Lanes marked 1x and 2x represent uninfected BHK cell lysates at 1 and 2 times the total loaded protein, respectively, versus the NV1 and NV2 lanes. Arrows indicate Norwalk virus antiserum-reactive protein bands, and arrowheads indicate background bands.
Self-assembly of NLV virus-like particles.
We previously demonstrated that infection of BHK cells with VRP-NV1 but not VRP-NV2 results in the production and self-assembly of intact Norwalk VLPs (Baric et al., submitted). Similar results are obtained after transfection of BHK cells with the respective replicon RNAs, although it appears that relative levels of heterologous protein production are reduced compared with infection with VRPs (data not shown).
Due to the potentially high levels of heterologous protein expression from alphavirus replicons (61), we hypothesized that the VRP-NV1 construct would produce high titers of Norwalk VLPs that could be harvested and used for NLV diagnostics and vaccine experiments. To evaluate the extent of VLP production from VRP-NV1, flasks of BHK cells were infected with either VRP-NV1 or VRP-NV2 as a negative control at an MOI of ≈2 for 36 h. Cell monolayers were lysed by freeze-thaw, and putative VLPs were collected by ultracentrifugation of clarified cell extracts through a sucrose cushion. Transmission EM analysis confirmed that infection with VRP-NV1 but not VRP-NV2 results in the self-assembly of Norwalk VLPs (VEE-Nor) (Fig. 2B). Using latex beads as a standard, VEE-Nor particle counts from VRP-NV1-infected BHK cells were approximately 1010 particles per ml (data not shown). Also, SDS-PAGE and Western analysis of purified VEE-Nor demonstrated production of Norwalk virus capsid proteins of the appropriate size, with no detectable background protein in the preparations (data not shown).
We also evaluated whether VEE expression of the NCFL capsid construct resulted in the production of VLPs. BHK cells were transfected with T7 transcripts of VEE-NCFL, and cell extracts were harvested for VLPs. Although IFA data demonstrated NCFL capsid expression in replicon-transfected BHK cells, EM analysis revealed that expression of NCFL did not result in the self-assembly of intact VLPs (Fig. 2B), suggesting the presence of certain debilitating amino acid codon mutations in the NCFL clone that alter the capsid assembly cascade. The Leu182Val, Ile333Val, and Val542Leu amino acid variations appear to be unique among several genetically similar GI capsid proteins and were likely introduced by PCR amplification (Fig. 1b and data not shown).
We hypothesized that potential folding differences between the capsid protein expressed from VRP-NV2 versus VRP-NV1, based on the inability to form intact VLPs, might render the protein more susceptible to proteolytic degradation, which may consequently alter its immunogenicity and presentation in vivo. To test this hypothesis, crude extracts from VRP-NV1- and VRP-NV2-infected BHK cells were analyzed by SDS-PAGE and Western blot. Both constructs clearly expressed Norwalk virus capsid proteins of the appropriate size that were recognized by human antisera (Fig. 2C). However, an additional band of smaller size (≈30 kDa) was evident from the VRP-NV2-infected cell extracts, suggesting that the lack of proper folding and/or assembly into intact VLPs results in detectable proteolytic cleavage of the NV2 capsid protein.
Mouse inoculations.
The availability of VEE VRPs and purified, high-titer VEE-Nor allows direct comparisons of immune induction in response to a variety of NLV vaccine regimens. We evaluated the immune induction in BALB/c mice either subcutaneously inoculated with VEE VRPs expressing Norwalk virus capsid proteins or orally dosed with VEE-Nor. Isogenic BALB/c mice were inoculated and then boosted at day 23 postinoculation by either footpad injection with VRPs or oral dosing with VEE-Nor suspensions according to Table 1. PBS and VRPs expressing influenza virus HA were injected into mice as controls. We quantitated and compared the systemic and mucosal immune induction in response to four different vaccination regimens and determined whether serum antibody produced after inoculation with VRP-NV1 would cross-react with the capsid protein from NCFL, a heterotypic NLV strain. Compiled serum IgG and intestinal IgA data for all mice on days 44 and 28, respectively, are included in Table 1.
Inoculation with VRP-NV1 induces serum IgM and IgG responses to VEE-Nor in mice.
VEE-Nor-specific serum IgM and IgG induction in response to inoculation was quantitated by ELISA. Mice immunized with VRP-NV1 (NV1:NV1) developed strong IgM titers after one inoculation (Fig. 3). The average IgM titer was highest at the day 7 time point and increased slightly after a second inoculation, although not as profoundly as the initial response. IgM levels were negligible by 21 days postboost, which is expected of a replicon-induced immune response, as the VRPs infect only once without long-term antigen production to stimulate continual IgM production.
FIG. 3.
Serum immune responses to Norwalk VLPs (VEE-Nor). Groups of four mice were inoculated on days 0 and 23 (indicated by arrows in IgG chart) according to Table 1. Serum samples were collected and analyzed by ELISA for VEE-Nor-specific serum IgM or IgG. Experimental regimens and sample time points are indicated. Error bars at each time point represent the standard deviation between mice. *, three of four mice represented; one mouse developed IgM levels nearly 10 times the average of the other three mice at this time point (data not shown). Data from this mouse are included for all remaining time points.
Immunization with VRP-NV1 also induced a strong serum IgG response to VEE-Nor (Fig. 3). Even as early as day 7 postinoculation, IgG induction was detected in all four mice. After a second inoculation, IgG titers increased by nearly 10-fold within 5 days, suggesting that repeat immunization with VRPs can be used to substantially boost (P < 0.01, Student’s paired t test) antibody levels. IgG titers after the second inoculation continued to increase through the end of the experiment, and the mean titer in serum samples taken at 121 days postboost remained at more than 60% of the titer at 21 days postboost (data not shown), demonstrating a robust, enduring IgG response.
VRP-NV1 immunization induces the production of VEE-Nor-specific mucosal IgA.
Because NLVs are mucosal pathogens, it is likely that the ability to induce IgA responses at mucosal surfaces will be a necessary feature of an effective NLV vaccine (18). Therefore, we examined whether subcutaneous inoculation with VRP-NV1 results in the production of antigen-specific mucosal IgA. To test the induction of a mucosal response, buffered fecal extracts from immunized mice were analyzed by ELISA for VEE-Nor-specific intestinal IgA. Evaluation of intestinal IgA levels by fecal IgA quantitation has been previously used to study mucosal immune induction to both NLVs and Norwalk VLPs (3, 55). Although levels varied somewhat between mice, a single inoculation of VRP-NV1 did not result in a significant increase (P > 0.1, Student’s paired t test) of detectable intestinal IgA by 14 or 21 days postinoculation (Fig. 4). A second inoculation with VRP-NV1, however, induced a significant increase (P = 0.031) in intestinal IgA in all four mice tested. These results suggest that subcutaneous inoculation of VRP-NV1 stimulated the production of VEE-Nor-specific IgA at mucosal surfaces in the digestive tract, the likely site of NLV infection (40).
FIG. 4.
Intestinal IgA responses to VEE-Nor. Fecal pellets were collected from all mice in each mouse group at the indicated time points, processed to obtain a clarified, buffered extract, and analyzed by ELISA. Samples are from the same mice inoculated on days 0 and 23 represented in Table 1 and Fig. 3. To correct for background, IgA values for each time point from mock-infected mice were subtracted from corresponding time points for all experimental groups.
Systemic and mucosal immune responses after inoculation with VRP-NV2 or oral inoculation of VEE-Nor particles.
At the same time mice were immunized with VRP-NV1, three additional groups of mice were immunized with different regimens to compare immune induction among all four NLV vaccination strategies (Table 1). To determine whether the potential for Norwalk VLP self-assembly might play a role in eliciting immune responses to VEE-Nor, a group of four mice were inoculated and boosted with VRP-NV2 (NV2:NV2), a construct that expresses Norwalk virus capsid proteins that fail to self-assemble into VLPs in vitro (Fig. 2). All mice inoculated with VRP-NV2 developed serum IgM and IgG responses to VEE-Nor, although IgG levels were considerably reduced compared with mice inoculated with VRP-NV1, especially after the second inoculation (P = 0.009 at day 28, Student’s t test) (Fig. 3).
Even more remarkable was the difference in intestinal IgA titers. Although one of the mice might have initially responded by day 14 postinoculation, immunization with VRP-NV2 did not result in significant levels (P > 0.1) of detectable intestinal IgA production at any time point tested (Fig. 4). These results suggest that the potential for Norwalk VLP self-assembly likely plays an important role for maximum immune induction to VEE-Nor after VRP inoculation.
The NV1:VEE-Nor regimen was designed to determine if oral inoculation with VEE-Nor can boost antibody responses in mice previously exposed to VRP-NV1. Evaluation of serum IgG results reveals a slight boost in serum IgG was evident after a 75-μg oral dose of VEE-Nor (Fig. 5). Statistical analysis of raw data indicates that IgG levels did not increase significantly (P > 0.1, Student’s paired t test) from day 14 to day 21, whereas VEE-Nor dosing on day 23 resulted in a significant increase (P < 0.01) in serum IgG titers from day 21 to day 28. These data suggest that antibodies produced after VRP-NV1 inoculation recognized VEE-Nor in the digestive tract, resulting in a slight boost in serum IgG titers within 5 days after VEE-Nor dosing. However, oral boosting with VEE-Nor did not result in significant (P > 0.1) detectable levels of intestinal IgA (Fig. 4), suggesting that additional inoculations and/or higher doses of VEE-Nor are likely needed to induce considerable production of intestinal IgA after priming with VRP-NV1.
FIG. 5.
Serum IgG boost responses from NV1:VEE-Nor mice. Average serum IgG titers of four mice on days 14, 21, and 28, which were inoculated on day 0 with VRP-NV1 and boosted orally on day 23 (arrow) with 75 μg of VEE-Nor. Error bars represent standard deviation. *, the increase in IgG titers from day 14 to day 21 was not statistically significant (P > 0.1); **, significant increase in IgG titers from day 21 to day 28 (P < 0.01; Student’s paired t test of data from two separate ELISA analyses).
A group of four mice were immunized and boosted orally with 75 μg of VEE-Nor particles (VEE-Nor:VEE-Nor), a regimen that was designed to compare the VRP-based method to an NLV oral vaccination strategy previously tested in mice and humans (2, 3). Although modest serum IgM titers were elicited after oral immunization with VEE-Nor, no serum IgG response was notable (Fig. 3). Also, one or two oral inoculations of VEE-Nor did not stimulate the production of intestinal IgA above background levels of detection (P > 0.1, Student’s t test) at any time point (Fig. 4). Ball et al. demonstrated that mice orally dosed with 75 μg of baculovirus-expressed, recombinant Norwalk VLPs (Bac-Nor) in the absence of adjuvant produced limited serum antibody responses, whereas more consistent responses were detected in mice dosed with at least 200 μg of Bac-Nor (3). Therefore, an additional four mice were similarly inoculated and boosted with 200 μg of purified VEE-Nor, resulting in IgG induction in only one mouse, with a titer more than 6- or 30-fold lower than the average response at the same time point after VRP-NV2 or VRP-NV1 inoculation, respectively (Table 1).
Heterotypic immune response.
Due to extensive sequence and antigenic variation among NLVs, an effective vaccine regimen should provide cross-protective immunity to a variety of GI and GII NLV strains. To test heterotypic immune induction, we determined whether serum from mice immunized with VRP-NV1 could cross-react with the capsid protein from NCFL, a heterotypic GI NLV strain isolated and characterized from a recent outbreak (4). BHK cells were transfected with RNA transcripts of a VEE replicon expressing the NCFL capsid and seeded onto slides. The presence of heterotypic serum IgG from VRP-NV1-inoculated mice (serum day 44 from Fig. 3) as well as from a Norwalk virus-infected human volunteer was evaluated by IFA (Fig. 6).
FIG. 6.
Heterotypic immune responses from VRP-NV1-inoculated mice. BHK cells were electroporated with NCFL-encoding VEE replicon RNA, and cross-reactive IgG responses were examined by IFA. Reactivity was detected using (A) serum from an NCFL-infected patient, (B) serum from a Norwalk virus-infected volunteer, (C) pooled high-titer serum from VRP-NV1-inoculated mice, and (D) preimmune mouse serum, followed with goat anti-human or goat anti-mouse IgG-FITC-conjugated secondary antibodies.
The results demonstrate that mice inoculated with VRP-NV1 as well as the individual infected with live Norwalk virus develop serum IgG that cross-reacts with the capsid protein from NCFL. The level of heterotypic IgG reactivity appears similar in both the immunized mice and the infected volunteer at the dilutions tested, but the significance of the signal similarity cannot be established using this assay because different FITC-conjugated secondary antibodies were used. Nevertheless, the overall signal was clearly reduced compared with the reactivity of the homotypic NCFL antiserum. Although these results do not demonstrate cross-protective immunity, the potential for heterotypic immune induction in response to VRP inoculation further establishes the VEE replicon system as a practical approach to NLV vaccine design and warrants further study to determine the extent of cross-reactivity to a variety of GI and GII NLVs.
DiSCUSSION
The most promising NLV vaccine designs currently being pursued are based on oral inoculation of NLV VLPs expressed from recombinant baculoviruses. There are several valuable features of this vaccination approach, including simple oral delivery of antigens, stability of the VLPs at various temperatures for ease in transport, and presentation of antigens at the sites of NLV infection (reviewed in reference 18). A recent phase I clinical trial established that baculovirus-expressed, recombinant Norwalk VLPs were safe in humans and induced dose-dependent increases in antigen-specific serum IgG titers in volunteers with existing serum IgG to Norwalk virus (2). However, mucosal immune responses were not evaluated in these individuals, and serum immune responses were inferior to those elicited after live viral infection (2, 45).
Possible reasons for the lack of a vigorous IgG response to orally dosed Norwalk VLPs include insufficient amounts of antigen, lack of virus replication or amplification, inadequate responses to antigen without the use of adjuvant, and the possibility of tolerance induction. Guerrero et al. have recently demonstrated that mice inoculated intranasally with Norwalk VLPs generate systemic and mucosal antibody responses (27). One major advantage of this vaccination strategy is that only about one-tenth of the amount of VLPs is needed to induce responses that are equivalent to, if not better than, those elicited after oral inoculation.
With either inoculation method, the addition of adjuvant greatly enhances and prolongs the systemic and mucosal responses (3, 27). However, it is not clear whether intranasal or oral inoculation with Norwalk VLPs, let alone infection with wild-type NLVs, confers long-term protective homotypic or heterotypic immunity. Consequently, additional experiments are needed to evaluate the efficacy of these mucosal vaccination methods. This paper describes a novel complementary NLV vaccine approach employing an alphavirus-based expression method to generate NLV VLPs in a mammalian system.
Using a VEE replicon expression system, we have shown that NLV capsid proteins self-assemble to generate large quantities of NLV VLPs in mammalian cells. Importantly, as a potential vaccine approach, and unlike mucosal or parenteral inoculation of VLPs, VEE expression of the Norwalk virus capsid protein provides a method for introducing endogenous NLV VLPs into mammalian cells in a way similar to a natural viral infection. Mice inoculated with VEE VRPs expressing Norwalk virus capsid proteins developed significant serum and mucosal immune responses to VEE-Nor, the strength of which correlates with the ability of the expressed proteins to self-assemble into VLPs. Serum IgG and IgM responses to VEE-Nor after immunization with VRP-NV1 rose to remarkably high levels, even after a single inoculation. VRP-NV1-immunized mice also generated intestinal IgA responses, a feature that is likely to be essential for protective immunity to NLV infection.
The presence of cross-reactive IgG to a different GI NLV capsid protein evokes the potential for heterotypic immune induction, and these data represent the first known demonstration of a cross-reactive antibody response induced in mice with a candidate NLV vaccine. Heterotypic immune induction will likely be a necessary feature of an effective vaccine regimen against the many genetically diverse NLV strains. Our results suggest that a VEE-based vaccine provides a powerful complementary approach to current NLV vaccine strategies.
We expect even greater heterotypic reactivity to NLV capsid proteins that assemble into intact VLPs, which are more likely to retain conserved conformational antigenic sites. Epitope mapping of baculovirus-expressed Norwalk VLPs using a panel of monoclonal antibodies has suggested that most antigenic epitopes of the Norwalk virus capsid protein are in fact discontinuous and therefore likely to be present only in at least partially assembled VLPs (28). Based on sequence data and primer design, the noted variations in the NCFL clone at amino acids 182, 333, and 542 were likely introduced by PCR. Interestingly, amino acids 182 and 333 map to β-strand and dimerization sites, respectively, in the Norwalk virus capsid crystal structure (54), and we therefore speculate that these mutations were debilitating to the capsid assembly cascade.
Although the responses observed in mice orally inoculated with VEE-Nor generally appear to be consistent with previous experiments described by Ball et al. (3), certain discrepancies exist between the results. In particular, mice orally dosed twice with 200 μg of Bac-Nor without adjuvant exhibited more consistent serum immune responses than we could detect after two 200-μg VEE-Nor doses (3). These differences might be attributed to the use of different mouse strains for inoculation, differences in the purity of VEE-Nor and Bac-Nor antigen preparations for animal dosing, and/or variability in ELISA detection sensitivity. Despite these potential discrepancies, our immunization and detection methods revealed that mice inoculated twice with VRP-NV1 developed considerably stronger serum and intestinal immune responses than those given two doses of either 75 or 200 μg of VEE-Nor particles.
The fact that subcutaneous inoculation of VEE VRPs resulted in the production of mucosal antibody is a conundrum to conventional perception of mucosal immune induction. One would expect that only immunization with live virus that can disseminate to mucosal tissues, or the use of a mucosal route of immunization, might result in a significant mucosal immune response. VRPs are designed to infect for only one round of replication and expression without packaging and spreading to neighboring cells (57). Therefore, it is not immediately anticipated that this vaccination strategy would be useful in stimulating protective immunity against a mucosal pathogen. However, our data demonstrate that antigen-specific IgA, the predominant antibody at mucosal surfaces, was present in the digestive tract of VRP-NV1-immunized mice. We expect the IgA present in fecal extracts from VRP-NV1-inoculated mice arose from antibody-secreting cells in lymphoid tissue of the gut, although this has not yet been confirmed.
Recent experiments have revealed that mice subcutaneously inoculated with VRP-HA develop antigen-specific IgA-secreting B cells in the gut and are protected against mucosal influenza virus challenge (57; E. Richmond, personal communication). Other groups have similarly observed mucosal immune responses after parenteral inoculation with nucleic acid or protein subunit vaccines (48, 50). The precise mechanism(s) for mucosal immune induction after parenteral inoculation with a nondisseminating vaccine is not known. Possible mechanisms that we are currently addressing with the VEE replicon system include the ideas that VRP-infected cells traffic to and present antigen at mucosal inductive sites and VEE VRPs infect cells in the draining lymph node, subsequently rendering the organ into a mucosal inductive site.
The VEE-NV1 vaccination system described in this paper may provide a valuable model for the study of replicon-induced mucosal immunity. Because a human challenge model for Norwalk virus infection is available, the use of VEE VRPs expressing NLV capsid proteins might provide a powerful tool for the study of mucosal immunity in humans. This model system would likely benefit researchers of a wide range of animal and human mucosal pathogens.
Several groups have reported enhanced mucosal and systemic immune responses using various combination mucosal/parenteral immunization regimens (reviewed in reference 50). Although the mucosal responses in mice primed with VRP-NV1 could not be detectably enhanced after oral dosing with 75 μg of VEE-Nor, we speculate that a combination prime-boost immunization strategy will induce a more effective systemic and mucosal immune response. In particular, we are interested in evaluating responses in mice immunized with a combination of parenterally administered VEE VRPs and intranasally administered VEE-Nor. Also, wild-type VEE will infect mice when administered intranasally (10, 14, 30, 31). Therefore, it seems appropriate to explore the efficacy of an intranasal VEE VRP inoculation as a means to enhance systemic and mucosal immune responses to NLV capsid proteins.
Specific requirements for protective immunity against NLV infection are not well established, with much of the available data being contradictory. Limited data obtained prior to the advent of recent molecular-based approaches to study NLV immunity have suggested that existing serum antibody to NLVs correlates with an increased risk of infection, while others have demonstrated short-term and occasionally long-term immunity to homologous rechallenge of NLV-dosed volunteers, as well as natural immunity to NLV infection upon repeated exposure within communities (reviewed in references 18 and 40). Longer-term immunity to NLV infection likely requires a mucosal response to localized areas of infection. The fact that no small-animal or tissue culture system is available to cultivate NLVs makes it difficult to identify important components of a protective immune response. The development of such systems and the design of innovative human challenge experiments are needed to fully address NLV infectivity and pathogenesis and to assay the effectiveness of candidate NLV vaccines.
VEE replicon systems have been used to design vaccines that induce systemic, mucosal, and cellular immune responses to heterologous viral proteins (9, 10, 15). Several VEE replicon vaccine regimens to date have been shown to be safe in a wide range of laboratory animals and provide mucosal and/or systemic protection against a number of viral pathogens, including influenza, Ebola, Marburg, and simian immunodeficiency viruses (16, 32, 56, 57). Also, immunity induced in mice by sequential immunization with distinct VRP constructs has confirmed the effectiveness of VEE-based vaccines for repeat immunizations against different viral pathogens (57).
A significant advantage of the VEE replicon vaccination system is the fact that VEE viruses naturally target professional antigen-presenting cells, particularly dendritic cells (15), which are potent stimulators of T-cell responses to virus-infected cells (34). As a result, VEE replicons stimulate high levels of antigen-specific, cytotoxic T lymphocytes (8, 9), which often play an important role in providing protective immunity against viral infection. To our knowledge, cellular immunity to NLV infection has not been examined. Therefore, the role of cellular immunity in protection against NLV infection is unknown. We are currently investigating the cytotoxic T-lymphocyte response to NLV capsid proteins in humans infected with NLVs, as well as in mice immunized with VEE VRPs.
VLP-based vaccines not only allow the ability to present conformational antigens that are morphologically and immunologically analogous to live virus counterparts, but also allow the presentation of viral antigens that are likely more resistant to proteolytic degradation (such as in the digestive tract) (3, 29). The additional lower-molecular-weight protein product observed from VRP-NV2-infected BHK cells is of particular interest (Fig. 2C). We hypothesize that it represents a Norwalk virus capsid protein cleavage product similar to those found in the stools of Norwalk virus-infected patients, as well as from protease-treated, baculovirus-expressed Norwalk VLPs (26, 29). This hypothesis would explain why the product is not detected from cells infected with VRP-NV1, which expresses capsid proteins that self-assemble into intact VLPs. Similarly, alkaline dissociation of baculovirus-expressed Norwalk VLPs exposes a trypsin cleavage site that is otherwise protected in fully assembled particles (69). Consequently, we cannot rule out the possibility that reduced immune responses after immunization with VRP-NV2 versus VRP-NV1 are the result of less full-size capsid protein present in VRP-NV2-infected cells of immunized mice. However, it is more likely that the presence of fully assembled VEE-Nor particles provides additional conformational and discontinuous epitopes that are not present in the full-length, unassembled capsid proteins.
VLPs are currently being examined for vaccine purposes for a number of other human pathogens, including rotavirus, papillomavirus, polyomavirus, and human immunodeficiency virus (HIV) (12, 23, 60, 67). A variety of vectors have been used to express VLPs, including vaccinia viruses, baculoviruses, alphaviruses, yeasts, and bacteria, among others (6, 49, 59).
Unlike soluble subunit counterpart vaccines, Pr55gag-based HIV VLPs have been shown to induce high titers of HIV-specific antibodies without the use of adjuvant (68). Also, denaturation of human papillomavirus VLPs prior to immunization abolishes the ability of the vaccine to induce protective immune responses in animal models (7, 41). VLPs also offer a greater possibility to induce cross-protective immunity, as mice vaccinated with rotavirus VLPs are protected from challenge with heterotypic strains (13, 35). These experiments clearly demonstrate the importance of authentic conformational epitopes that can only be presented on VLPs and live virions to elicit maximum levels of virus-neutralizing antibodies.
It is important to consider the potential for certain dissimilarities in VLPs attributable to nonmammalian expression, as the proteins might be posttranslationally processed in a different manner in mammalian and insect cell expression systems. Although we have not observed any differences between baculovirus and VEE-expressed Norwalk VLPs (Baric et al., submitted), previous experiments have demonstrated subtle variations between insect and mammalian posttranslational modification patterns of recombinant proteins (33, 64). For example, baculovirus- and vaccinia virus-expressed papillomavirus VLPs were recently shown to possess notable variations in phosphorylation and glycosylation properties (19). Such variations attributable to insect cell expression might be extremely important, as posttranslational modifications have been shown to play a vital role in the immunogenic manifestation of recombinant proteins, especially notable for cytotoxic T-lymphocyte induction (43, 51, 52).
As a model system, the VEE-based approach described in this paper represents an advantageous method for the expression of heterologous proteins in a more natural cellular environment, which will likely increase the chances of generating biologically authentic proteins for vaccine purposes. In addition, since VEE replicons do not reassemble after infection, this method provides a means for producing high-titer VLPs in the absence of contaminating helper virus. VEE-based expression of VLPs might also prove useful for researchers studying other viruses, as structural proteins from many viruses can be expressed to generate VLPs.
Because NLVs have not been successfully cultured in vitro, little is known about the expression, subcellular location, and processing of NLV capsid proteins. Systems for the expression and self-assembly of NLV capsid proteins in mammalian cells are needed to fully understand NLV replication and pathogenesis. We believe that VEE replicon-driven Norwalk virus capsid self-assembly will offer new insights into NLV entry, assembly, encapsidation, and release and allow potential reverse genetic approaches to recover infectious NLV particles from mammalian cells. VEE replicons shall provide a complementary approach to baculovirus expression systems for studying calicivirus molecular biology and pathogenesis and will likely provide useful diagnostic tools for future NLV research.
Acknowledgments
We thank Vicky Madden and Bob Bagnell for their assistance in electron microscopy. We also acknowledge Martha Collier for her services in making VEE VRPs and Shermalyn Greene and Fan-Chen Tseng for providing the NV1 and NV2 capsid clones. Also, we thank Kris Curtis, Jan Vinjé, and Lisa Lindesmith for their helpful discussions in writing the manuscript.
This work was supported by research grants from the National Institutes of Health (RSB-AI23946) and the North Carolina Biotechnology Center.
REFERENCES
- 1.Arness, M. K., B. H. Feighner, M. L. Canham, D. N. Taylor, S. S. Monroe, T. J. Cieslak, E. L. Hoedebecke, C. S. Polyak, J. C. Cuthie, R. L. Fankhauser, C. D. Humphrey, T. L. Barker, C. D. Jenkins, and D. R. Skillman. 2000. Norwalk-like viral gastroenteritis outbreak in U.S. Army trainees. Emerg. Infect. Dis. 6:204–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ball, J. M., D. Y. Graham, A. R. Opekun, M. A. Gilger, R. A. Guerrero, and M. K. Estes. 1999. Recombinant Norwalk virus-like particles given orally to volunteers: phase I study. Gastroenterology 117:40–48. [DOI] [PubMed] [Google Scholar]
- 3.Ball, J. M., M. E. Hardy, R. L. Atmar, M. E. Conner, and M. K. Estes. 1998. Oral immunization with recombinant Norwalk virus-like particles induces a systemic and mucosal immune response in mice. J. Virol. 72:1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker, K. M., C. L. Moe, K. L. Southwick, and J. N. MacCormack. 2000. Transmission of Norwalk virus during a football game. N. Engl. J. Med. 343:1223–1227. [DOI] [PubMed] [Google Scholar]
- 5.Bernard, K. A., W. B. Klimstra, and R. E. Johnston. 2000. Mutations in the E2 glycoprotein of Venezuelan equine encephalitis virus confer heparan sulfate interaction, low morbidity, and rapid clearance from blood of mice. Virology 276:93–103. [DOI] [PubMed] [Google Scholar]
- 6.Betenbaugh, M., M. Yu, K. Kuehl, J. White, D. Pennock, K. Spik, and C. Schmaljohn. 1995. Nucleocapsid- and virus-like particles assemble in cells infected with recombinant baculoviruses or vaccinia viruses expressing the M and the S segments of Hantaan virus. Virus Res. 38:111–124. [DOI] [PubMed] [Google Scholar]
- 7.Breitburd, F., R. Kirnbauer, N. L. Hubbert, B. Nonnenmacher, C. Trin-Dinh-Desmarquet, G. Orth, J. T. Schiller, and D. R. Lowy. 1995. Immunization with virus-like particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 69:3959–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caley, I. J., M. R. Betts, N. L. Davis, R. Swanstrom, J. A. Frelinger, and R. E. Johnston. 1999. Venezuelan equine encephalitis virus vectors expressing HIV-1 proteins: vector design strategies for improved vaccine efficacy. Vaccine 17:3124–3135. [DOI] [PubMed] [Google Scholar]
- 9.Caley, I. J., M. R. Betts, D. M. Irlbeck, N. L. Davis, R. Swanstrom, J. A. Frelinger, and R. E. Johnston. 1997. Humoral, mucosal, and cellular immunity in response to a human immunodeficiency virus type 1 immunogen expressed by a Venezuelan equine encephalitis virus vaccine vector. J. Virol. 71:3031–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charles, P. C., K. W. Brown, N. L. Davis, M. K. Hart, and R. E. Johnston. 1997. Mucosal immunity induced by parenteral immunization with a live attenuated Venezuelan equine encephalitis virus vaccine candidate. Virology 228:153–160. [DOI] [PubMed] [Google Scholar]
- 11.Clarke, I. N., and P. R. Lambden. 2000. Organization and expression of calicivirus genes. J Infect. Dis. 181(Suppl. 2):S309–316. [DOI] [PubMed] [Google Scholar]
- 12.Conner, M. E., C. D. Zarley, B. Hu, S. Parsons, D. Drabinski, S. Greiner, R. Smith, B. Jiang, B. Corsaro, V. Barniak, H. P. Madore, S. Crawford, and M. K. Estes. 1996. Virus-like particles as a rotavirus subunit vaccine. J.Infect. Dis. 174(Suppl. 1):S88–92. [DOI] [PubMed] [Google Scholar]
- 13.Crawford, S. E., M. K. Estes, M. Ciarlet, C. Barone, C. M. O’Neal, J. Cohen, and M. E. Conner. 1999. Heterotypic protection and induction of a broad heterotypic neutralization response by rotavirus-like particles. J. Virol. 73:4813–4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis, N. L., K. W. Brown, G. F. Greenwald, A. J. Zajac, V. L. Zacny, J. F. Smith, and R. E. Johnston. 1995. Attenuated mutants of Venezuelan equine encephalitis virus containing lethal mutations in the PE2 cleavage signal combined with a second-site suppressor mutation in E1. Virology. 212:102–110. [DOI] [PubMed] [Google Scholar]
- 15.Davis, N. L., K. W. Brown, and R. E. Johnston. 1996. A viral vaccine vector that expresses foreign genes in lymph nodes and protects against mucosal challenge. J.Virol. 70:3781–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis, N. L., I. J. Caley, K. W. Brown, M. R. Betts, D. M. Irlbeck, K. M. McGrath, M. J. Connell, D. C. Montefiori, J. A. Frelinger, R. Swanstrom, P. R. Johnson, and R. E. Johnston. 2000. Vaccination of macaques against pathogenic simian immunodeficiency virus with Venezuelan equine encephalitis virus replicon particles. J. Virol. 74:371–378. (Erratum, J. Virol. 74:3430.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis, N. L., N. Powell, G. F. Greenwald, L. V. Willis, B. J. Johnson, J. F. Smith, and R. E. Johnston. 1991. Attenuating mutations in the E2 glycoprotein gene of Venezuelan equine encephalitis virus: construction of single and multiple mutants in a full-length cDNA clone. Virology 183:20–31. [DOI] [PubMed] [Google Scholar]
- 18.Estes, M. K., J. M. Ball, R. A. Guerrero, A. R. Opekun, M. A. Gilger, S. S. Pacheco, and D. Y. Graham. 2000. Norwalk virus vaccines: challenges and progress. J. Infect. Dis. 181(Suppl. 2):S367–373. [DOI] [PubMed] [Google Scholar]
- 19.Fang, N., I. H. Frazer, and G. J. Fernando. 2000. Differences in the posttranslational modifications of human papillomavirus type 6b major capsid protein expressed from a baculovirus system compared with a vaccinia virus system. Biotechnol.Appl. Biochem. 32:27–33. [DOI] [PubMed] [Google Scholar]
- 20.Fankhauser, R. L., J. S. Noel, S. S. Monroe, T. Ando, and R. I. Glass. 1998. Molecular epidemiology of “Norwalk-like viruses”in outbreaks of gastroenteritis in the United States. J. Infect. Dis. 178:1571–1578. [DOI] [PubMed] [Google Scholar]
- 21.Glass, P. J., L. J. White, J. M. Ball, I. Leparc-Goffart, M. E. Hardy, and M. K. Estes. 2000. Norwalk virus open reading frame 3 encodes a minor structural protein. J. Virol. 74:6581–6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glass, R. I., J. Noel, T. Ando, R. Fankhauser, G. Belliot, A. Mounts, U. D. Parashar, J. S. Bresee, and S. S. Monroe. 2000. The epidemiology of enteric caliciviruses from humans: a reassessment using new diagnostics. J. Infect. Dis. 181(Suppl. 2):S254–261. [DOI] [PubMed] [Google Scholar]
- 23.Goldmann, C., H. Petry, S. Frye, O. Ast, S. Ebitsch, K. D. Jentsch, F. J. Kaup, F. Weber, C. Trebst, T. Nisslein, G. Hunsmann, T. Weber, and W. Luke. 1999. Molecular cloning and expression of major structural protein VP1 of the human polyomavirus JC virus: formation of virus-like particles useful for immunological and therapeutic studies. J. Virol. 73:4465–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green, J., J. Vinje, C. I. Gallimore, M. Koopmans, A. Hale, and D. W. Brown. 2000. Capsid protein diversity among Norwalk-like viruses. Virus Genes 20:227–236. [DOI] [PubMed] [Google Scholar]
- 25.Green, K. Y., J. F. Lew, X. Jiang, A. Z. Kapikian, and M. K. Estes. 1993. Comparison of the reactivities of baculovirus-expressed recombinant Norwalk virus capsid antigen with those of the native Norwalk virus antigen in serologic assays and some epidemiologic observations. J. Clin. Microbiol. 31:2185–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberg, H. B., J. R. Valdesuso, A. R. Kalica, R. G. Wyatt, V. J. McAuliffe, A. Z. Kapikian, and R. M. Chanock. 1981. Proteins of Norwalk virus. J. Virol. 37:994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guerrero, R. A., J. M. Ball, S. S. Krater, S. E. Pacheco, J. D. Clements, and M. K. Estes. 2001. Recombinant norwalk virus-like particles administered intranasally to mice induce systemic and mucosal (fecal and vaginal) immune responses. J. Virol. 75:9713–9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardy, M. E., T. N. Tanaka, N. Kitamoto, L. J. White, J. M. Ball, X. Jiang, and M. K. Estes. 1996. Antigenic mapping of the recombinant Norwalk virus capsid protein using monoclonal antibodies. Virology 217:252–261. [DOI] [PubMed] [Google Scholar]
- 29.Hardy, M. E., L. J. White, J. M. Ball, and M. K. Estes. 1995. Specific proteolytic cleavage of recombinant Norwalk virus capsid protein. J. Virol. 69:1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hart, M. K., K. Caswell-Stephan, R. Bakken, R. Tammariello, W. Pratt, N. Davis, R. E. Johnston, J. Smith, and K. Steele. 2000. Improved mucosal protection against Venezuelan equine encephalitis virus is induced by the molecularly defined, live-attenuated V3526 vaccine candidate. Vaccine 18:3067–3075. [DOI] [PubMed] [Google Scholar]
- 31.Hart, M. K., W. Pratt, F. Panelo, R. Tammariello, and M. Dertzbaugh. 1997. Venezuelan equine encephalitis virus vaccines induce mucosal IgA responses and protection from airborne infection in BALB/c, but not C3H/HeN mice. Vaccine 15:363–369. [DOI] [PubMed] [Google Scholar]
- 32.Hevey, M., D. Negley, P. Pushko, J. Smith, and A. Schmaljohn. 1998. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology 251:28–37. [DOI] [PubMed] [Google Scholar]
- 33.Hoss, A., I. Moarefi, K. H. Scheidtmann, L. J. Cisek, J. L. Corden, I. Dornreiter, A. K. Arthur, and E. Fanning. 1990. Altered phosphorylation pattern of simian virus 40 T antigen expressed in insect cells by using a baculovirus vector. J. Virol. 64:4799–4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janeway, C. A., et. al. 1999. T-cell mediated immunity, p.263–305. In C. A. Janeway et al. (ed.), Immunobiology, 4th ed. Elsevier Science Ltd/Garland Publishing, London, United Kingdom.
- 35.Jiang, B., M. K. Estes, C. Barone, V. Barniak, C. M. O’Neal, A. Ottaiano, H. P. Madore, and M. E. Conner. 1999. Heterotypic protection from rotavirus infection in mice vaccinated with virus-like particles. Vaccine 17:1005–1013. [DOI] [PubMed] [Google Scholar]
- 36.Jiang, X., M. Wang, D. Y. Graham, and M. K. Estes. 1992. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 66:6527–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang, X., M. Wang, K. Wang, and M. K. Estes. 1993. Sequence and genomic organization of Norwalk virus. Virology. 195:51–61. [DOI] [PubMed] [Google Scholar]
- 38.Johnson, P. C., J. J. Mathewson, H. L. DuPont, and H. B. Greenberg. 1990. Multiple-challenge study of host susceptibility to Norwalk gastroenteritis in US adults. J. Infect. Dis. 161:18–21. [DOI] [PubMed] [Google Scholar]
- 39.Johnston, R. E., and C. J. Peters. 1996. Alphaviruses, p.843–898. In B. Fields (ed.), Virology, 3rd ed., vol. 2. Lippincott-Raven, Philadelphia, Pa.
- 40.Kapikian, A. Z., M. K. Estes, and R. M. Chanock. 1996. Norwalk group of viruses, p.783–810. In B. Fields (ed.), Virology, 3rd ed., vol. 2. Lippincott-Raven, Philadelphia, Pa.
- 41.Kirnbauer, R., F. Booy, N. Cheng, D. R. Lowy, and J. T. Schiller. 1992. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 89:12180–12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koopmans, M., J. Vinje, M. de Wit, I. Leenen, W. van der Poel, and Y. van Duynhoven. 2000. Molecular epidemiology of human enteric caliciviruses in The Netherlands. J Infect. Dis. 181(Suppl. 2):S262–269. [DOI] [PubMed] [Google Scholar]
- 43.Larson, J. K., L. Otvos, Jr., and H. C. Ertl. 1992. Posttranslational side chain modification of a viral epitope results in diminished recognition by specific T cells. J. Virol. 66:3996–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mason, H. S., J. M. Ball, J. J. Shi, X. Jiang, M. K. Estes, and C. J. Arntzen. 1996. Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc. Natl. Acad. Sci. USA 93:5335–5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsui, S. M. 1999. A new model system to study Norwalk virus immunity. Gastroenterology 117:255–257. [DOI] [PubMed] [Google Scholar]
- 46.Matsui, S. M., and H. B. Greenberg. 2000. Immunity to calicivirus infection. J Infect. Dis. 181(Suppl. 2):S331–335. [DOI] [PubMed] [Google Scholar]
- 47.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mittal, S. K., N. Aggarwal, G. Sailaja, A. van Olphen, H. HogenEsch, A. North, J. Hays, and S. Moffatt. 2000. Immunization with DNA, adenovirus or both in biodegradable alginate microspheres: effect of route of inoculation on immune response. Vaccine 19:253–263. [DOI] [PubMed] [Google Scholar]
- 49.Nardelli-Haefliger, D., R. B. Roden, J. Benyacoub, R. Sahli, J. P. Kraehenbuhl, J. T. Schiller, P. Lachat, A. Potts, and P. De Grandi. 1997. Human papillomavirus type 16 virus-like particles expressed in attenuated Salmonella typhimurium elicit mucosal and systemic neutralizing antibodies in mice. Infect. Immun. 65:3328–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ogra, P. L., H. Faden, and R. C. Welliver. 2001. Vaccination strategies for mucosal immune responses. Clin. Microbiol Rev. 14:430–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otvos, L., Jr., B. Cappelletto, I. Varga, J. D. Wade, Z. Q. Xiang, K. Kaiser, L. J. Stephens, and H. C. Ertl. 1996. The effects of posttranslational side-chain modifications on the stimulatory activity, serum stability and conformation of synthetic peptides carrying T helper cell epitopes. Biochim. Biophys. Acta 1313:11–19. [DOI] [PubMed] [Google Scholar]
- 52.Otvos, L., Jr., L. Urge, Z. Q. Xiang, G. R. Krivulka, L. Nagy, G. I. Szendrei, and H. C. Ertl. 1994. Glycosylation of synthetic T helper cell epitopic peptides influences their antigenic potency and conformation in a sugar location-specific manner. Biochim. Biophys. Acta 1224:68–76. [DOI] [PubMed] [Google Scholar]
- 53.Parks, C. G., C. L. Moe, D. Rhodes, A. Lima, L. Barrett, F. Tseng, R. Baric, A. Talal, and R. Guerrant. 1999. Genomic diversity of “Norwalk like viruses” (NLVs): pediatric infections in a Brazilian shantytown. J. Med. Virol. 58:426–434. [DOI] [PubMed] [Google Scholar]
- 54.Prasad, B. V., M. E. Hardy, T. Dokland, J. Bella, M. G. Rossmann, and M. K. Estes. 1999. X-ray crystallographic structure of the Norwalk virus capsid. Science 286:287–290. [DOI] [PubMed] [Google Scholar]
- 55.Pujol, F. H., G. Vasquez, A. M. Rojas, M. E. Fuenmayor, C. L. Loureiro, I. Perez-Schael, M. K. Estes, and F. Liprandi. 1998. Norwalk virus infection in Venezuela. Ann.Trop. Med. Parasitol. 92:205–211. [PubMed] [Google Scholar]
- 56.Pushko, P., M. Bray, G. V. Ludwig, M. Parker, A. Schmaljohn, A. Sanchez, P. B. Jahrling, and J. F. Smith. 2000. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine 19:142–153. [DOI] [PubMed] [Google Scholar]
- 57.Pushko, P., M. Parker, G. V. Ludwig, N. L. Davis, R. E. Johnston, and J. F. Smith. 1997. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology 239:389–401. [DOI] [PubMed] [Google Scholar]
- 58.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning, 2nd ed., vol. 3, p. 18.47–18.75. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 59.Sasagawa, T., P. Pushko, G. Steers, S. E. Gschmeissner, M. A. Hajibagheri, J. Finch, L. Crawford, and M. Tommasino. 1995. Synthesis and assembly of virus-like particles of human papillomaviruses type 6 and type 16 in fission yeast Schizosaccharomyces pombe. Virology 206:126–135. [DOI] [PubMed] [Google Scholar]
- 60.Schiller, J. T. 1999. Papillomavirus-like particle vaccines for cervical cancer. Mol. Med. Today 5:209–215. [DOI] [PubMed] [Google Scholar]
- 61.Schlesinger, S., and T. W. Dubensky. 1999. Alphavirus vectors for gene expression and vaccines. Curr. Opin.Biotechnol. 10:434–439. [DOI] [PubMed] [Google Scholar]
- 62.Someya, Y., N. Takeda, and T. Miyamura. 2000. Complete nucleotide sequence of the chiba virus genome and functional expression of the 3C-like protease in Escherichia coli. Virology 278:490–500. [DOI] [PubMed] [Google Scholar]
- 63.Tacket, C. O., H. S. Mason, G. Losonsky, M. K. Estes, M. M. Levine, and C. J. Arntzen. 2000. Human immune responses to a novel Norwalk virus vaccine delivered in transgenic potatoes. J. Infect. Dis. 182:302–305. [DOI] [PubMed] [Google Scholar]
- 64.Thomsen, D. R., L. E. Post, and A. P. Elhammer. 1990. Structure of O-glycosidically linked oligosaccharides synthesized by the insect cell line Sf9. J. Cell Biochem. 43:67–79. [DOI] [PubMed] [Google Scholar]
- 65.Tubulekas, I., P. Berglund, M. Fleeton, and P. Liljestrom. 1997. Alphavirus expression vectors and their use as recombinant vaccines: a minireview. Gene 190:191–195. [DOI] [PubMed] [Google Scholar]
- 66.Vinje, J., J. Green, D. C. Lewis, C. I. Gallimore, D. W. Brown, and M. P. Koopmans. 2000. Genetic polymorphism across regions of the three open reading frames of “Norwalk-like viruses”. Arch. Virol. 145:223–241. [DOI] [PubMed] [Google Scholar]
- 67.Wagner, R., L. Deml, F. Notka, H. Wolf, R. Schirmbeck, J. Reimann, V. Teeuwsen, and J. Heeney. 1996. Safety and immunogenicity of recombinant human immunodeficiency virus-like particles in rodents and rhesus macaques. Intervirology 39:93–103. [DOI] [PubMed] [Google Scholar]
- 68.Wagner, R., L. Deml, R. Schirmbeck, M. Niedrig, J. Reimann, and H. Wolf. 1996. Construction, expression, and immunogenicity of chimeric HIV-1 virus-like particles. Virology 220:128–140. [DOI] [PubMed] [Google Scholar]
- 69.White, L. J., M. E. Hardy, and M. K. Estes. 1997. Biochemical characterization of a smaller form of recombinant Norwalk virus capsids assembled in insect cells. J. Virol. 71:8066–8072. [DOI] [PMC free article] [PubMed] [Google Scholar]