Abstract
Essential to the oncogenic properties of human papillomavirus type 16 (HPV-16) are the activities encoded by the early gene product E7. HPV-16 E7 (E7.16) binds to cellular factors involved in cell cycle regulation and differentiation. These include the retinoblastoma tumor suppressor protein (Rb) and histone deacetylase (HDAC) complexes. While the biological significance of these interactions remains unclear, E7 is believed to help maintain cells in a proliferative state, thus establishing an environment that is conducive to viral replication. Most pathways that govern cell growth converge on downstream effectors. Among these is the cdc25A tyrosine phosphatase. cdc25A is required for G1/S transition, and its deregulation is associated with carcinogenesis. Considering the importance of cdc25A in cell cycle progression, it represents a relevant target for viral oncoproteins. Accordingly, the present study focuses on the putative deregulation of cdc25A by E7.16. Our results indicate that E7.16 can impede growth arrest induced during serum starvation and keratinocyte differentiation. Importantly, these E7-specific phenotypes correlate with elevated cdc25A steady-state levels. Reporter assays performed with NIH 3T3 cell lines and human keratinocytes indicate that E7 can transactivate the cdc25A promoter. In addition, transcriptional activation by E7.16 requires the distal E2F site within the cdc25A promoter. We further demonstrate that the ability of E7 to abrogate cell cycle arrest, activate cdc25A transcription, and increase cdc25A protein levels requires intact Rb and HDAC-1 binding domains. Finally, by using the cdk inhibitor roscovitine, we reveal that E7 activates the cdc25A promoter independently of cell cycle progression and cdk activity. Consequently, we propose that E7.16 can directly target cdc25A transcription and maintains cdc25A gene expression by disrupting Rb/E2F/HDAC-1 repressor complexes.
Human papillomavirus (HPV) is a small DNA tumor virus predominantly infecting the cutaneous and mucosal epithelium of the genital tract (51, 52, 58). Subcategorization of HPVs can be made based on disease association, with low-risk HPVs (HPV type 6 [HPV-6] and HPV-11) causing benign genital warts and high-risk HPVs (HPV-16 and HPV-18) contributing to more aggressive forms of malignancies such as cervical cancer (51). In the case of HPV, a direct etiological link can be established between viral infection and the onset of carcinogenesis. This is highlighted by the fact that the HPV genome can be found in over 90% of cervical malignancies (8, 21). As such, HPV represents a relevant model system for the study of viral oncogenesis.
The HPV genome is a mere 8 kb, consisting of eight open reading frames carrying the late genes L1, L2, and E4 and five early genes designated E1, E2, E5, E6, and E7. The activities of E5, E6, and E7 seem to be especially important for viral oncogenesis. These three oncoproteins are known to disrupt key components of various growth pathways. For instance, E5 interacts with the vacuolar ATPase and increases epidermal growth factor signaling (2, 79, 80). E6 can bind to the tumor suppressor gene p53 and lead to its degradation (25, 33, 46), while other studies demonstrate that E6 can interact with the family of coactivators CBP/p300 and disrupt transcriptional activation (65, 88). The present study focuses on the properties of HPV-16 E7 (E7.16).
E7.16 is a 98-amino-acid protein, which is divided into three regions (CR1, CR2, and CR3) based on homology to the adenoviral oncoprotein E1A (5, 23). E7 induces significant biological changes, particularly in regard to cellular growth. Indeed, E7.16 can immortalize human foreskin keratinocytes (HFKs), extend proliferation of rat embryo fibroblasts, and, in conjunction with Ha-RAS, immortalize baby rat kidney cells (1, 16, 31, 36, 40). Moreover, E7 can transform a variety of cell lines, including NIH 3T3 mouse fibroblasts (15, 32, 67). The mechanisms underlying these E7-induced phenotypes remain for the most part unclear, but perhaps the most well-characterized biochemical property of E7 is its ability to bind to the retinoblastoma tumor suppressor protein (Rb) (24, 59). The retinoblastoma protein family members play a central role in the regulation of the eukaryotic cell cycle. More specifically, in its hypophosphorylated state, Rb can bind to transcription factors such as the E2F family members and repress the transcription of particular genes. As cells progress from G0 through G1 and into S phase, Rb family members become progressively hyperphosphorylated by G1 cyclin-cyclin-dependent kinases (cdks), consequently releasing the transcription factor E2F, which in turn activates genes involved in DNA synthesis and cell cycle progression (22). Since E7 is able to bind to unphosphorylated Rb, it is believed that E7 can prematurely induce cells into S phase by disrupting Rb-E2F complexes (35, 23, 66).
Chromatin remodeling through histone acetylation is emerging as an important mechanism by which gene transcription is regulated. Actively transcribed genes show a high level of histone acetylation, while repressed genes do not (30). In addition, it has been demonstrated that Rb can associate with histone deacetylase 1 (HDAC-1) and that both Rb and HDAC-1 cooperate in repressing transcription from E2F-regulated genes (10, 48). These observations suggest that HDAC complexes are potential targets for viral oncoproteins. As predicted, the CR3 zinc finger domain of E7 has been found to interact with HDAC-1 (10, 11). Although this association is Rb independent, E7 binding to HDAC-1 occurs indirectly through Mi2β, a component of the nucleosome remodelling deacetylase (NURD) complex (11, 87). Importantly, the activities provided by the CR3 domain of E7 are essential for immortalization of keratinocytes, indicating that the association between HDAC-1 complexes and E7 is biologically relevant.
Given the known biochemical functions of E7 binding targets in cell proliferation, it is generally presumed that the main function of E7 is to disrupt the cell cycle, thus establishing an environment that is more conducive for viral replication and subsequently promoting tumorigenesis. The mammalian cell cycle involves multiple tiers of regulation. Various signaling pathways that influence cellular growth converge at two major checkpoints, designated the G1/S boundary and the G2/M transition point. Relevant to the process of viral oncogenesis is the characterization of the G1/S point, which involves the initiation of cellular DNA synthesis. Since DNA tumor viruses rely on the host’s replication machinery to duplicate their own genome, infected cells must be shifted into S phase, even in the presence of growth arrest signals. In accordance with this rationale, E7 molecules of oncogenic HPVs, such as E7.16, have been found to induce cellular DNA synthesis in the presence of antimitogenic signals such as suprabasal differentiation, transforming growth factor beta (TGF-β), DNA damage, and serum deprivation (17). In light of the ability of E7 to bypass these biological signals, a more comprehensive examination of the mediators of such signaling pathways is warranted.
The downstream effectors of G1/S progression are the G1 cyclin-cdks. These include cyclin D-cdk4/6, cyclin E-cdk2, and cyclin A-cdk2. Negative regulation of these protein complexes involves binding to two classes of inhibitory subunits: the INK4 family and the Cip/Kip class of inhibitors (77). Activation of cyclin-cdk complexes depends on the phosphorylation state of critical residues in the cdk subunit. As such, phosphorylation of cdks at threonine 16 by the cdk-activating kinase is essential for cdk activation (61). Conversely, phosphorylation of tyrosine 15 by the wee1 and mik1 kinases leads to cdk inhibition (47, 64), while dephosphorylation of the same residue by the cdc25A phosphatase induces activation (41). Interestingly, the cdc25A phosphatase itself can be activated via cyclin E-cdk2 phosphorylation, suggesting the existence of a positive feedback loop at the G1/S boundary (34). The cdc25A multigene family is particularly important in regulating cell proliferation. Overexpression of the cdc25 phosphatases is believed to contribute to the development of some human cancers (12, 19, 27, 29). In addition, cdc25A and cdc25B (but not cdc25C) cooperate with either Ha-RAS or loss of RB1 in mammalian oncogenic focus formation (29). The cdc25B and cdc25C phosphatases are required for G2 regulation and entry into mitosis (63). Of the three cdc25 members, cdc25A is critical for G1/S progression. Its expression is predominant in late G1 (34), and ectopic expression of cdc25A leads to premature activation of cyclin E-cdk2 and cyclin A-cdk2 complexes, thereby accelerating the G1/S transition (7).
In light of its rate-limiting function in cell cycle progression, the cdc25A tyrosine phosphatase represents a potential target for viral proteins during the process of oncogenesis. Supporting this hypothesis is the discovery that expression of the adenovirus E1A oncoprotein in human fibroblasts leads to a rapid increase in cdc25A expression and phosphatase activity, potentially mediating E1A’s ability to induce quiescent cells into S phase (78). In the present study, experiments were designed to study the putative effects of E7.16 on the cdc25A tyrosine phosphatase. Our results indicate that E7.16 alters cdc25A gene expression and that this deregulation is linked to disruption of cell cycle arrest.
MATERIALS AND METHODS
Cell culture and establishment of cell lines.
Pooled HFKs were purchased from Clonetics BioWhittaker (San Diego, Calif.) and grown in KGM (BioWhittaker) supplemented with a low concentration of Ca2+ (90 μM). Experiments involving HFKs were restricted to use of subconfluent early-passage cells (sixth passage or lower). NIH 3T3 cells were kindly provided by Hartmut Land (University of Rochester, Rochester, N.Y.) and cultured in Dulbecco modified Eagle medium (DMEM) (GIBCO-BRL) supplemented with 10% bovine calf serum (BCS) (HyClone). U2OS cell lines were purchased from the American Type Culture Collection and grown in DMEM supplemented with 10% fetal calf serum.
Cell lines stably expressing E7.16 or the appropriate mutants were established via the pBabe amphotropic retroviral infection system (57). HFKs were infected at the third passage, and E7-HFKs or vector control HFKs were selected with 1.25 μg of puromycin per ml and replated for experimentation immediately following selection, thus ensuring the usage of early-passage cell lines. NIH 3T3 lines expressing E7 were similarly infected, selected, and maintained in medium containing 2.5 μg of puromycin per ml and were also used up to passage 10.
Growth inhibition, differentiation, and cell cycle analysis.
Asynchronous 3T3 cell lines were seeded at approximately 2 × 106 cells per 100-mm-diameter dish, and 24 h later, when at 70% confluence, cells were washed with phosphate-buffered saline and incubated in DMEM-0.1% BCS for the indicated amounts of time. Serum restimulation refers to NIH 3T3 cell lines synchronized in 0.1% BCS for 32 h and restimulated to enter the cell cycle in medium containing 10% BCS for 14 h. Keratinocyte differentiation was conducted following a protocol established by Poumay and Pittelkow (71). Briefly, subconfluent early-passage HFK cell lines were grown and seeded onto a 100-mm-diameter plate at either 5 × 105 cells/plate (low density) or 2 × 106 cells/plate (high density). Low-density plates were harvested the next day at 25% confluence and were designated cycling samples. High-density plates reached 90% confluence within 2 days and were subsequently incubated in KBM (deprived of growth factors) supplemented with high concentrations of calcium (1.5 mM) and harvested at the indicated time points.
Cell cycle analysis of treated NIH 3T3 and HFK cells was conducted by first pulsing with 10 μM bromodeoxyuridine (BrdU) for 30 min. Samples were then harvested and fixed with 70% ethanol. Finally, cells were labeled with fluorescein isothiocyanate-conjugated anti-BrdU antibody (Boehringer Mannheim), treated with RNase A, and stained with propidium iodide (20 μg/ml) by standard protocols. Data collection and analysis were performed with FACSCalibur (ELITE) and Multicycle software.
Plasmids.
pBabe retroviral constructs and pSG5 plasmids expressing E7.16 and E7C24G were generated and are described elsewhere (62). Plasmids encoding E7L67R and E7R77G were a generous gift from T. Kouzarides, University of Cambridge (11). Reporter constructs of the cdc25A promoter, including NPGL2-460/+129, NPGL2mE2F-A, and NPGL2mE2F-B, were provided by A. Iavarone, Albert Einstein College of Medicine, New York, N.Y. (38).
Transfections and reporter assays.
Reporter assays using HFKs were performed by seeding cells in six-well dishes at a density of 3 × 105 cells/well. Cells were transfected 24 h later with 200 ng of the indicated cdc25A reporter plasmid along with 300 ng of pSG5-E7-expressing constructs or pSG5 alone, using FUGENE 6 (Roche) according to the manufacturer’s instructions. After a 6-h incubation period, the transfection medium was removed and cells were maintained in KGM for 24 h, at which time luciferase activity was measured and standardized as previously described (3, 65). Reporter assays with NIH 3T3 cell lines were similarly conducted. Cells were seeded at 1.5 × 105 cells/well in a six-well plate. The next day samples were transfected with 100 ng of reporter plasmid and 100 ng of pSG5-E7 or vector control via Lipofectamine 2000 (GIBCO-BRL) according to the manufacturer’s instructions. Five hours later, cells were washed with phosphate-buffered saline and incubated in DMEM-0.1% BCS, and luciferase activity was measured after 24 to 48 h. In experiments using trichostatin A (TSA) (Sigma), the drug was added to the starvation medium at the indicated concentrations.
Semiquantitative RT-PCR.
Reverse transcription-PCRs (RT-PCRs) were carried out essentially as previously described (12). Quantitation of cdc25A expression levels was done by comparison to the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) housekeeping gene. Primer sequences were as follows: cdc25A, 5′-AGC CCC AAA GAG TCA ACT AAT CCA GA-3′ (forward) and 5′-CCG GTA GCT AGG GGG CTC ACA-3′ (reverse); GAPDH gene, 5′-CCC CTC TGC TGA TGC CCC CAT GTT-3′ (forward) and 5′-GAG CTT CCC GTC TAG CTC AGG GAT-3′ (reverse); and E7.16 gene, 5′-GCA TGG AGA AGA TAC ACC TAC ATT G-3′ (forward) and 5′-TGG TTT CTG AGA ACA GAT GGG-3′ (reverse). PCRs for cdc25A and the GAPDH gene were performed in a 25-μl volume for 25 cycles (denaturation at 95°C for 30 s, annealing at 53°C for 30 s, and extension at 72°C for 30 s), yielding amplification products of approximately 570 and 350 bp for cdc25A and the GAPDH gene, respectively. Confirmation of E7.16 gene expression was conducted similarly but with an annealing temperature of 46°C.
Immunoblots.
The antibodies used in this study included anti-E7.16 (Zymed), anti-cdc25A (sc-7389; Santa Cruz), anti-cyclin E (sc-481; Santa Cruz), anti-cyclin B1 (sc-245; Santa Cruz), anti-p27 (sc-528; Santa Cruz), anti-p21 (sc-6246; Santa Cruz), and fluorescein anti-BrdU (Chemicon). Western blot analysis was performed by lysing cell pellets for 1 h in 50 mM HEPES (pH 7.5)-250 mM NaCl-5 mM EDTA-0.5% NP-40-1:100 dilution of protease inhibitor cocktail (Sigma P8340). Protein extracts were quantified by standard Bio-Rad Bradford assay, and 75 μg was loaded and resolved on a sodium dodecyl sulfate-10% polyacrylamide gel. Following protein transfer, immunodetection of cdc25A (≅67 kDa) was achieved by blotting with anti-cdc25A antibody and developing with Super Signal West Femto chemiluminescent reagent (Pierce). All other immunoblots were developed by using the enhanced luminol chemiluminescent kit from NEN (Nalgene). Densitometric analysis was done using ChemiImager 5500 (Alpha Innotech) and AlphaEaseFC software.
RESULTS
Abolition of growth arrest and increased proliferative potential by E7.16 correlates with elevated cdc25A steady-state levels.
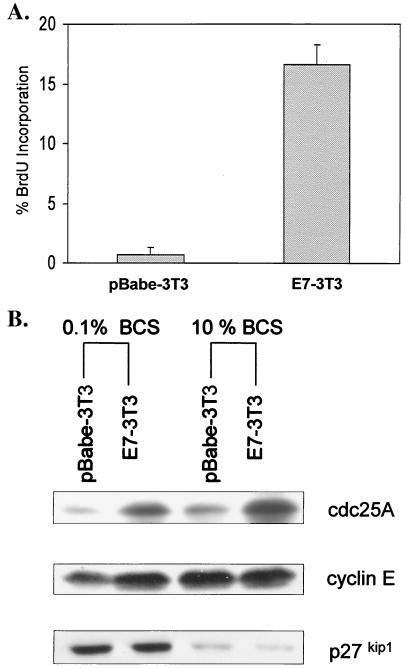
In NIH 3T3 fibroblasts, serum starvation is a potent signal that induces cellular quiescence via a G0/G1 arrest and, in the process, can modulate cdc25A steady-state levels and activity (13, 34, 38, 41). This relatively well-defined cellular system was used to assess the relevance of E7.16-induced effects on the cdc25A tyrosine phosphatase in relation to enforced cell cycle progression. As described previously (13), when NIH 3T3 cells were grown to 70% confluence and maintained in starvation medium for 48 h, S-phase entry was blocked, as a majority of cells (87.1%) arrested in G1 and 10.3% were in G2 (Fig. 1A and data not shown). After performing Western blot analysis of these extracts, we could detect low levels of cdc25A protein in growth-arrested samples (Fig. 1B). As cells are restimulated with 10% serum to enter S phase, however, cdc25A transcription increases (13), with protein steady-state levels peaking at around 14 to 16 h (Fig. 1B). This increase in cdc25A levels has been shown to be required for cell cycle reentry (34), and at 15 h following serum stimulation it correlates with S-phase reentry of 60% of cells that were previously in G1 (data not shown).
FIG. 1.
Abolition of cell cycle arrest by E7.16 correlates with increased cdc25A steady-state levels. (A) NIH 3T3 cells were infected with amphotropic virus encoding either E7.16 or vector alone. Following selection with puromycin, E7-3T3 and pBabe-3T3 cells were grown to 70% confluence, starved in 0.1% BCS for 32 h, and then maintained in starvation medium or restimulated with 10% BCS for an additional 14 to 16 h. Cells were pulsed with BrdU for 30 min and harvested for flow cytometric analysis. Error bars indicate standard deviations. (B) Samples treated as described for panel A were harvested and lysed, and then 75 μg of lysates was resolved on a sodium dodecyl sulfate-12% polyacrylamide gel and transferred overnight onto a nitrocellulose membrane for Western blot analysis. Equal loading was verified via Ponceau S staining.
In order to assess the effects of E7 within this system, stable cell lines were generated by infecting NIH 3T3 cells with amphotropic virus encoding E7.16. Pooled NIH 3T3 cells lines expressing E7.16 or a vector control (pBabe) were subsequently grown to 70% confluence, starved for 48 h in 0.1% BCS, and pulsed with BrdU. Previous work has shown that E7 can promote cell cycle progression in NIH 3T3 cells (4). Accordingly, following flow cytometric analysis, we showed that rather than arresting in G1, approximately 18% of E7.16-expressing cells can maintain S-phase DNA synthesis, while control cell lines are rendered completely quiescent. Therefore, we conclude that within this system, cells stably expressing E7.16 are able to maintain S-phase entry and bypass serum withdrawal-mediated growth arrest.
Past work has identified p27kip1 as an important mediator of cell cycle arrest via contact inhibition and serum starvation in NIH 3T3 cells (69, 70). When examining protein expression in our cell lines, we found that E7-3T3 and control cell lines express equally elevated levels of the cyclin cdk inhibitor p27kip1 upon serum deprivation, indicating that signaling is intact in both cell lines. Importantly, however, differences were detected with respect to gene products associated with cell cycle progression. As shown in Fig. 1B, there is a slight increase in cyclin E levels in E7-3T3 cells relative to control cells. More significantly, serum-starved E7-3T3 cells express fourfold-greater amounts of cdc25A protein than control cells (Fig. 1B). Finally, when restimulated with 10% serum, cells expressing E7 have an accelerated cell cycle (data not shown) and continue to express elevated cdc25A steady-state levels.
Taken together, these results indicate that despite being signaled to arrest, cells expressing E7.16 possess an increased proliferative potential, and this phenotype correlates with elevated levels of cdc25A. Our experimental results are consistent with observations made with NIH 3T3 lines in which cdc25A was overexpressed, revealing a rate-limiting function for cdc25A in S-phase entry (76) and further suggesting that the induction of cdc25A gene expression by E7 may contribute to enforced cell cycle progression.
E7.16 transactivates the cdc25A promoter under conditions of normal growth arrest.
The modulation of the cdc25A in response to antimitogenic signals is in part contingent on transcriptional regulation. Using reporter assays with the minimal cdc25A promoter, we confirm that in NIH 3T3 fibroblasts, the cdc25A gene is activated after serum restimulation of quiescent cells (Fig. 2A). This activation has been shown to necessitate relief from Rb/E2F repression (13). The cdc25A promoter contains two putative E2F binding sites, and the distal site (E2F-A) confers responsiveness to TGF-β signaling (38). The distal site is also required during serum deprivation, as a mutation in this area eliminates most of the responsiveness to serum induction while a mutation in the proximal E2F site (E2F-B) does not affect regulation by serum (Fig. 2A). It has consequently been suggested that under antimitogenic conditions, E2F/p130 complexes assemble at the E2F-A site and repress cdc25A transcription. In the context of TGF-β arrest, optimal repression of cdc25A requires HDAC-1 (38). To determine if histone deacetylation plays a similar role in regulating gene expression during serum withdrawal, we transfected the minimal cdc25A promoter into NIH 3T3 cells and starved them in the presence of the HDAC inhibitor TSA. Similarly to TGF-β-mediated arrest, we demonstrate here that repression of the cdc25A promoter via serum starvation necessitates HDAC activity, as titrating amounts of TSA caused cdc25A derepression (Fig. 2B). Our results lend credence to the idea that antiproliferative signals can repress cdc25A promoter activity through Rb/E2F/HDAC complexes. Moreover, this repression may contribute to cell cycle arrest and maintenance of cellular quiescence.
FIG. 2.
E7.16 transactivates the cdc25A promoter. (A) NIH 3T3 cells were seeded in six-well dishes, and 24 h later cells were transfected with luciferase constructs containing either the wild-type (wt) cdc25A promoter region (−468/+129) or mutations at putative E2F sites. Luciferase activity was measured after samples were cultured in 0.1% BCS for 32 h followed by either induction with DMEM-10% BCS or maintenance in starvation medium for an additional 16 h. Results from a representative of three independent experiments are shown. Error bars indicate standard deviations. RLU, relative light units. (B) Same as for panel A except that samples were also treated with the indicated concentrations of the HDAC inhibitor TSA for the full 48 h. (C) Vector control or pSG5-E7.16 was transfected into NIH 3T3 cells along with the indicated cdc25A promoter-luciferase constructs. mutE2F-A and mutE2F-B refer to the minimal cdc25A promoter containing point mutations in the distal and proximal E2F sites, respectively. Luciferase activity was measured after samples were treated as described for panel A. Data from three independent experiments were pooled, standardized, and represented as fold induction by E7.16 relative to samples transfected with the empty vector control.
In view of the fact that E7 associates with pRb family members (including p130) and HDAC-1 complexes, we attempted to determine if E7 could induce cdc25A transcription. Accordingly, when transfected along with the cdc25A promoter reporter plasmid, E7.16 can transactivate the cdc25A promoter up to 20-fold under conditions of serum starvation. To further address the effects of E7.16 on cdc25A, we cotransfected E7.16 with promoter constructs containing mutations at either one of the two putative E2F sites. Transient transfection of E7.16 along with a mutE2F-A cdc25A reporter did not result in any activation, while E7.16 could still activate mutE2F-B (Fig. 2C). Consequently, E7.16-mediated activation of the cdc25A promoter requires an intact E2F-A site, whereas the E2F-B site is dispensable. The results presented here are contrary to those obtained in a recent study by Katich et al., in which it was shown that a mutation at the E2F-A site was not sufficient to prevent further transactivation by E7.16 (44). It should be noted, however, that other groups (13, 38), including ourselves (Fig. 2A), have reproducibly demonstrated that mutations at the proximal site are sufficient to cause constitutive cdc25A promoter activity, making it unlikely that any further activation by E7 is necessary or possible. Based on these observations, we conclude that E7 increases cdc25A gene expression by transactivating the cdc25A promoter. Furthermore, E7 most likely accomplishes this by disrupting transcriptional repressor complexes located at the distal E2F-A site during cell cycle arrest.
The ability of E7.16 to abolish cell cycle arrest, activate cdc25A transcription, and increase cdc25A steady-state levels requires intact Rb and HDAC-1 binding domains.
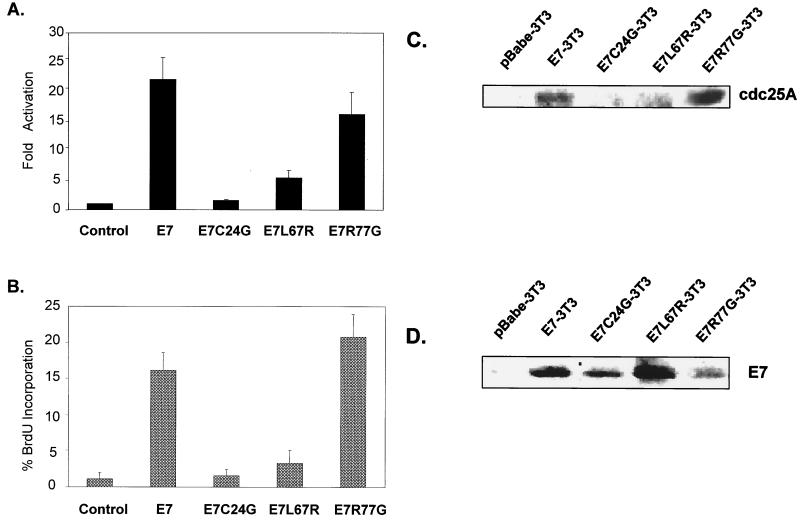
To gain further insight into the mechanism by which E7.16 affects cdc25A, a series of E7.16 point mutants were generated. The point mutants used in this study included the CR2 domain mutant E7C24G, which is deficient in pRb/p107/130 binding due to an alteration in the LXCXE motif (67). In addition, point mutations were introduced in the CR3 zinc finger domain of E7, generating mutants E7L67R and E7R77G. Previous assays indicate that both zinc finger mutants retain their ability to bind Rb but that E7L67R is compromised in its ability to bind Mi2β, a component of the NURD HDAC complex, while E7R77G is not (11).
Wild-type E7.16 can significantly transactivate the cdc25A promoter in serum-starved cells. Under the same conditions, E7C24G caused minimal promoter activation (Fig. 3A). E7L67R also poorly activated cdc25A, at 30% of wild-type levels, while E7R77G behaved similarly to wild-type E7 by activating the cdc25A promoter 15- to 20-fold (Fig. 3A). In addition, there was little or no difference between the various mutants when samples were restimulated with serum (data not shown). These results illustrate how specific mutations in the LXCXE motif as well as in the zinc finger domain of E7.16 abolish its ability to activate cdc25A. Moreover, because E7C24G and E7L67R poorly activate the cdc25A promoter during serum deprivation, it is inferred that the ability of E7 to bind pRb family members as well as associate with HDAC-1 complexes is essential for derepression or activation of the cdc25A promoter under specific antimitogenic conditions.
FIG. 3.
Maintenance of DNA synthesis and cdc25A activation by E7.16 requires intact Rb and HDAC-1 binding domains. (A) Expression plasmids encoding wild-type E7.16, mutants deficient in Rb binding (E7C24G) or HDAC-1 association (E7L67R), or a control zinc finger mutant (E7R77G) were transfected along with the cdc25A luciferase-promoter. Luciferase was measured 48 h following serum starvation. Results are expressed as fold induction by the various E7.16 constructs relative to the vector control. Error bars indicate standard deviations. (B) Cell lines stably expressing wild-type E7.16, mutant E7C24G, mutant E7L67R, mutant E7R77G, or the vector control were cultured as described for panel A, and S-phase DNA synthesis was measured by BrdU incorporation and flow cytometric analysis. (C and D) cdc25A (C) and E7.16 (D) expression was examined via Western blot analysis. Equal loading was verified via Ponceau S staining.
To further assess the effects of E7 point mutations on cdc25A and cell cycle progression, NIH 3T3 cell lines stably expressing the various E7.16 mutants were established via retroviral infection. As illustrated in Fig. 3B, cell lines expressing E7C24G or E7L67R could not maintain cellular DNA synthesis, while wild-type E7-3T3 cells and E7R77G-3T3 cells clearly blocked cell cycle arrest by maintaining up to 20% of cells in S phase upon serum withdrawal. Western blot analysis of these cells lines confirmed similar levels of E7 expression (Fig. 3D) yet revealed elevated cdc25A protein levels in serum-starved E7-3T3 and E7R77G-3T3 cells, whereas control, E7C24G, and E7L67R cell lines exhibited smaller amounts of cdc25A (Fig. 3C). The correlation between the abilities of E7 to bypass quiescence, activate cdc25A transcription, and cause elevated cdc25A steady-state levels suggests that E7-induced cell cycle progression requires increased cdc25A gene expression.
E7.16-mediated activation of cdc25A transcription is independent of cell cycle progression and cdk activity.
It is generally presumed that the most important downstream targets of cdc25A are the cdk complexes, most notably cyclin A-cdk2 and cyclin E-cdk2 (7). Cyclin E-cdk2 activity is involved in the transcriptional control of several other cell cycle-regulated genes, such as the cyclin A gene (85). In addition, it has previously been shown that cdc25A is itself regulated via phosphorylation by cyclin E-cdk2 (34). It is thus believed that both cdc25A and cyclin E-cdk2 participate in a positive feedback loop, synergistically regulating the transition from G1 to S. Given the fact that E7 can cause increased cyclin E gene expression (Fig. 1B) as well as cyclin E-cdk2 activity (50), we wondered whether the effects of E7 on cdc25A gene expression were actually a result of enforced cell cycle progression and/or increased cdk activity.
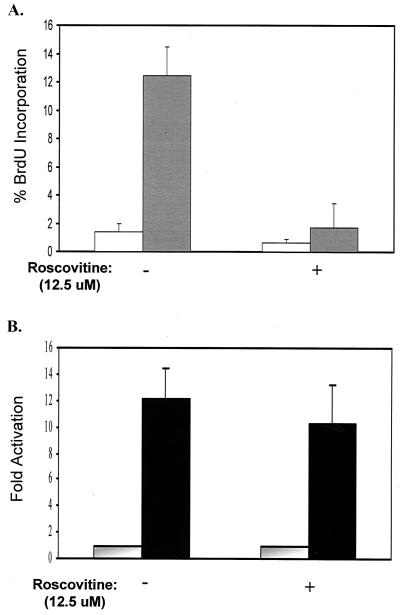
In order to examine the effects of cdc25A and cdks on cell proliferation, a series of experiments involving the use of the well-characterized drug roscovitine were conducted. Roscovitine is a specific inhibitor of cdk1 and cdk2 activity and therefore can cause cell cycle arrest (55). In this series of experiments, E7.16 induced 13% of the cells to maintain cellular DNA synthesis, while matching control cells completely arrested (Fig. 4A). However, when E7.16-expressing cells were starved in medium containing active concentrations of roscovitine, they could no longer maintain DNA synthesis (Fig. 4A). Consequently, we conclude that cdk activity is necessary for E7.16-mediated cell cycle progression. It can additionally be reasoned that in order to abolish cell cycle arrest, E7.16 must be targeting cdk activity and/or upstream elements involved in cdk activation.
FIG. 4.
Cdc25A Transcriptional activation by E7.16 is independent of cell cycle progression and cdk2 activity. (A) Cell lines stably expressing E7.16 were sustained in starvation medium containing the indicated amounts of the cdk inhibitor roscovitine for 48 h, and DNA synthesis was measured via BrdU incorporation. Open bars, pBabe-3T3; shaded bars, E7-3T3. Error bars indicate standard deviations. (B) Cells stably expressing E7.16 (solid bars) or vector control (shaded bars) were transfected with the cdc25A promoter and cultured in the presence of roscovitine as for panel A. Luciferase activity was measured after 48 h and standardized as described in the text.
To assess the effects of roscovitine on cdc25A transcription, stable E7.16 or Babe control cell lines were transiently transfected with the wild-type cdc25A promoter. The transfected cells were then starved in medium with or without 12.5 μM roscovitine, and luciferase activity was measured after 48 h. As illustrated in Fig. 4B, under conditions of normal cellular arrest, stably E7-expressing cells maintained cdc25A promoter activity approximately 10-fold relative to control cells. More significantly, roscovitine treatment of these cells does not reduce E7 mediated cdc25A transcription. Since roscovitine can abolish E7.16-enforced DNA synthesis, we conclude that E7.16 can activate cdc25A transcription independently of cell cycle progression. We therefore infer that E7.16 can directly target cdc25A gene expression and that this initial induction is independent of cdk activity.
E7.16 causes elevated cdc25A gene expression in primary human keratinocytes.
Our initial characterization of the effects of E7 on cell cycle progression and cdc25A expression was conducted with the NIH 3T3 model because this system is well defined and is more amenable to genetic and biochemical experimentation than other cell lines. This allowed for accurate delineation of the mechanisms involved in E7-mediated cdc25A deregulation and enforced G1/S transition. Ultimately, however, the study of cdc25A must be confirmed within a physiologically relevant setting. This entails investigating cdc25A regulation in the natural host cells of HPVs, namely, primary human keratinocytes.
To this end, early-passage pooled HFKs were infected with amphotropic virus encoding either E7.16 or an empty pBabe vector control. Following selection, early-passage cells were replated and harvested at 50% confluence. These samples were lysed, and immunoblot analysis was performed on the resulting extracts. In agreement with other studies (42, 43, 50, 73), our results confirm that asynchronous E7-HFKs contain moderately larger amounts of the cyclin cdk inhibitor p21cip1 (data not shown). More importantly, expression of E7.16 in keratinocytes leads to a fourfold increase in cdc25A levels as measured by densitometry (Fig. 5A). This increase in cdc25A steady-state levels is likely due to transcriptional activation, since E7 induces a concomitant increase in RNA levels as measured via semiquantitative RT-PCR (Fig. 5A). These results are consistent with experiments done with NIH 3T3 cells, in which E7.16 can also maintain elevated cdc25A gene expression.
FIG. 5.
E7.16 increases cdc25A gene expression in primary human keratinocytes. (A) Human keratinocytes isolated from pooled neonatal foreskins were infected with amphotropic virus encoding empty vector (pBabe-HFK) or E7.16 (E7-HFK). Asynchronous cells were harvested at 40 to 50% confluence, lysed, and analyzed via semiquantitative RT-PCR or Western blotting as described in the text. (B) HFKs were seeded onto six-well dishes at 3 × 106 cells per well. After 24 h, cells were at approximately 30% confluence and were transfected with luciferase constructs containing either the wild-type (wt) cdc25A promoter region (−468/+129) or mutations at putative E2F sites. In addition, the vector control or pSG5-E7.16 was transfected into HFKs along with the indicated cdc25A promoter-luciferase constructs. Reporter activity was measured 24 h later, when HFKs reached 50% confluence. Results from a representative of three independent experiments are shown. Error bars indicate standard deviations. RLU, relative light units. (C and D) E7 mutational analysis of primary keratinocyte gene expression and promoter activity was accomplished via semiquantitative RT-PCR and reporter assays as described for panels A and B.
In order to see if increased cdc25A steady-state levels in keratinocytes are due to transcriptional activation of the cdc25A promoter, reporter assays were conducted. In these experiments, the confluence of keratinocyte samples was carefully monitored to ensure that transfections and luciferase measurements were performed on subconfluent (40 to 50%) HFKs (see Materials and Methods). Similarly to what is seen in NIH 3T3 cells, E7.16 could cause a fivefold activation of the cdc25A promoter in asynchronous HFKs (Fig. 5B). Interestingly, the cdc25A promoter seems to be tightly regulated in cycling HFKs, as a point mutation in the E2F-A site results in increased cdc25A luciferase activity compared to the wild-type minimal promoter. On the other hand, mutation in the proximal E2F site (E2F-B) has no discernible effect relative to basal activity in asynchronous keratinocytes (Fig. 5B). Not surprisingly, then, when E7.16 is cotransfected with mutE2F-A, there is no further promoter activation (Fig. 5B), demonstrating once again that E7.16 targets factors located at E2F-A and that disruptions at this site cause derepression and constitutive cdc25A transcription. Finally, similar to what is seen in the NIH 3T3 system, E7 mutational analysis conducted with primary keratinocytes revealed that activation of the cdc25A promoter and E7-mediated increases in cdc25A gene expression require binding to Rb and HDAC-1-containing complexes (Fig. 5C). In summary, our experiments with HFKs reveal that E7.16 can cause increased cdc25A gene expression in the primary host cell of HPV.
E7.16 delays growth arrest and maintains cdc25A steady-state levels during keratinocyte differentiation.
To extend our studies with primary cells, additional experiments were aimed at examining cdc25A regulation within the context of keratinocyte differentiation. Cell density and calcium concentrations are believed to be the most important factors in regulating keratinocyte commitment towards differentiation and expression of suprabasal keratins (71). Considering these properties, we utilized an in vitro differentiation protocol developed by Poumay and Pittelkow that mimicked the important aspects of squamous epithelial stratification (71). Pooled HFK cells lines were grown and seeded at either low density (25% confluence) or high density (90% confluence). Low-density plates were subsequently harvested as cycling samples. High-density samples were washed and incubated in medium lacking growth factors but supplemented with elevated calcium concentrations. Finally, differentiated samples were harvested at various time points for cell cycle analysis.
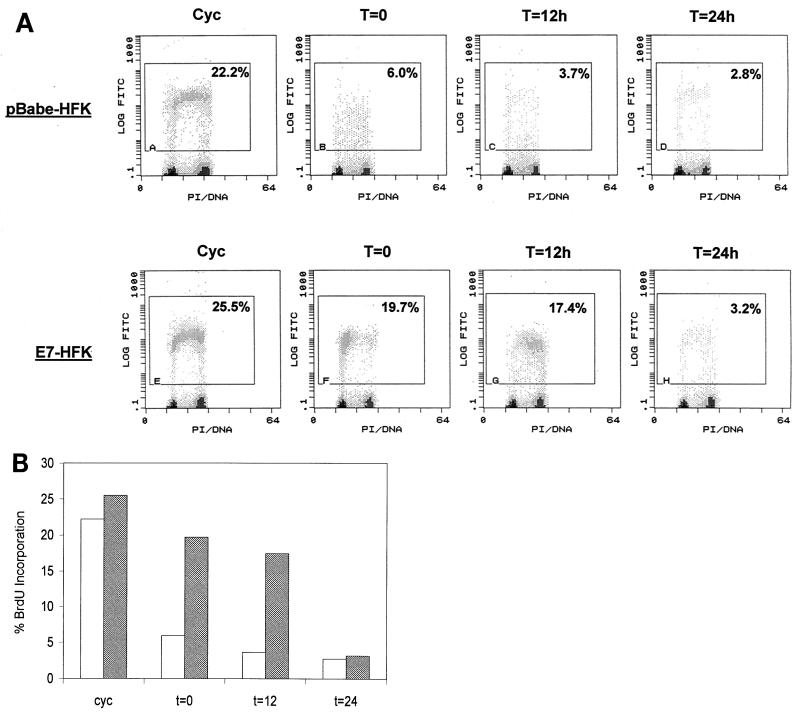
As shown in Fig. 6, control HFKs arrest in G1 immediately upon confluence. The confluence signal most likely induces terminal growth arrest, since confluent HFKs have been found to loose their clonogenic potential (71). Significantly, E7.16 can impede this differentiation-induced growth arrest, because 17% (as measured via BrdU incorporation) of E7-HFKs can sustain active DNA synthesis and S-phase entry for up to 12 h following the switch to growth factor-deprived medium (Fig. 6). Interestingly, the observed disruption of cell cycle arrest by E7.16 is transient, as E7-HFKs eventually arrest in G1 by 24 h. Based on these results, we conclude that E7.16 can delay differentiation-induced growth arrest by disrupting the initial signaling pathways triggering commitment towards differentiation. In view of the fact that E7-expressing cells do eventually arrest, E7 activity by itself is not sufficient for complete elimination of differentiation-induced growth arrest. This indicates that total uncoupling of cell cycle progression from the keratinocyte differentiation program requires additional factors that may include activities provided by other HPV oncoproteins.
FIG. 6.
E7.16 delays differentiation-induced cell cycle arrest in primary keratinocytes. Pooled human keratinocytes were infected with amphotropic virus encoding E7.16 or empty vector. The resulting cell lines were then harvested at subconfluence (cycling [Cyc]) or grown to confluence, differentiated, and harvested at various time points for flow cytometric analysis. (A) Represented are dual-parameter histograms for log fluorescein isothiocyanate (FITC) and propidium iodide (PI) staining. The indicated gates encompass cell populations entering S phase and actively synthesizing DNA. These experiment have subsequently been repeated with cell lines generated from a single foreskin donor and have yielded similar trends. (B) Bar graph representation of results in panel A. Open bars, pBabe-3T3 cells; shaded bars, E7-3T3 cells.
After cell cycle analysis, we analyzed the steady-state levels of several known mediators of cell cycle progression and keratinocyte differentiation. As illustrated in Fig. 7A, both E7 and matched control cell lines expressed the early differentiation marker keratin 1 at 24 h following the switch to growth factor-deprived medium with large amounts of calcium. Furthermore, the cdk inhibitor p21cip1, one of the earliest detectable modifications in cell cycle-regulatory molecules during keratinocyte differentiation (18, 20, 56, 81), is also similarly expressed in both cell lines (data not shown). The fact that the expression patterns of keratin 1 and p21 are similar in E7-HFKs and pBabe-HFKs confirms that both cell lines are signaled to differentiate and suggests that E7.16 does not interfere with differentiation-specific gene expression.
FIG. 7.
E7.16 maintains cdc25A gene expression and steady-state levels during keratinocyte differentiation. E7.16-expressing cells and matching controls were differentiated and harvested in parallel with flow cytometric samples (Fig. 6). Cells pellets were then lysed and run on a 10 to 12% polyacrylamide gel for Western blot analysis. Equal loading was verified via Ponceau S staining. (A) Immunoblot of keratin 1. Cyc, cycling. (B) Immunoblot of cdc25A. (C) Similar experiments were performed in order to assess endogenous cdc25A expression during HFK differentiation via semiquantitative RT-PCR.
Since the focus of this study was to investigate cdc25A regulation, we next examined cdc25A steady-state levels in differentiating keratinocytes. Interestingly, cdc25A levels decreased significantly in control HFKs upon confluence, remaining practically undetectable as differentiation proceeded (Fig. 7B). More importantly, there was a less dramatic decrease in cdc25A protein levels in E7-expressing cells. Furthermore, cdc25A steady-state levels were maintained during differentiation of E7-HFKs, indicating that E7.16 may be causing deregulated cdc25A expression. Indeed, this was confirmed by semiquantitative RT-PCR analysis, as E7.16 expression correlated with maintenance of cdc25A transcripts during differentiation of primary keratinocytes (Fig. 7C). Taken together, our results suggest that cdc25A down regulation is associated with keratinocyte differentiation-induced growth arrest. Considering that the ability of E7.16 to delay differentiation-induced growth arrest correlates with increased cdc25A levels, deregulated cdc25A gene expression may be an important step in uncoupling cell cycle progression from keratinocyte differentiation during HPV-16 infection.
DISCUSSION
In the present study, we sought to elucidate the effects of E7.16 on the cdc25A tyrosine phosphatase. cdc25A is considered essential for cell cycle progression, and recent studies have focused on the regulation of cdc25A in response to various biological signals. cdc25A was thus found to interact with the proto-oncogene Raf-1, linking the former to various extracellular signals that promote cell growth (28). Furthermore, cdc25A gene transcription can be repressed in response to serum starvation and TGF-β-mediated arrest (9, 13, 38, 39). Finally, other potent arrest signals such as DNA damage lead to cdc25A protein degradation (6, 49). Interestingly, many of these signals are abolished in cells expressing E7.16 (17). These observations prompted us to investigate whether cdc25A deregulation is directly associated with E7.16-induced cell cycle progression.
Accordingly, we demonstrate that E7.16 can maintain DNA synthesis in normally quiescent cells and that this correlates with increased cdc25A gene expression (Fig. 1). The association between increased cdc25A gene expression and enforced cell cycle progression suggests that cdc25A is an important mediator of E7-induced S-phase entry. Since many biological signals converge on cdc25A, deregulation at the level of such downstream effectors would be an efficient way for viral oncoproteins to disrupt multiple signaling pathways and maintain a cellular environment that is more conducive to viral replication. Prior experiments carried out with keratinocyte cell lines (HaCat) revealed that cdc25A transcription is repressed in response to antimitogenic signals such as TGF-β1 and serum deprivation (38, 39). This regulation requires the activities provided by Rb, E2F, and HDAC-1, which form a repressor complex at the distal E2F site within the cdc25A promoter (7, 13, 38, 82, 83). In addition, Iavarone and Massague have shown that this E2F site had no enhancer or repressor function in cycling cells, serving only to down regulate cdc25A transcription upon growth arrest (38). Contrary to data obtained from keratinocyte cell lines, we find that basal transcription of cdc25A in asynchronous primary cells is already regulated by E2F-associated factors, since a point mutation in the distal E2F site causes increased promoter activity (Fig. 5B). Our observations highlight a fundamental difference between primary keratinocytes and transformed keratinocyte cells lines, indicating that cdc25A expression is more stringently controlled in cycling HFKs. Importantly, E7.16 can cause transcriptional activation of the cdc25A promoter in primary cells (Fig. 5B) and NIH 3T3 cell lines (Fig. 2C.), demonstrating that increases in cdc25A steady-state levels by E7 are due to direct transcriptional activation and elevated gene expression. In addition, E7.16-mediated activation requires an intact distal E2F site (E2F-A) (Fig. 2C and 5B). Finally, since the data from primary keratinocytes correlate with those obtained from a 3T3-based cell system, the observed E7-associated effects are not simply due to immortalization. Collectively, data from both NIH 3T3 and HFK cellular systems lend credence to the idea that E7.16 can bypass growth arrest signals by inducing cdc25A gene expression.
A considerable amount of evidence points to the importance of Rb binding in the ability of E7.16 to transform cells (32, 60, 68). Recent data, however, suggest that association with Rb may not be essential for E7.16-induced immortalization. Within the context of the whole HPV genome, mutations in the CR2 region of E7, which abolish binding to Rb, do not affect the ability of E7.16 to immortalize HFKs (40). Conversely, mutations in the CR3 zinc finger motif of E7 led to complete elimination of immortalization capabilities (40). This has prompted the search for Rb-independent interaction, involving E7 and other cellular factors that may prove to be essential for cellular immortalization and carcinogenesis. One potentially important E7 function, independent of Rb binding, is the E7.16-specific association with HDAC complexes (11). Disruption of HDAC-1 activity is all the more pertinent considering that Rb and HDAC-1 can bind to each other and potently repress transcription of cell cycle-regulated genes (10, 48, 74, 86). Results from our mutational analysis provide evidence that binding to both Rb and HDAC-1 is required for E7.16 to induce cdc25A transcriptional activation and increase cdc25A expression in fibroblasts and primary keratinocytes (Fig. 3 and 5). Moreover, the E7.16 point mutants that are unable to activate cdc25A are equally deficient in their capacity to maintain cellular DNA synthesis (Fig. 3B). Altogether, our experiments reveal that E7.16 can independently derepress Rb and HDAC-1 and that both functions are essential for optimal cdc25A transcription, gene expression, and subsequent S-phase entry. These observations also strengthen the notion that cdc25A is an important mediator of E7-induced cell cycle progression and viral tumorigenesis.
Because of the extremely complex nature of the eukaryotic cell cycle, it remained unclear to us whether the observed activation of cdc25A by E7 was a direct effect or simply a by-product of enforced cell cycle progression. This is of considerable importance, since cdc25A is suggested to participate in a positive feedback loop with cyclin E-cdk2 (34). Furthermore, E7.16 is known to cause increases in cyclin E- and cyclin A-associated cdk2 activity (42, 54, 72, 73, 84). As a result, rather than being a mediator of E7.16-induced cell cycle progression, it could be argued that cdc25A activation may be due to E7.16-enforced S-phase entry and/or increased cdk2 activity. To resolve this question, experiments taking advantage of the cdk inhibitor roscovitine were used to demonstrate that E7.16 could activate the cdc25A promoter independently of its ability to maintain cellular DNA synthesis (Fig. 4). During preparation of this paper, Katich et al. published a similar study in which the use of an inducible E7 molecule led to rapid induction of cdc25A transcription, prior to the onset of DNA synthesis (44). In sum, these results convincingly demonstrate that E7.16 can directly target cdc25A transcription and that induction of the cdc25A gene precedes the onset of cellular DNA synthesis. The effects of roscovitine in our system also illustrate the requirement for cdk activity in engendering the E7-specific proliferative phenotype. Therefore, it can be reasoned that in order to maintain cell cycle progression, E7 must be targeting cdk activity and/or upstream elements involved in cdk activation. Since cyclin-cdk complexes are known targets of the cdc25A phosphatase, it is tempting to suggest a paradigm wherein E7.16 induces cdc25A gene expression, which in turn activates cdk2 activity and promotes cell cycle progression. Evidence for this hypothesis, however, remains circumstantial, and further experiments regarding this question are being pursued.
The HPV life cycle is intimately linked to epithelial cell growth and differentiation. During keratinocyte differentiation, cells in the basal layer of the epithelium initially divide and synthesize DNA but progressively undergo a program of irreversible arrest as stratification occurs. Given that optimal viral replication takes place in the stratified layer of the epithelium, HPV oncoproteins are believed to maintain infected cells in a proliferative state that is more conducive to viral replication. On the other hand, HPV-infected cells must be allowed to differentiate, as expression of late viral genes requires differentiation-specific factors (14, 26, 37, 45, 53, 72). Consistent with this hypothesis, we find that E7.16-expressing keratinocytes can delay the growth arrest associated with terminal differentiation without affecting differentiation-specific gene expression. Indeed, upon in vitro differentiation, both E7-HFKs and pBabe-HFKs begin expressing the early marker keratin 1 at approximately the same time (24 h).
An essential aim of the present study was to ascertain the regulatory pattern of cdc25A during keratinocyte differentiation. Interestingly, we find that cdc25A transcript and protein levels in control HFK cell lines drop dramatically upon confluence, remaining undetectable as keratinocytes differentiate. Decreases in cdc25A levels have been observed in other primary cell systems as well, most notably during senescence of mammary epithelial cells (75). Furthermore, studies by Poumay and Pittelkow indicate that a large majority of proliferative keratinocytes are committed to irreversible growth arrest upon cell confluence (71). Consequently, we conclude that cdc25A down regulation is associated with terminal cell cycle arrest induced via keratinocyte differentiation. Of note is the fact that Rb and p130 become progressively hypophosphorylated as HFKs differentiate (data not shown) (42). Since we and others have shown that Rb/E2F can repress cdc25A transcription, the down regulation of cdc25A upon keratinocyte arrest is predicted to occur via repression of the cdc25A promoter. Considering that E7.16 can activate cdc25A transcription and maintain cdc25A steady-state levels following growth arrest and/or differentiation (Fig. 7B and C), we propose that the subsequent deregulation of the cdc25A tyrosine phosphatase by E7 may serve to delay the initial step of keratinocyte differentiation, thus promoting the uncoupling of cell cycle progression from differentiation.
In summary, we have shown that the E7.16 oncoprotein can impede growth arrest signals in both NIH 3T3 cells and HFKs and that this correlates with elevated cdc25A gene expression. E7.16 can transactivate the cdc25A promoter by disrupting or derepressing Rb/E2F/HDAC-1 complexes, thus preventing transcriptional repression of cdc25A upon growth arrest signals. As such, cdc25A is likely to be an important mediator of E7-enforced cell cycle progression. Since many of the functional aspects of cdc25A remain unknown, additional experiments will be needed in order to more specifically assess the consequences of E7-mediated cdc25A activation in relation to viral oncogenesis.
Acknowledgments
We thank Antonio Iavarone, Tony Kouzarides, Hartmut Land, Jiri Lukas, and Neils Mailand for providing us with various plasmids, antibodies, and cell lines. We are also indebted to Peter Keng for expert assistance in performing flow cytometry. Finally, we thank Laurel Baglia for scientific advice, Shih-Min Huang for help on the figures, and Helene McMurray and Daksha Patel for review of the manuscript.
This work was funded by a grant from the National Institute of Allergy and Infectious Diseases (AI 30798) to D.J.M.
REFERENCES
- 1.Alunni-Fabbroni, M., T. Littlewood, L. Deleu, S. Caldeira, M. Giarre, M. Dell’ Orco, and M. Tommasino. 2000. Induction of S phase and apoptosis by the human papillomavirus type 16 E7 protein are separable events in immortalized rodent fibroblasts. Oncogene 19:2277–2285. [DOI] [PubMed] [Google Scholar]
- 2.Andresson, T., J. Sparkowski, D. J. Goldstein, and R. Schlegel. 1995. Vacuolar H(+)-ATPase mutants transform cells and define a binding site for the papillomavirus E5 oncoprotein. J. Biol. Chem. 270:6830–6837. [DOI] [PubMed] [Google Scholar]
- 3.Antinore, M. J., M. J. Birrer, D. Patel, L. Nader, and D. J. McCance. 1996. The human papillomavirus type 16 E7 gene product interacts with and trans-activates the AP1 family of transcription factors. EMBO J. 15:1950–1960. [PMC free article] [PubMed] [Google Scholar]
- 4.Banks, L., C. Edmonds, and K. Vousden. 1990. Ability of the HPV16 E7 protein to bind RB and induce DNA synthesis is not sufficient for efficient transforming activity in NIH3T3 cells. Oncogene 5:1383–1389. [PubMed] [Google Scholar]
- 5.Barbosa, M. S., C. Edmonds, C. Fisher, J. T. Schiller, D. R. Lowy, and K. H. Vousden. 1990. The region of the HPV E7 oncoprotein homologous to adenovirus E1a and Sv40 large T antigen contains separate domains for Rb binding and casein kinase II phosphorylation. EMBO J. 9:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernardi, R., D. A. Liebermann, and B. Hoffman. 2000. Cdc25A stability is controlled by the ubiquitin-proteasome pathway during cell cycle progression and terminal differentiation. Oncogene 19:2447–2454. [DOI] [PubMed] [Google Scholar]
- 7.Blomberg, I., and I. Hoffmann. 1999. Ectopic expression of Cdc25A accelerates the G1/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases. Mol. Cell. Biol. 19:6183–6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boshart, M., L. Gissmann, H. Ikenberg, A. Kleinheinz, W. Scheurlen, and H. zur Hausen. 1984. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 3:1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouzahzah, B., M. Fu, A. Iavarone, V. M. Factor, S. S. Thorgeirsson, and R. G. Pestell. 2000. Transforming growth factor-beta1 recruits histone deacetylase 1 to a p130 repressor complex in transgenic mice in vivo. Cancer Res. 60:4531–4537. [PubMed] [Google Scholar]
- 10.Brehm, A., E. A. Miska, D. J. McCance, J. L. Reid, A. J. Bannister, and T. Kouzarides. 1998. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature 391:597–601. [DOI] [PubMed] [Google Scholar]
- 11.Brehm, A., S. J. Nielsen, E. A. Miska, D. J. McCance, J. L. Reid, A. J. Bannister, and T. Kouzarides. 1999. The E7 oncoprotein associates with Mi2 and histone deacetylase activity to promote cell growth. EMBO J. 18:2449–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cangi, M. G., B. Cukor, P. Soung, S. Signoretti, G. Moreira, Jr., M. Ranashinge, B. Cady, M. Pagano, and M. Loda. 2000. Role of the Cdc25A phosphatase in human breast cancer. J. Clin. Invest 106:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, X., and R. Prywes. 1999. Serum-induced expression of the cdc25A gene by relief of E2F-mediated repression. Mol. Cell. Biol. 19:4695–4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng, S., D. C. Schmidt-Grimminger, T. Murant, T. R. Broker, and L. T. Chow. 1995. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 9:2335–2349. [DOI] [PubMed] [Google Scholar]
- 15.Chesters, P. M., and D. J. McCance. 1989. Human papillomavirus types 6 and 16 in cooperation with Ha-ras transform secondary rat embryo fibroblasts. J. Gen. Virol. 70:353–365. [DOI] [PubMed] [Google Scholar]
- 16.Chesters, P. M., K. H. Vousden, C. Edmonds, and D. J. McCance. 1990. Analysis of human papillomavirus type 16 open reading frame E7 immortalizing function in rat embryo fibroblast cells. J. Gen. Virol. 71:449–453. [DOI] [PubMed] [Google Scholar]
- 17.Demers, G. W., E. Espling, J. B. Harry, B. G. Etscheid, and D. A. Galloway. 1996. Abrogation of growth arrest signals by human papillomavirus type 16 E7 is mediated by sequences required for transformation. J. Virol. 70:6862–6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Cunto, F., G. Topley, E. Calautti, J. Hsiao, L. Ong, P. K. Seth, and G. P. Dotto. 1998. Inhibitory function of p21Cip1/WAF1 in differentiation of primary mouse keratinocytes independent of cell cycle control. Science 280:1069–1072. [DOI] [PubMed] [Google Scholar]
- 19.Dixon, D., T. Moyana, and M. J. King. 1998. Elevated expression of the cdc25A protein phosphatase in colon cancer. Exp. Cell Res. 240:236–243. [DOI] [PubMed] [Google Scholar]
- 20.Dotto, G. P. 1999. Signal transduction pathways controlling the switch between keratinocyte growth and differentiation. Crit. Rev. Oral Biol. Med. 10:442–457. [DOI] [PubMed] [Google Scholar]
- 21.Durst, M., L. Gissmann, H. Ikenberg, and H. zur Hausen. 1983. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc. Natl. Acad. Sci. USA 80:3812–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dyson, N. 1998. The regulation of E2F by pRB-family proteins. Genes Dev. 12:2245–2262. [DOI] [PubMed] [Google Scholar]
- 23.Dyson, N., P. Guida, K. Munger, and E. Harlow. 1992. Homologous sequences in adenovirus E1A and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. J. Virol. 66:6893–6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dyson, N., P. M. Howley, K. Munger, and E. Harlow. 1989. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243:934–937. [DOI] [PubMed] [Google Scholar]
- 25.Foster, S. A., G. W. Demers, B. G. Etscheid, and D. A. Galloway. 1994. The ability of human papillomavirus E6 proteins to target p53 for degradation in vivo correlates with their ability to abrogate actinomycin D-induced growth arrest. J. Virol. 68:5698–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frattini, M. G., H. B. Lim, and L. A. Laimins. 1996. In vitro synthesis of oncogenic human papillomaviruses requires episomal genomes for differentiation-dependent late expression. Proc. Natl. Acad. Sci. USA 93:3062–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frey, R. S., J. Li, and K. W. Singletary. 2001. Effects of genistein on cell proliferation and cell cycle arrest in nonneoplastic human mammary epithelial cells: involvement of Cdc2, p21(waf/cip1), p27(kip1), and Cdc25C expression. Biochem. Pharmacol. 61:979–989. [DOI] [PubMed] [Google Scholar]
- 28.Galaktionov, K., C. Jessus, and D. Beach. 1995. Raf1 interaction with Cdc25 phosphatase ties mitogenic signal transduction to cell cycle activation. Genes Dev. 9:1046–1058. [DOI] [PubMed] [Google Scholar]
- 29.Galaktionov, K., A. K. Lee, J. Eckstein, G. Draetta, J. Meckler, M. Loda, and D. Beach. 1995. CDC25 phosphatases as potential human oncogenes. Science 269:1575–1577. [DOI] [PubMed] [Google Scholar]
- 30.Grunstein, M. 1997. Histone acetylation in chromatin structure and transcription. Nature 389:349–352. [DOI] [PubMed] [Google Scholar]
- 31.Halbert, C. L., G. W. Demers, and D. A. Galloway. 1991. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J. Virol. 65:473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heck, D. V., C. L. Yee, P. M. Howley, and K. Munger. 1992. Efficiency of binding the retinoblastoma protein correlates with the transforming capacity of the E7 oncoproteins of the human papillomaviruses. Proc. Natl. Acad. Sci. USA 89:4442–4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hickman, E. S., S. M. Picksley, and K. H. Vousden. 1994. Cells expressing HPV16 E7 continue cell cycle progression following DNA damage induced p53 activation. Oncogene 9:2177–2181. [PubMed] [Google Scholar]
- 34.Hoffmann, I., G. Draetta, and E. Karsenti. 1994. Activation of the phosphatase activity of human cdc25A by a cdk2-cyclin E dependent phosphorylation at the G1/S transition. EMBO J. 13:4302–4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang, P. S., D. R. Patrick, G. Edwards, P. J. Goodhart, H. E. Huber, L. Miles, V. M. Garsky, A. Oliff, and D. C. Heimbrook. 1993. Protein domains governing interactions between E2F, the retinoblastoma gene product, and human papillomavirus type 16 E7 protein. Mol. Cell. Biol. 13:953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hudson, J. B., M. A. Bedell, D. J. McCance, and L. A. Laiminis. 1990. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J. Virol. 64:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hummel, M., J. B. Hudson, and L. A. Laimins. 1992. Differentiation-induced and constitutive transcription of human papillomavirus type 31b in cell lines containing viral episomes. J. Virol. 66:6070–6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iavarone, A., and J. Massague. 1999. E2F and histone deacetylase mediate transforming growth factor beta repression of cdc25A during keratinocyte cell cycle arrest. Mol. Cell. Biol. 19:916–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iavarone, A., and J. Massague. 1997. Repression of the CDK activator Cdc25A and cell-cycle arrest by cytokine TGF-beta in cells lacking the CDK inhibitor p15. Nature 387:417–422. [DOI] [PubMed] [Google Scholar]
- 40.Jewers, R. J., P. Hildebrandt, J. W. Ludlow, B. Kell, and D. J. McCance. 1992. Regions of human papillomavirus type 16 E7 oncoprotein required for immortalization of human keratinocytes. J. Virol. 66:1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jinno, S., K. Suto, A. Nagata, M. Igarashi, Y. Kanaoka, H. Nojima, and H. Okayama. 1994. Cdc25A is a novel phosphatase functioning early in the cell cycle. EMBO J. 13:1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones, D. L., R. M. Alani, and K. Munger. 1997. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 11:2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones, D. L., D. A. Thompson, E. Suh-Burgmann, M. Grace, and K. Munger. 1999. Expression of the HPV E7 oncoprotein mimics but does not evoke a p53-dependent cellular DNA damage response pathway. Virology 258:406–414. [DOI] [PubMed] [Google Scholar]
- 44.Katich, S., K. Zerfass-Thome, and I. Hoffman. 2001. Regulation of the Cdc25A gene by the human papillomavirus Type 16 E7 oncogene. Oncogene 20:543–550. [DOI] [PubMed] [Google Scholar]
- 45.Kyo, S., D. J. Klumpp, M. Inoue, T. Kanaya, and L. A. Laimins. 1997. Expression of AP1 during cellular differentiation determines human papillomavirus E6/E7 expression in stratified epithelial cells. J. Gen. Virol. 78:401–411. [DOI] [PubMed] [Google Scholar]
- 46.Lechner, M. S., D. H. Mack, A. B. Finicle, T. Crook, K. H. Vousden, and L. A. Laimins. 1992. Human papillomavirus E6 proteins bind p53 in vivo and abrogate p53-mediated repression of transcription. EMBO J. 11:3045–3052. (Erratum, 11:248.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lundgren, K., N. Walworth, R. Booher, M. Dembski, M. Kirschner, and D. Beach. 1991. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell 64:1111–1122. [DOI] [PubMed] [Google Scholar]
- 48.Magnaghi-Jaulin, L., R. Groisman, I. Naguibneva, P. Robin, S. Lorain, J. P. Le Villain, F. Troalen, D. Trouche, and A. Harel-Bellan. 1998. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature 391:601–605. [DOI] [PubMed] [Google Scholar]
- 49.Mailand, N., J. Falck, C. Lukas, R. G. Syljuasen, M. Welcker, J. Bartek, and J. Lukas. 2000. Rapid destruction of human Cdc25A in response to DNA damage. Science 288:1425–1429. [DOI] [PubMed] [Google Scholar]
- 50.Martin, L. G., G. W. Demers, and D. A. Galloway. 1998. Disruption of the G1/S transition in human papillomavirus type 16 E7-expressing human cells is associated with altered regulation of cyclin E. J. Virol. 72:975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCance, D. J. 1994. Human papillomaviruses. Infect. Dis. Clin. N. Am. 8:751–767. [PubMed] [Google Scholar]
- 52.McCance, D. J. 1998. Human papillomaviruses and cervical cancer. J. Med. Microbiol. 47:371–373. [DOI] [PubMed] [Google Scholar]
- 53.McCance, D. J., R. Kopan, E. Fuchs, and L. A. Laimins. 1988. Human papillomavirus type 16 alters human epithelial cell differentiation in vitro. Proc. Natl. Acad. Sci. USA 85:7169–7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McIntyre, M. C., M. N. Ruesch, and L. A. Laimins. 1996. Human papillomavirus E7 oncoproteins bind a single form of cyclin E in a complex with cdk2 and p107. Virology 215:73–82. [DOI] [PubMed] [Google Scholar]
- 55.Meijer, L., A. Borgne, O. Mulner, J. P. Chong, J. J. Blow, N. Inagaki, M. Inagaki, J. G. Delcros, and J. P. Moulinoux. 1997. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J. Biochem. 243:527–536. [DOI] [PubMed] [Google Scholar]
- 56.Missero, C., F. Di Cunto, H. Kiyokawa, A. Koff, and G. P. Dotto. 1996. The absence of p21Cip1/WAF1 alters keratinocyte growth and differentiation and promotes ras-tumor progression. Genes Dev. 10:3065–3075. [DOI] [PubMed] [Google Scholar]
- 57.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munger, K. 1995. The molecular biology of cervical cancer. J. Cell. Biochem. Suppl. 23:55–60. [DOI] [PubMed] [Google Scholar]
- 59.Munger, K., B. A. Werness, N. Dyson, W. C. Phelps, E. Harlow, and P. M. Howley. 1989. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 8:4099–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Munger, K., C. L. Yee, W. C. Phelps, J. A. Pietenpol, H. L. Moses, and P. M. Howley. 1991. Biochemical and biological differences between E7 oncoproteins of the high- and low-risk human papillomavirus types are determined by amino-terminal sequences. J. Virol. 65:3943–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagahara, H., S. A. Ezhevsky, A. M. Vocero-Akbani, P. Kaldis, M. J. Solomon, and S. F. Dowdy. 1999. Transforming growth factor beta targeted inactivation of cyclin E:cyclin-dependent kinase 2 (Cdk2) complexes by inhibition of Cdk2 activating kinase activity. Proc. Natl. Acad. Sci. USA 96:14961–14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nead, M. A., L. A. Baglia, M. J. Antinore, J. W. Ludlow, and D. J. McCance. 1998. Rb binds c-Jun and activates transcription. EMBO J. 17:2342–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nilsson, I., and I. Hoffmann. 2000. Cell cycle regulation by the Cdc25 phosphatase family. Prog. Cell Cycle Res. 4:107–114. [DOI] [PubMed] [Google Scholar]
- 64.O’Connell, M. J., J. M. Raleigh, H. M. Verkade, and P. Nurse. 1997. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 16:545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patel, D., S. M. Huang, L. A. Baglia, and D. J. McCance. 1999. The E6 protein of human papillomavirus type 16 binds to and inhibits co-activation by CBP and p300. EMBO J. 18:5061–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patrick, D. R., A. Oliff, and D. C. Heimbrook. 1994. Identification of a novel retinoblastoma gene product binding site on human papillomavirus type 16 E7 protein. J. Biol. Chem. 269:6842–6850. [PubMed] [Google Scholar]
- 67.Phelps, W. C., K. Munger, C. L. Yee, J. A. Barnes, and P. M. Howley. 1992. Structure-function analysis of the human papillomavirus type 16 E7 oncoprotein. J. Virol. 66:2418–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Phelps, W. C., C. L. Yee, K. Munger, and P. M. Howley. 1988. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell 53:539–547. [DOI] [PubMed] [Google Scholar]
- 69.Polyak, K., J. Y. Kato, M. J. Solomon, C. J. Sherr, J. Massague, J. M. Roberts, and A. Koff. 1994. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 8:9–22. [DOI] [PubMed] [Google Scholar]
- 70.Polyak, K., M. H. Lee, H. Erdjument-Bromage, A. Koff, J. M. Roberts, P. Tempst, and J. Massague. 1994. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78:59–66. [DOI] [PubMed] [Google Scholar]
- 71.Poumay, Y., and M. R. Pittelkow. 1995. Cell density and culture factors regulate keratinocyte commitment to differentiation and expression of suprabasal K1/K10 keratins. J. Invest. Dermatol. 104:271–276. [DOI] [PubMed] [Google Scholar]
- 72.Ruesch, M. N., and L. A. Laimins. 1998. Human papillomavirus oncoproteins alter differentiation-dependent cell cycle exit on suspension in semisolid medium. Virology 250:19–29. [DOI] [PubMed] [Google Scholar]
- 73.Ruesch, M. N., and L. A. Laimins. 1997. Initiation of DNA synthesis by human papillomavirus E7 oncoproteins is resistant to p21-mediated inhibition of cyclin E-cdk2 activity. J. Virol. 71:5570–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sambucetti, L. C., D. D. Fischer, S. Zabludoff, P. O. Kwon, H. Chamberlin, N. Trogani, H. Xu, and D. Cohen. 1999. Histone deacetylase inhibition selectively alters the activity and expression of cell cycle proteins leading to specific chromatin acetylation and antiproliferative effects. J. Biol. Chem. 274:34940–34947. [DOI] [PubMed] [Google Scholar]
- 75.Sandhu, C., J. Donovan, N. Bhattacharya, M. Stampfer, P. Worland, and J. Slingerland. 2000. Reduction of Cdc25A contributes to cyclin E1-Cdk2 inhibition at senescence in human mammary epithelial cells. Oncogene 19:5314–5323. [DOI] [PubMed] [Google Scholar]
- 76.Sexl, V., J. A. Diehl, C. J. Sherr, R. Ashmun, D. Beach, and M. F. Roussel. 1999. A rate limiting function of cdc25A for S phase entry inversely correlates with tyrosine dephosphorylation of Cdk2. Oncogene 18:573–582. [DOI] [PubMed] [Google Scholar]
- 77.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13:1501–1512. [DOI] [PubMed] [Google Scholar]
- 78.Spitkovsky, D., P. Jansen-Durr, E. Karsenti, and I. Hoffman. 1996. S-phase induction by adenovirus E1A requires activation of cdc25a tyrosine phosphatase. Oncogene 12:2549–2554. [PubMed] [Google Scholar]
- 79.Straight, S. W., B. Herman, and D. J. McCance. 1995. The E5 oncoprotein of human papillomavirus type 16 inhibits the acidification of endosomes in human keratinocytes. J. Virol. 69:3185–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Straight, S. W., P. M. Hinkle, R. J. Jewers, and D. J. McCance. 1993. The E5 oncoprotein of human papillomavirus type 16 transforms fibroblasts and effects the downregulation of the epidermal growth factor receptor in keratinocytes. J. Virol. 67:4521–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Topley, G. I., R. Okuyama, J. G. Gonzales, C. Conti, and G. P. Dotto. 1999. p21(WAF1/Cip1) functions as a suppressor of malignant skin tumor formation and a determinant of keratinocyte stem-cell potential. Proc. Natl. Acad. Sci. USA 96:9089–9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vigo, E., H. Muller, E. Prosperini, G. Hateboer, P. Cartwright, M. C. Moroni, and K. Helin. 1999. CDC25A phosphatase is a target of E2F and is required for efficient E2F-induced S phase. Mol. Cell. Biol. 19:6379–6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu, L., E. C. Goodwin, L. K. Naeger, E. Vigo, K. Galaktionov, K. Helin, and D. DiMaio. 2000. E2F-Rb complexes assemble and inhibit cdc25A transcription in cervical carcinoma cells following repression of human papillomavirus oncogene expression. Mol. Cell Biol 20:7059–7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zariwala, M., J. Liu, and Y. Xiong. 1998. Cyclin E2, a novel human G1 cyclin and activating partner of CDK2 and CDK3, is induced by viral oncoproteins. Oncogene 17:2787–2798. [DOI] [PubMed] [Google Scholar]
- 85.Zerfass-Thome, K., A. Schulze, W. Zwerschke, B. Vogt, K. Helin, J. Bartek, B. Henglein, P. Jansen-Durr, B. Mannhardt, R. Tindle, and J. W. Botz. 1997. p27KIP1 blocks cyclin E-dependent transactivation of cyclin A gene expression: inactivation of the cdk inhibitor p27KIP1 by the human papillomavirus type 16 E7 oncoprotein. Mol. Cell. Biol. 17:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang, H. S., M. Gavin, A. Dahiya, A. A. Postigo, D. Ma, R. X. Luo, J. W. Harbour, and D. C. Dean. 2000. Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell 101:79–89. [DOI] [PubMed] [Google Scholar]
- 87.Zhang, Y., G. LeRoy, H. P. Seelig, W. S. Lane, and D. Reinberg. 1998. The dermatomyositis-specific autoantigen Mi2 is a component of a complex containing histone deacetylase and nucleosome remodeling activities. Cell 95:279–289. [DOI] [PubMed] [Google Scholar]
- 88.Zimmermann, H., R. Degenkolbe, H. U. Bernard, and M. J. O’Connor. 1999. The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300. J. Virol. 73:6209–6219. [DOI] [PMC free article] [PubMed] [Google Scholar]